Molecular Dynamics Simulation on the Effect of Bonding Pressure on Thermal Bonding of Polymer Microfluidic Chip
Abstract
:1. Introduction
2. Simulation Models and Methods
2.1. Materials and model Constructing
2.2. Force Field and Simulation Procedure
3. Results and Discussion
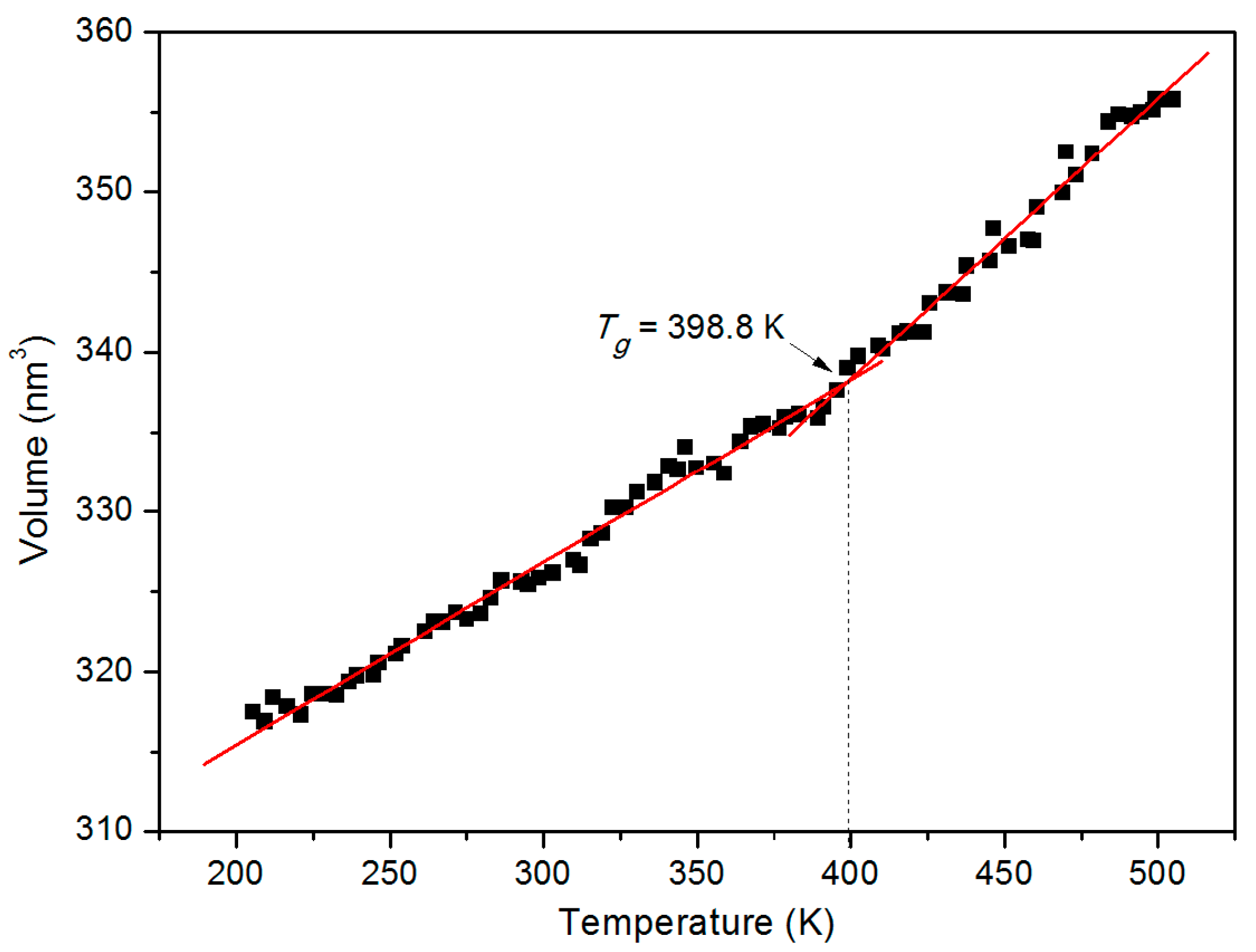
3.1. Glass Transition Temperature
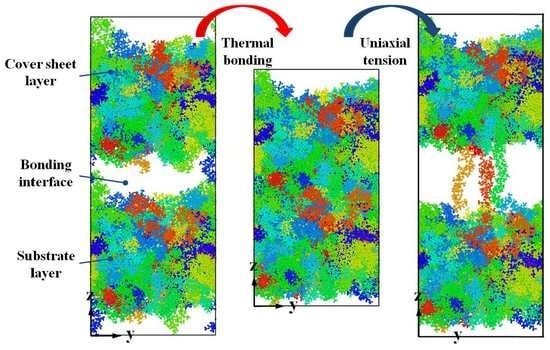
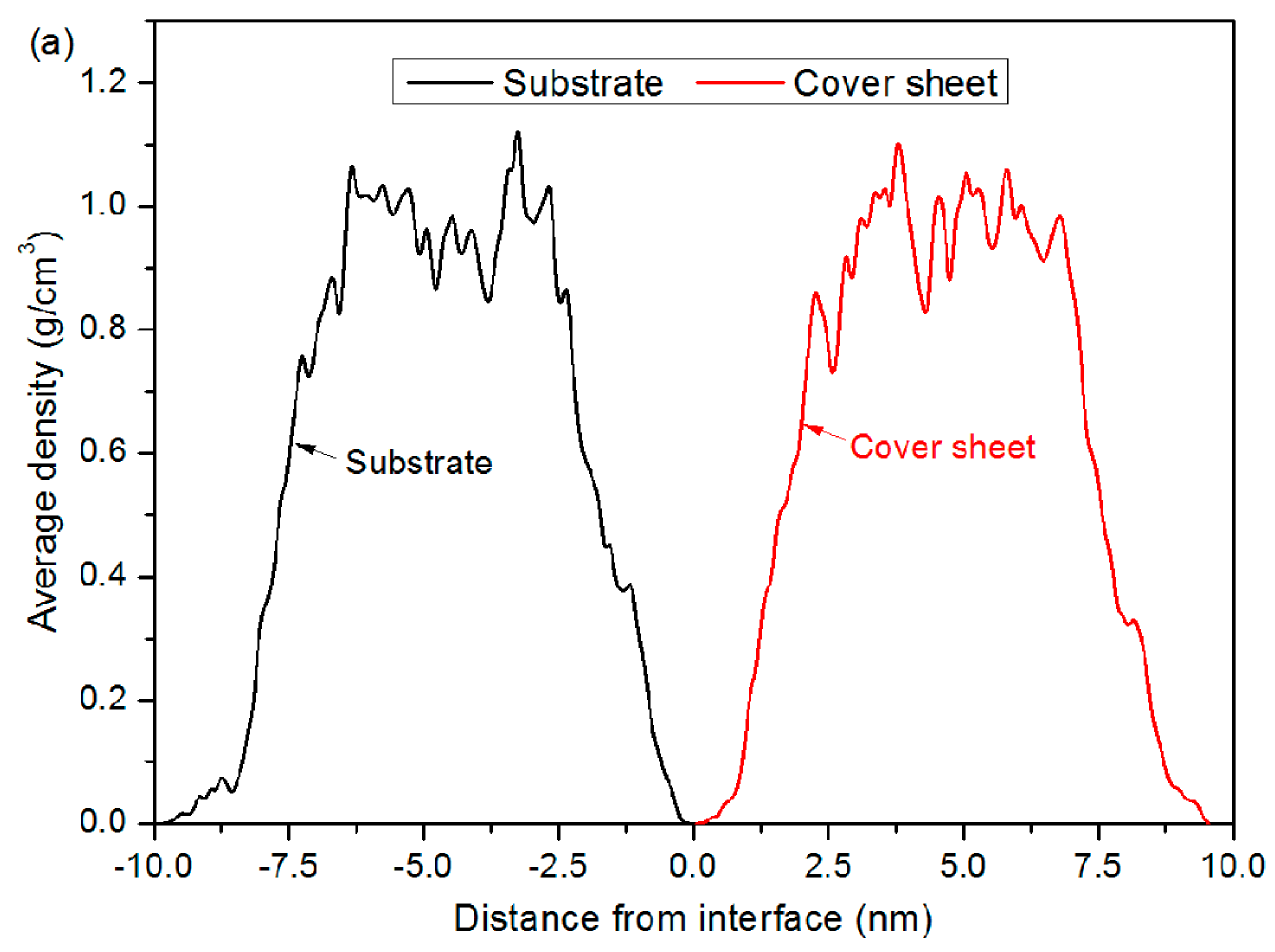
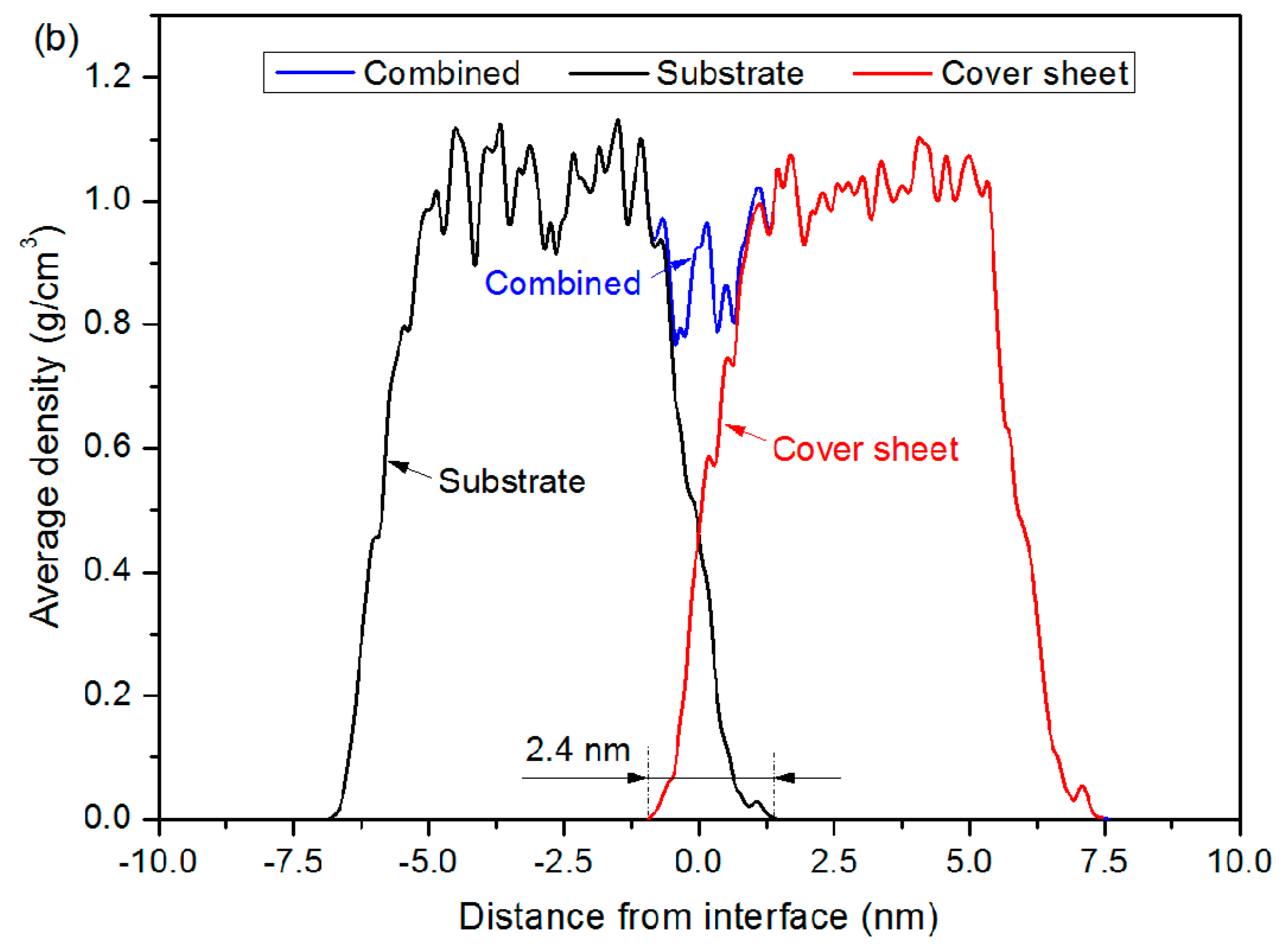
3.2. Bonding Process
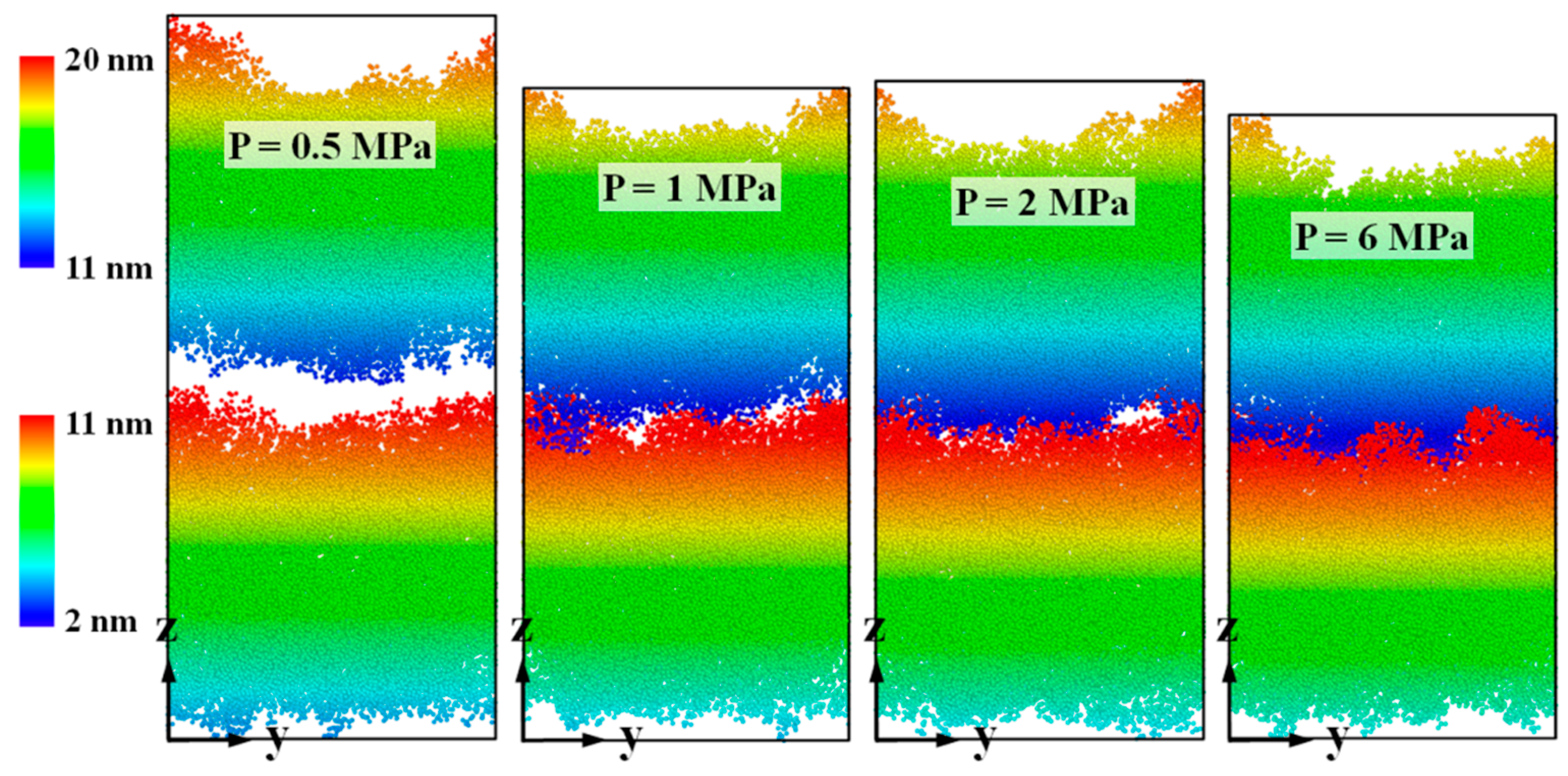
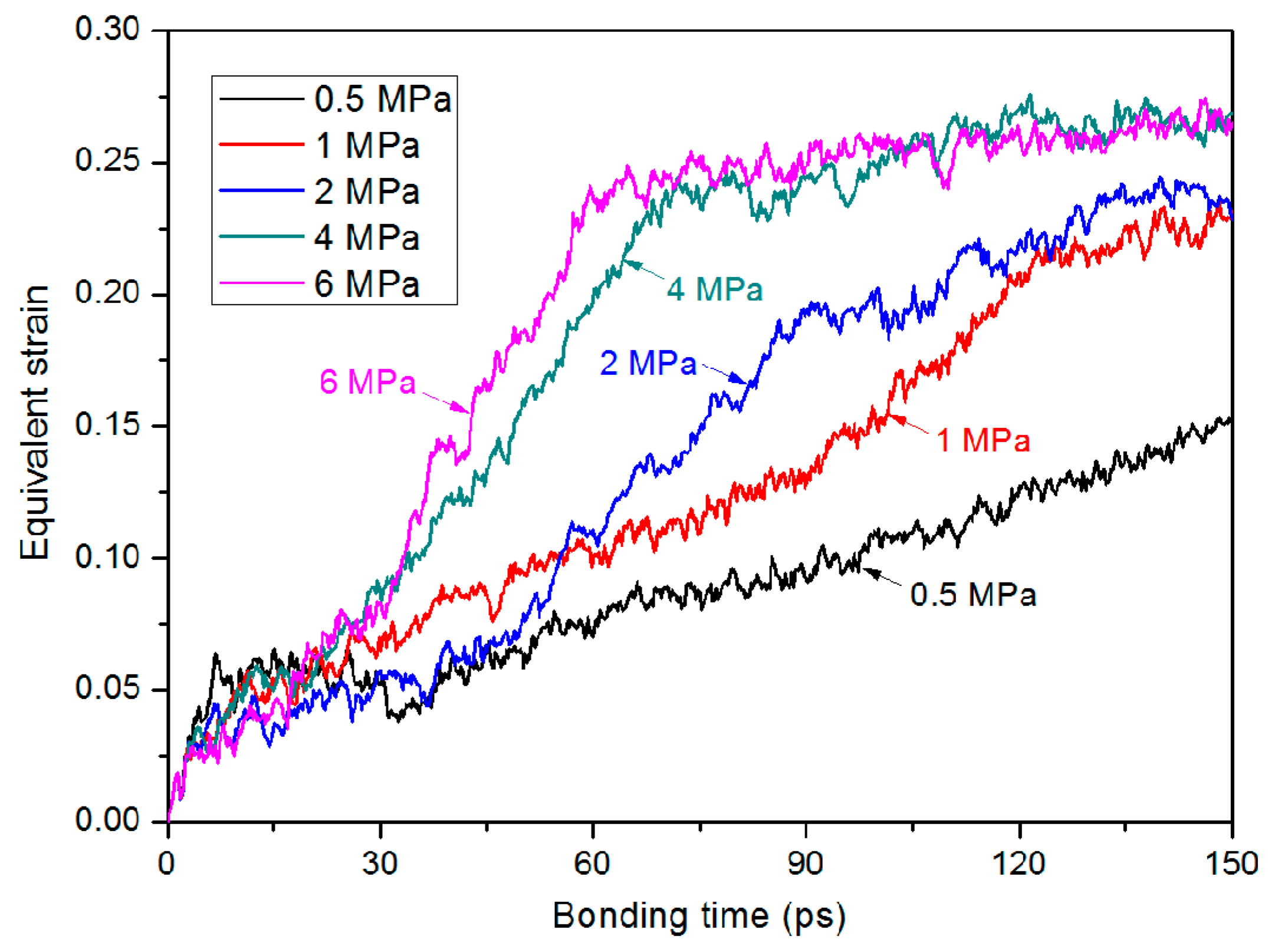
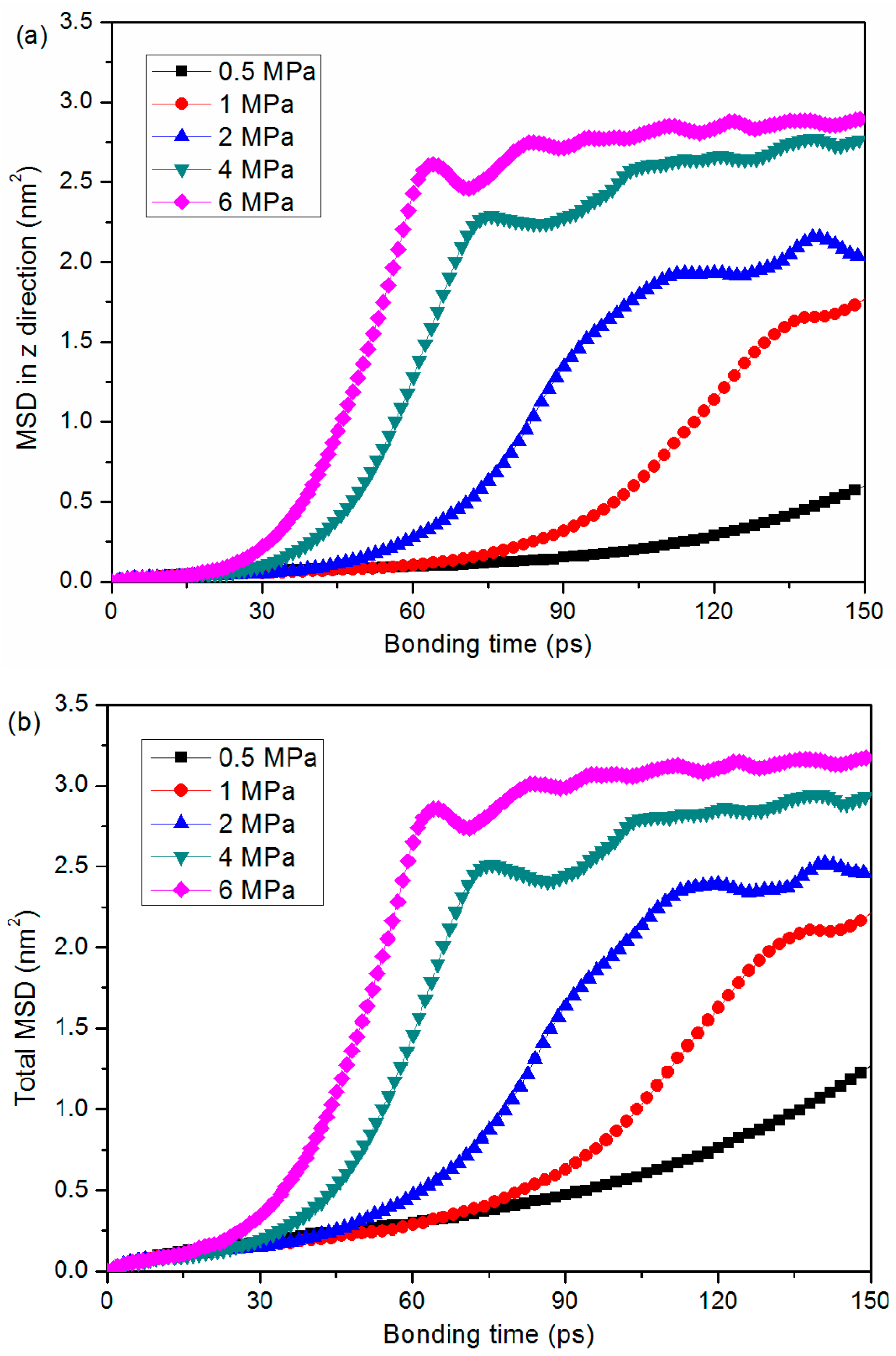
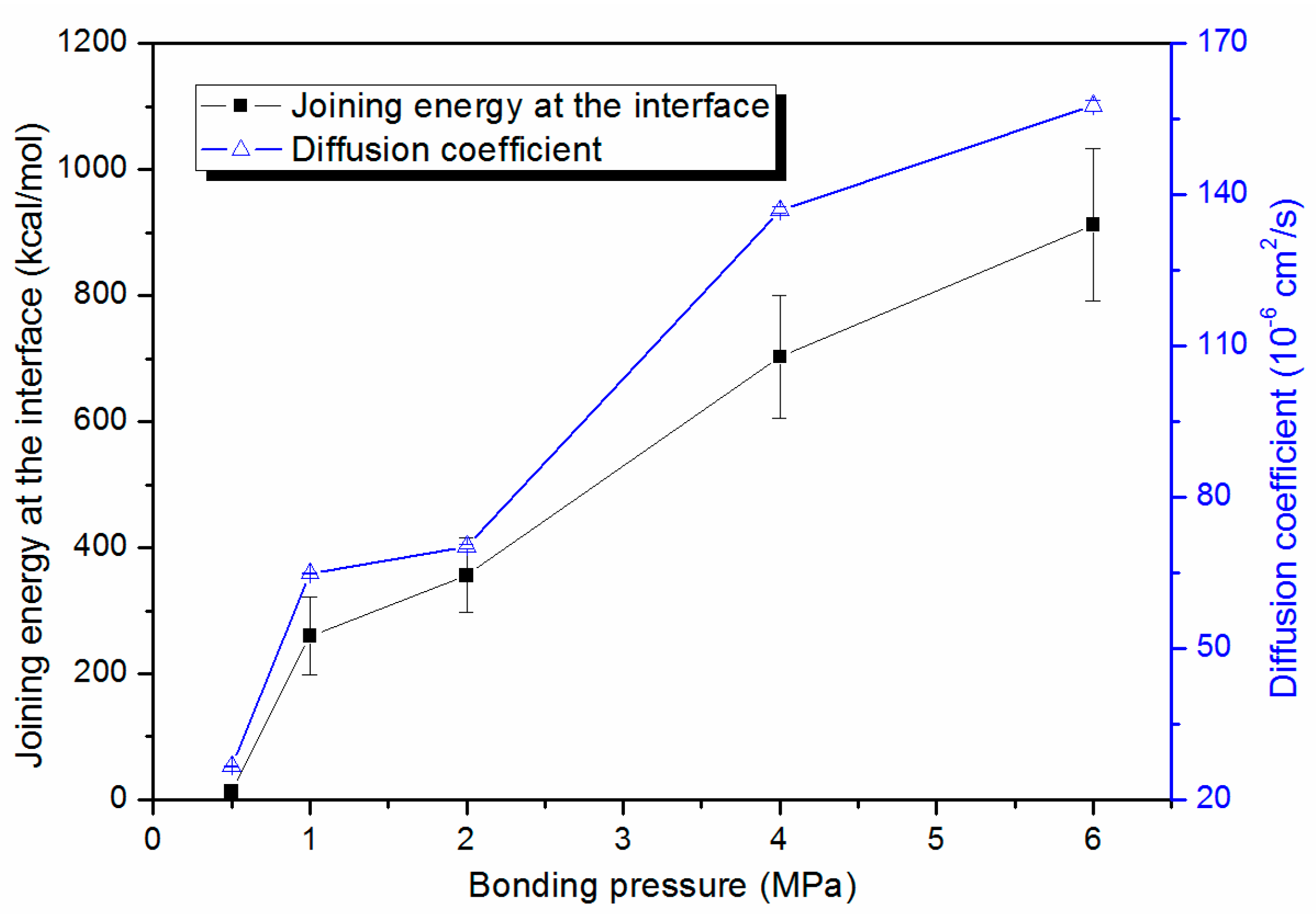
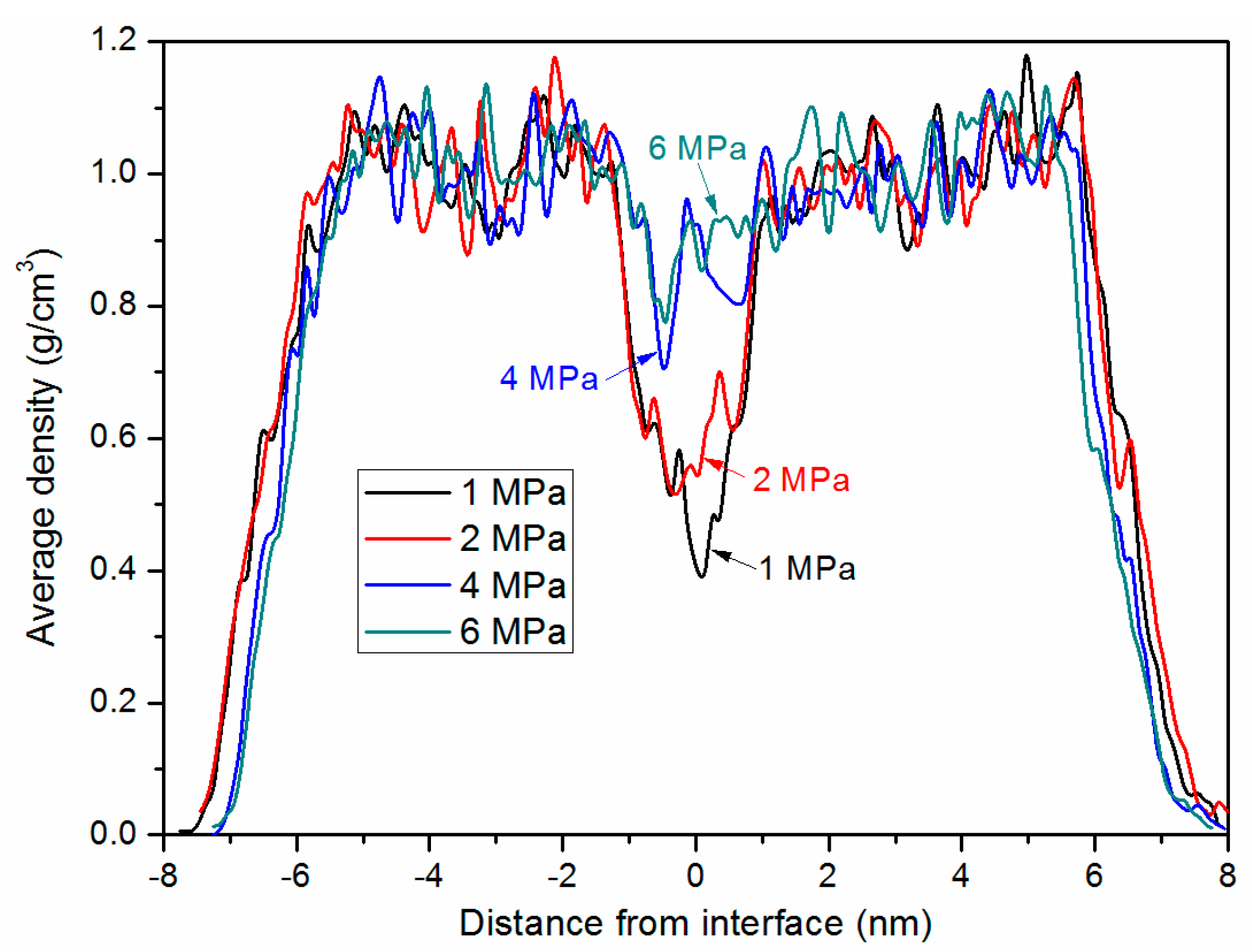
3.3. Bonding Pressure Dependence
3.4. Debonding Process
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Duarte-Guevara, P.; Duarte-Guevara, C.; Ornob, A.; Bashir, R. On-chip pma labeling of foodborne pathogenic bacteria for viable qpcr and qlamp detection. Microfluid. Nanofluid. 2016, 20, 114. [Google Scholar] [CrossRef]
