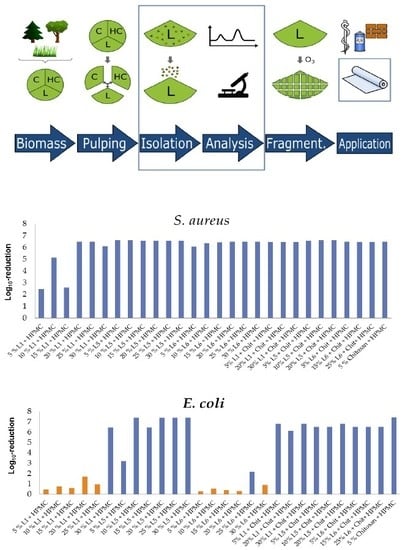
Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Lignin Isolation and Purification
2.2. Lignin Purity, Ash and Sugar Content via NREL Measurements
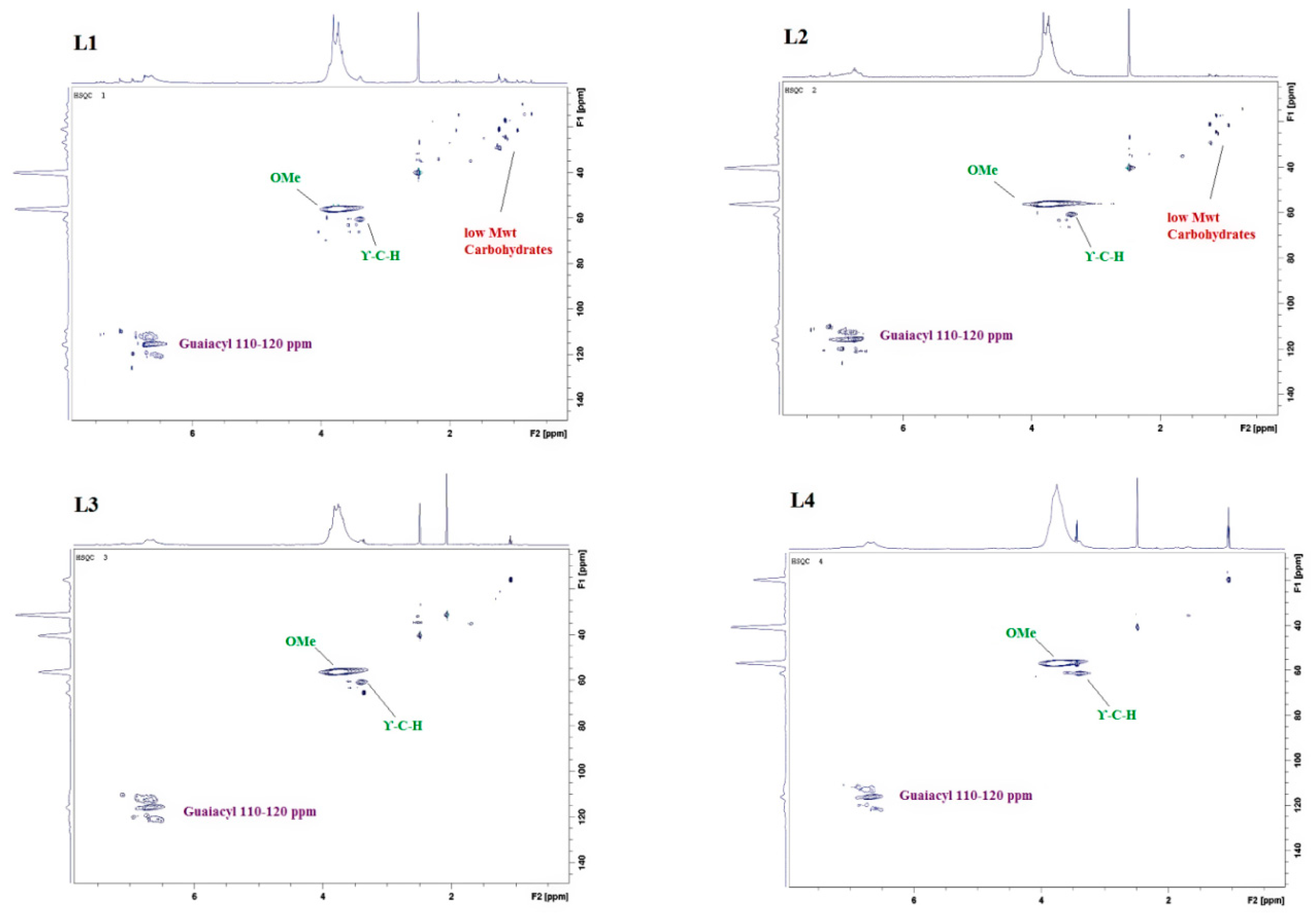
2.3. 2D Heteronuclear Single Quantum Coherence (HSQC) NMR Analysis
2.4. 31P NMR Analysis
2.5. Antioxidant Activity (DPPH Assay)
2.6. Antimicrobial Activity of Lignins (Zone of Inhibition Test)
2.7. Film Formation
2.8. Antioxidant Activity (DPPH Test)
2.9. Antimicrobial Activity of the Films
3. Results and Discussion
3.1. Antimicrobial Activity of Lignin
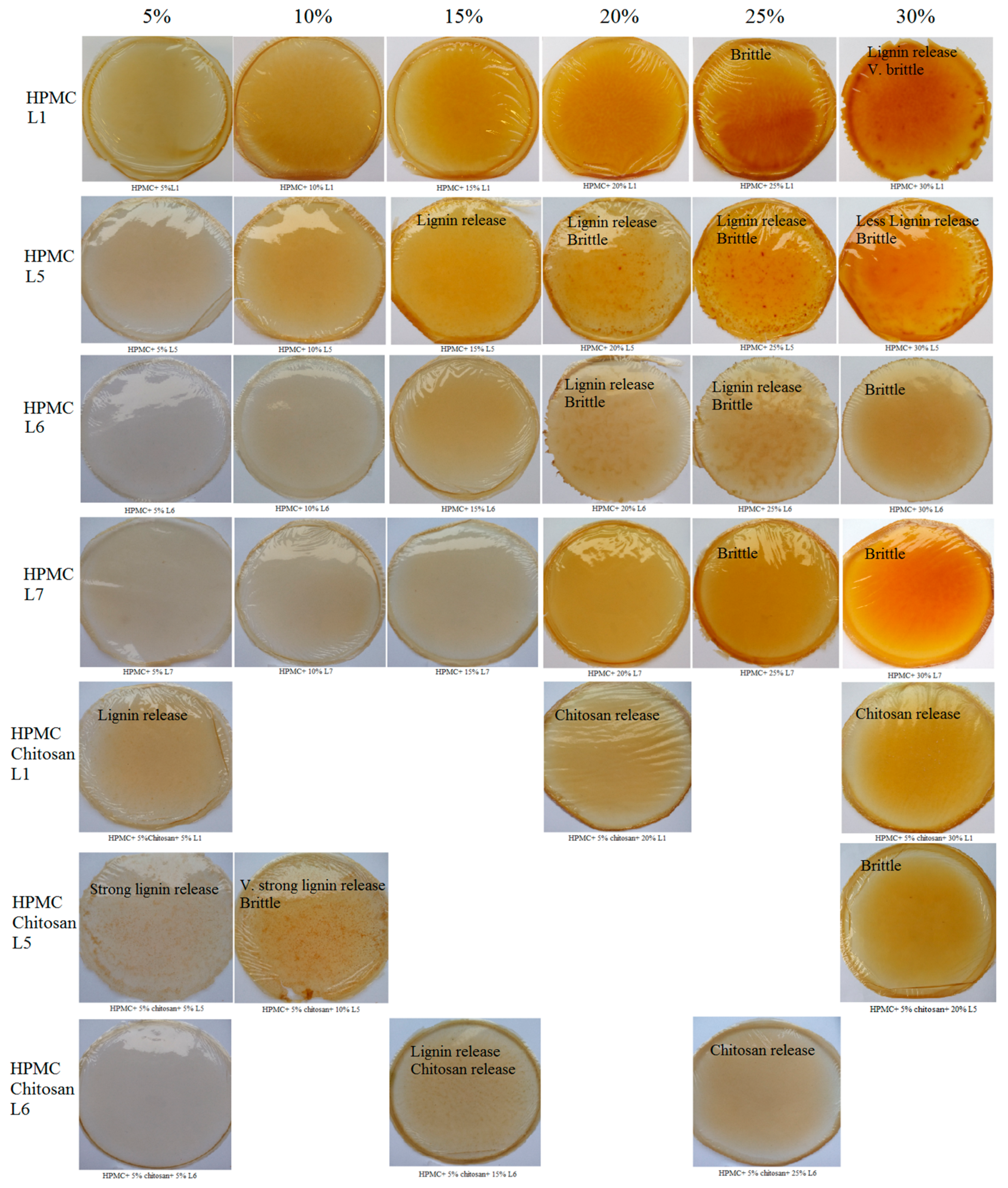
3.2. Preparation of Lignin-Derived Composites
3.3. Antiradical Activity of Lignin-Derived Films
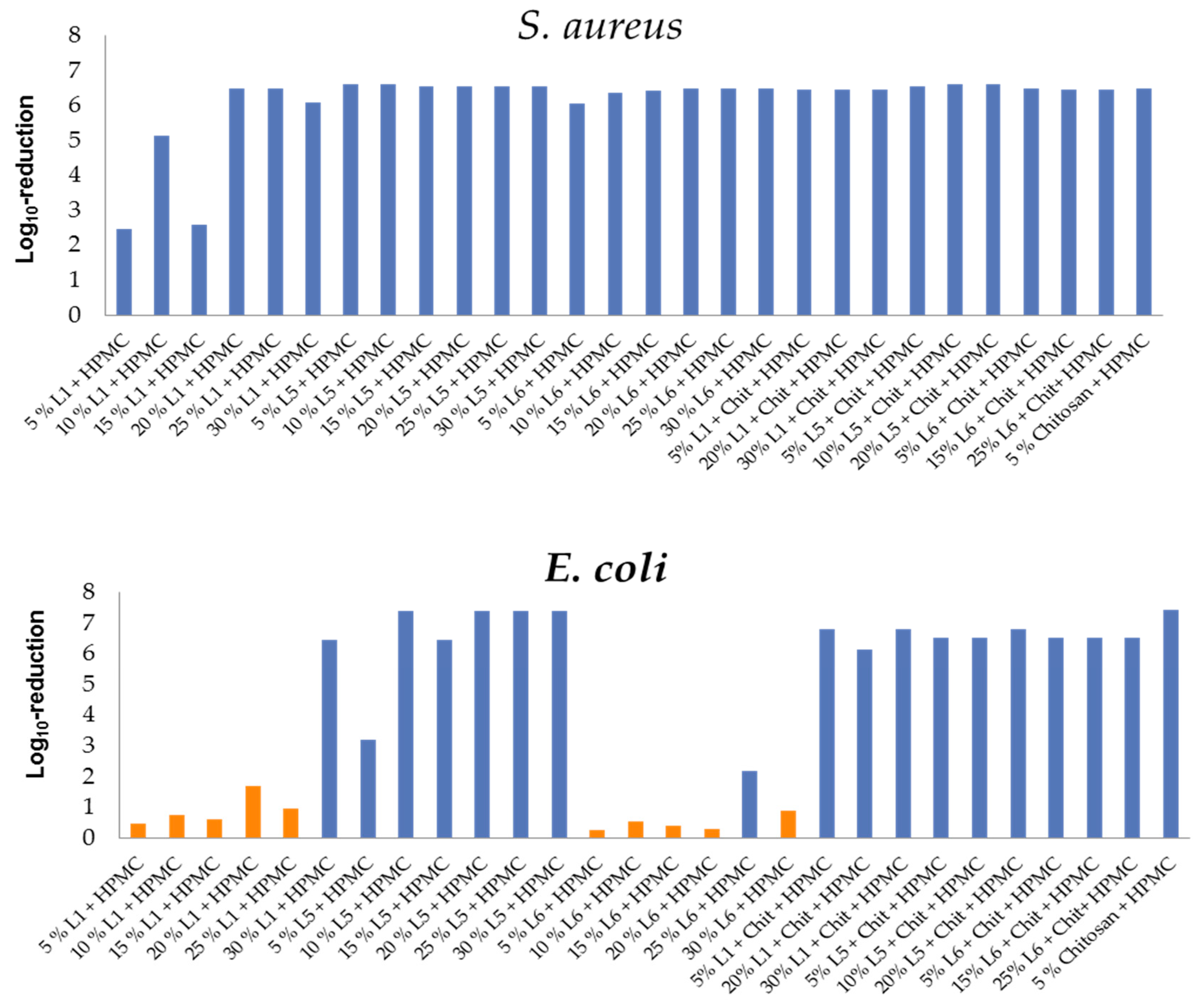
3.4. Antimicrobial Activity of HPMC/Lignin and HPMC/Lignin/Chitosan Composites
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, G.-G.; Fu, G.-Q.; Wang, X.-J.; Gong, X.-D.; Niu, Y.-S.; Peng, F.; Yao, C.-L.; Sun, R.-C. Facile synthesis of high strength hot-water wood extract films with oxygen-barrier performance. Sci. Rep. 2017, 7, 41075. [Google Scholar] [CrossRef]
- Dilkes-Hoffman, L.S.; Pratt, S.; Lant, P.A.; Laycock, B. The Role of Biodegradable Plastic in Solving Plastic Solid Waste Accumulation. In Plastics to Energy; Fuel, Chemicals, and Sustainability Implications Plastics Design Library 2019; William Andrew Publishing: Norwich, NY, USA, 2019; pp. 469–505. [Google Scholar]
- Muthuraj, R.; Misra, M.; Kumar, A. Biodegradable compatibilized polymer blends for packaging applications: A literature review. J. Appl. Polym. Sci. 2018, 135, 45726. [Google Scholar] [CrossRef]
- Abdul Khalil, H.P.S.; Tye, Y.Y.; Leh, C.P.; Saurabh, C.K.; Ariffin, F.; Fizree, H.M.; Mohamed, A.; Suriani, A.B. Cellulose Reinforced Biodegradable Polymer Composite Film for Packaging Applications. In Bionanocomposites for Packaging Applications; Jawaid, M., Swain, S., Eds.; Springer: Cham, The Netherlands, 2018. [Google Scholar]
- Shankar, S.; Reddy, J.P.; Rhim, J.-W. Effect of lignin on water vapor barrier, mechanical, and structural properties of agar/lignin composite films. Int. J. Biolog. Macromol. 2015, 81, 267–273. [Google Scholar] [CrossRef] [PubMed]