- Gitlin, L.; Schulze, P.; Ohla, S.; Bongard, H.J.; Belder, D. Surface modification of pdms microfluidic devices by controlled sulfuric acid treatment and the application in chip electrophoresis. Electrophoresis 2015, 36, 449–456. [Google Scholar] [CrossRef]
- Lee, G.B.; Chen, S.H.; Huang, G.R.; Sung, W.C.; Lin, Y.H. Microfabricated plastic chips by hot embossing methods and their applications for DNA separation and detection. Sens. Actuators B 2001, 75, 142–148. [Google Scholar] [CrossRef] [Green Version]
- Utko, P.; Persson, F.; Kristensen, A.; Larsen, N.B. Injection molded nanofluidic chips: Fabrication method and functional tests using single-molecule DNA experiments. Lab Chip 2011, 11, 303–308. [Google Scholar] [CrossRef]
- Wang, X.; Liedert, C.; Liedert, R.; Papautsky, I. A disposable, roll-to-roll hot-embossed inertial microfluidic device for size-based sorting of microbeads and cells. Lab Chip 2016, 16, 1821–1830. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, Y.-L.; Wang, W.; Ding, H.; Sun, H.-B. On-chip laser processing for the development of multifunctional microfluidic chips. Laser Photonics Rev. 2017, 11, 1600116. [Google Scholar] [CrossRef]
- Guckenberger, D.J.; de Groot, T.E.; Wan, A.M.; Beebe, D.J.; Young, E.W. Micromilling: A method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 2015, 15, 2364–2378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domansky, K.; Sliz, J.D.; Wen, N.; Hinojosa, C.; Thompson, G.; Fraser, J.P.; Hamkins-Indik, T.; Hamilton, G.A.; Levner, D.; Ingber, D.E. Sebs elastomers for fabrication of microfluidic devices with reduced drug absorption by injection molding and extrusion. Microfluid. Nanofluid. 2017, 21, 107. [Google Scholar] [CrossRef]
- Maghsoudi, K.; Jafari, R.; Momen, G.; Farzaneh, M. Micro-nanostructured polymer surfaces using injection molding: A review. Mater. Today Commun. 2017, 13, 126–143. [Google Scholar] [CrossRef]
- Norouzi, A.R.; Nikfarjam, A.; Hajghassem, H. Pdms–pmma bonding improvement using sio2 intermediate layer and its application in fabricating gas micro valves. Microsyst. Technol. 2018, 24, 2727–2736. [Google Scholar] [CrossRef]
- Song, I.H.; Park, T. Pmma solution assisted room temperature bonding for pmma(-)pc hybrid devices. Micromachines 2017, 8, 284. [Google Scholar] [CrossRef] [PubMed]
- Duffy, D.C.; McDonald, J.C.; Schueller, O.J.; Whitesides, G.M. Rapid prototyping of microfluidic systems in poly(dimethylsiloxane). Anal. Chem. 1998, 70, 4974–4984. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.; Jiang, B.; Zhu, L.; Jiang, F. A process analysis for microchannel deformation and bonding strength by in-mold bonding of microfluidic chips. J. Polym. Eng. 2014, 35, 267–275. [Google Scholar] [CrossRef]
- Chow, W.W.Y.; Lei, K.F.; Shi, G.; Li, W.J.; Huang, Q. Microfluidic channel fabrication by pdms-interface bonding. Smart Mater. Struct. 2006, 15, S112–S116. [Google Scholar] [CrossRef]
- Sun, S.; Chen, S.; Weng, X.; Shan, F.; Hu, S. Effect of carbon nanotube addition on the interfacial adhesion between graphene and epoxy: A molecular dynamics simulation. Polymers 2019, 11, 121. [Google Scholar] [CrossRef]
- Hossain, D.; Tschopp, M.A.; Ward, D.K.; Bouvard, J.L.; Wang, P.; Horstemeyer, M.F. Molecular dynamics simulations of deformation mechanisms of amorphous polyethylene. Polymer 2010, 51, 6071–6083. [Google Scholar] [CrossRef]
- Wei, Q.; Wang, Y.; Rao, Y.; Jiang, A.; Zhang, K.; Lu, T.; Chen, X. Evaluating the effects of nanosilica on mechanical and tribological properties of polyvinyl alcohol/polyacrylamide polymer composites for artificial cartilage from an atomic level. Polymers 2019, 11, 76. [Google Scholar]
- Zhou, M.; Jiang, B.; Weng, C. Molecular dynamics study on polymer filling into nano-cavity by injection molding. Comput. Mater.Sci. 2016, 120, 36–42. [Google Scholar] [CrossRef]
- Awasthi, A.P.; Lagoudas, D.C.; Hammerand, D.C. Modeling of graphene–polymer interfacial mechanical behavior using molecular dynamics. Model. Simul. Mater. Sci. Eng. 2009, 17, 015002. [Google Scholar] [CrossRef]
- Yokomizo, K.; Banno, Y.; Kotaki, M. Molecular dynamics study on the effect of molecular orientation on polymer welding. Polymer 2012, 53, 4280–4286. [Google Scholar] [CrossRef]
- Yang, H.; Yu, K.; Mu, X.; Wei, Y.; Guo, Y.; Qi, H.J. Molecular dynamics studying on welding behavior in thermosetting polymers due to bond exchange reactions. RSC Adv. 2016, 6, 22476–22487. [Google Scholar] [CrossRef] [Green Version]
- Ge, T.; Pierce, F.; Perahia, D.; Grest, G.S.; Robbins, M.O. Molecular dynamics simulations of polymer welding: Strength from interfacial entanglements. Phys. Rev. Lett. 2013, 110, 098301. [Google Scholar] [CrossRef] [PubMed]
- Ekdawi-Sever, N.C.; Conrad, P.B.; de Pablo, J.J. Molecular simulation of sucrose solutions near the glass transition temperature. J. Phys. Chem. A 2001, 105, 734–742. [Google Scholar] [CrossRef]
- LAMMPS Molecular Dynamics Simulator. Available online: https://lammps.sandia.gov/ (accessed on 25 June 2014).
- Kang, J.-H.; Kim, K.-S.; Kim, K.-W. Molecular dynamics study on the effects of stamp shape, adhesive energy, and temperature on the nanoimprint lithography process. Appl. Surf. Sci. 2010, 257, 1562–1572. [Google Scholar] [CrossRef]
- Chu, C.P.; Jiang, B.Y.; Weng, C.; Jiang, F.Z. Microchannel deformation of polymer chip in in-mold bonding. Int. Polym. Process. 2014, 29, 245–251. [Google Scholar] [CrossRef]
- Jaramillo, E.; Wilson, N.; Christensen, S.; Gosse, J.; Strachan, A. Energy-based yield criterion for pmma from large-scale molecular dynamics simulations. Phys. Rev. B 2012, 85, 024114. [Google Scholar] [CrossRef]


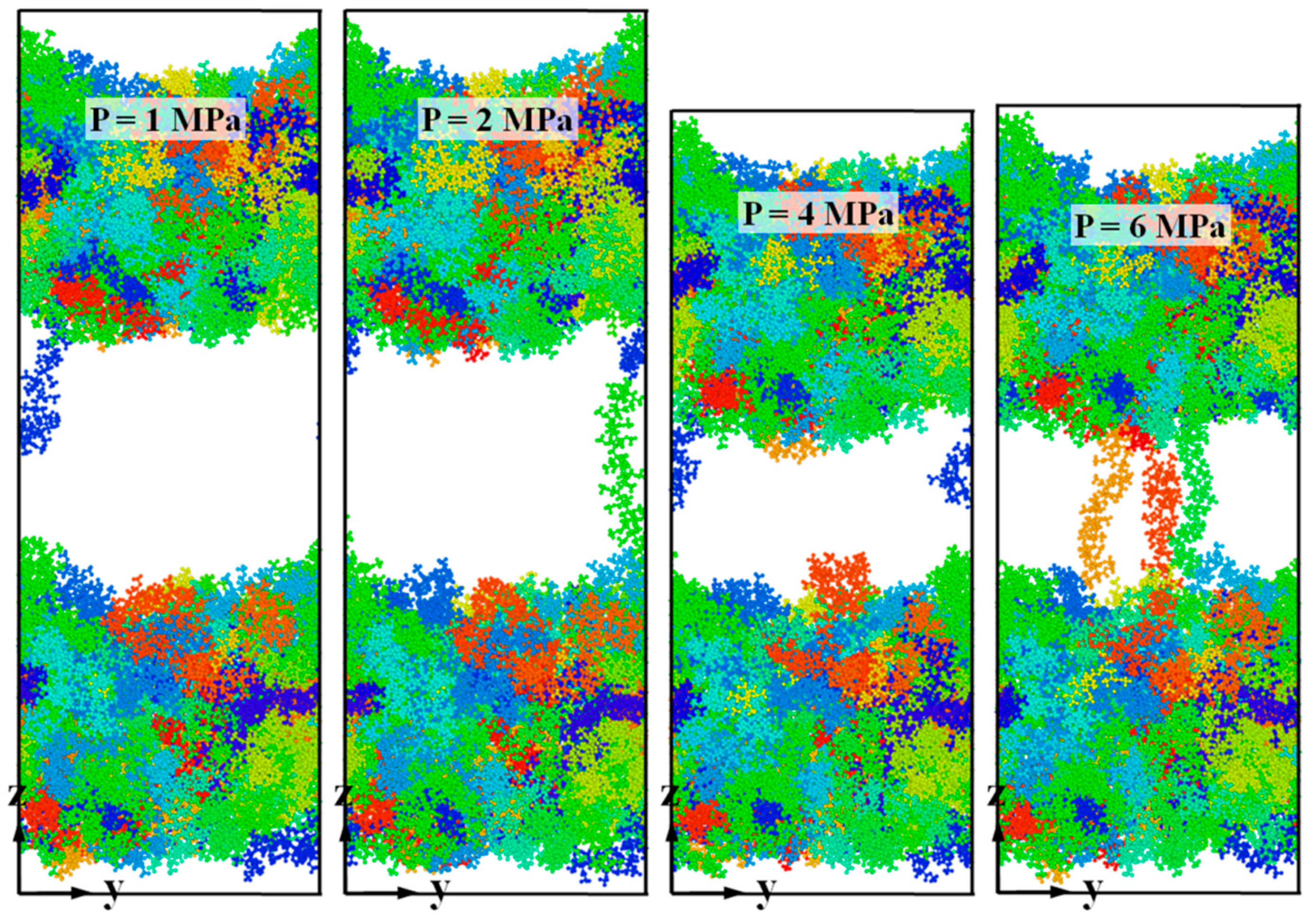
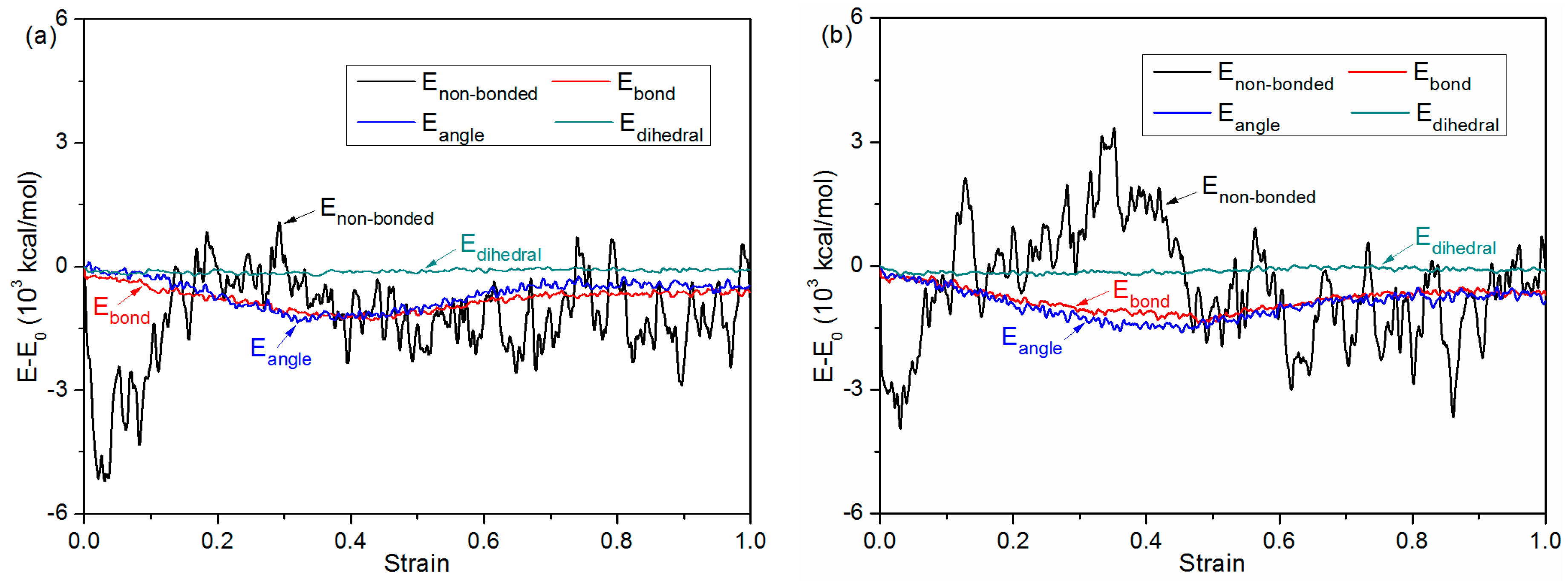
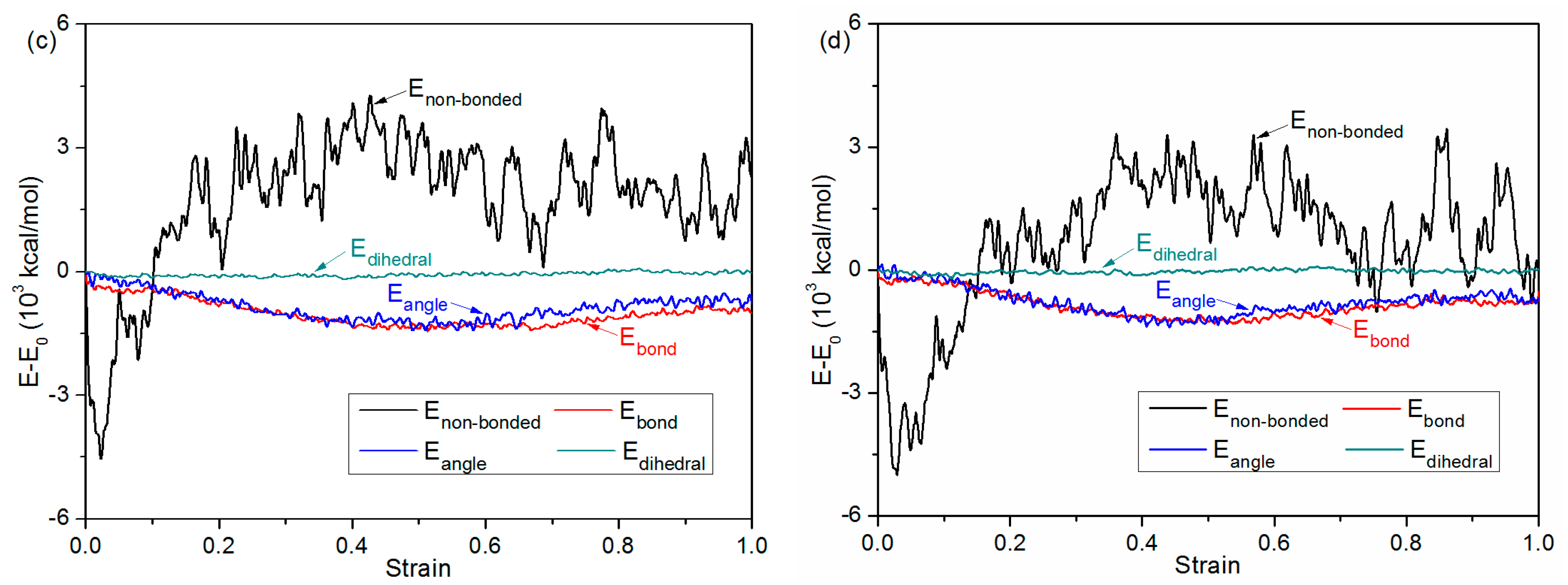
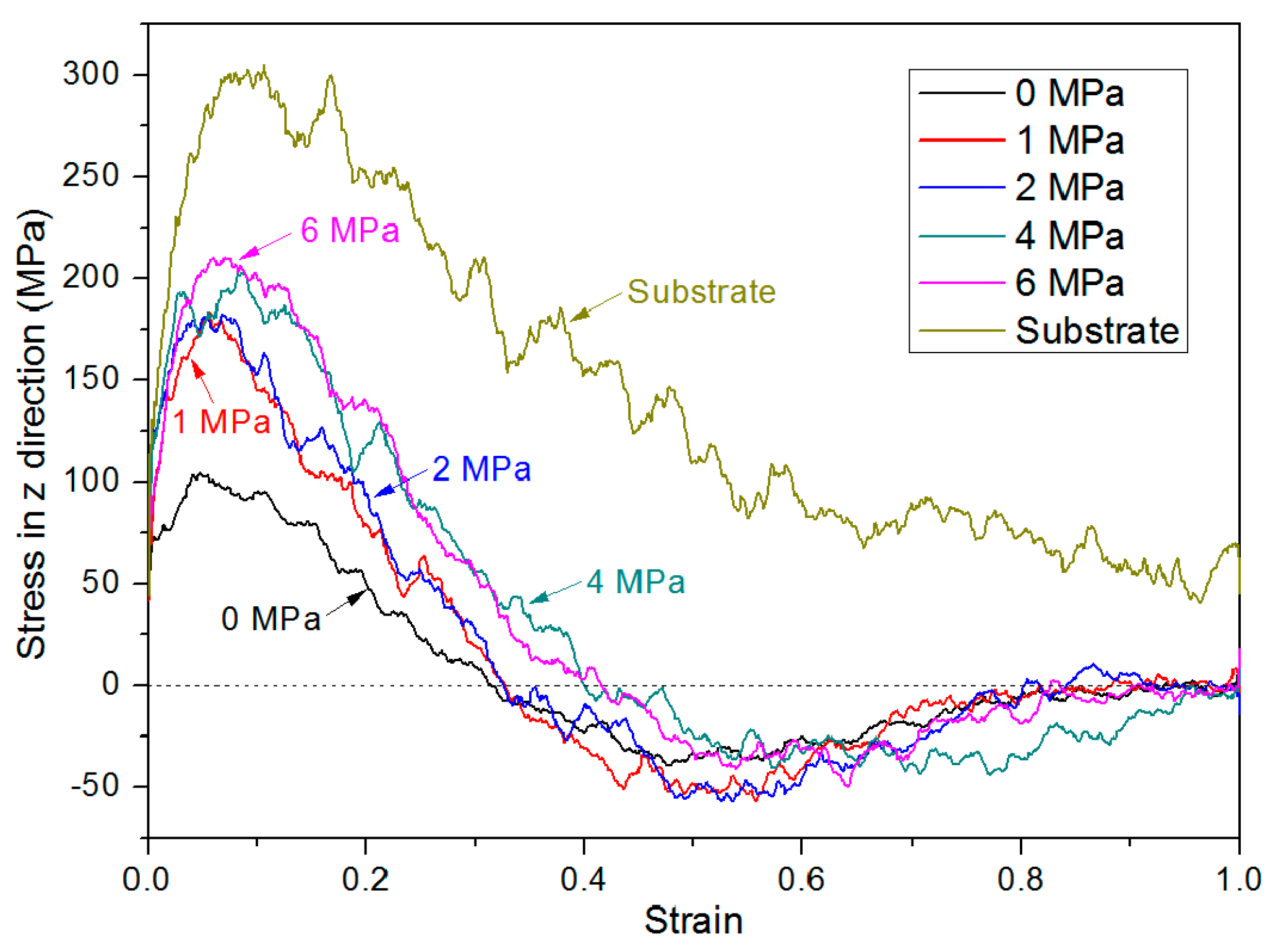
| Simulation model/Bonding Pressure (MPa) | Substrate | 0 | 1 | 2 | 4 | 6 |
|---|---|---|---|---|---|---|
| Peak yield stress (MPa) | 305.3 | 104.0 | 184.0 | 182.9 | 203.3 | 210.4 |
| Yield strain | 0.106 | 0.048 | 0.057 | 0.068 | 0.085 | 0.070 |
| Strain at zero stress | N/A | 0.314 | 0.327 | 0.326 | 0.400 | 0.416 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, M.; Xiong, X.; Drummer, D.; Jiang, B. Molecular Dynamics Simulation on the Effect of Bonding Pressure on Thermal Bonding of Polymer Microfluidic Chip. Polymers 2019, 11, 557. https://doi.org/10.3390/polym11030557
Zhou M, Xiong X, Drummer D, Jiang B. Molecular Dynamics Simulation on the Effect of Bonding Pressure on Thermal Bonding of Polymer Microfluidic Chip. Polymers. 2019; 11(3):557. https://doi.org/10.3390/polym11030557
Chicago/Turabian StyleZhou, Mingyong, Xiang Xiong, Dietmar Drummer, and Bingyan Jiang. 2019. "Molecular Dynamics Simulation on the Effect of Bonding Pressure on Thermal Bonding of Polymer Microfluidic Chip" Polymers 11, no. 3: 557. https://doi.org/10.3390/polym11030557