- Kamm, B.; Kamm, M.; Hirth, T.; Schulze, M. Lignocelluloses Based Chemical Products and Product Family Trees. In Biorefineries-Industrial Processes and Products; Kamm, M., Kamm, B., Gruber, P.C., Eds.; Wiley-VCH: Weinheim, Germany, 2006; pp. 97–150. ISBN 3-527-31027-4. [Google Scholar]
- Kamm, B.; Gruber, P.R.; Kamm, M. Biorefineries-Industrial Processes and Products. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: Weinheim, Germany, 2016; ISBN 9783527306732. [Google Scholar]
- Su, Y.; Yang, B.; Liu, J.; Sun, B.; Cao, C.; Zou, X.; Lutes, R.; He, Z. Prospects for Replacement of Some Plastics in Packaging with Lignocellulose Materials: A Brief Review. BioResources 2018, 13, 4550–4576. [Google Scholar] [CrossRef]
- Ko, F.K.; Goudarzi, A.; Lin, L.-T.; Li, Y.; Kadla, J.F. Lignin-Based Composite Carbon Nanofibers. Lignin Polym. Compos. 2016, 167–194. [Google Scholar] [CrossRef]
- Rinaldi, R.; Jastrzebski, R.; Clough, M.T.; Ralph, J.; Kennema, M.; Bruijnincx, P.C.A.; Weckhuysen, B.M. Paving the Way for Lignin Valorisation: Recent Advances in Bioengineering, Biorefining and Catalysis. Angew. Chem. Int. Ed. 2016, 55, 2–54. [Google Scholar] [CrossRef]
- Liu, H.L.; Chung, H.Y. Lignin-Based Polymers via Graft Copolymerization. J. Polym. Sci. 2017, 55, 3515–3528. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Kenny, J.M.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Puglia, D. Effect of cellulose and lignin on disintegration, antimicrobial and antioxidant properties of PLA active films. Int. J. Biol. Macromol. 2016, 89, 360–368. [Google Scholar] [CrossRef] [PubMed]
- Alzagameem, A.; El Khaldi-Hansen, B.; Büchner, D.; Larkins, M.; Kamm, B.; Witzleben, S.; Schulze, M. Lignocellulosic Biomass as Source for Lignin-Based Environmentally Benign Antioxidants. Molecules 2018, 23, 2664. [Google Scholar] [CrossRef]
- Witzler, M.; Alzagameem, A.; Bergs, M.; El Khaldi-Hansen, B.; Klein, S.E.; Hielscher, D.; Kamm, B.; Kreyenschmidt, J.; Tobiasch, E.; Schulze, M. Lignin-Derived Biomaterials for Drug Release and Tissue Engineering. Molecules 2018, 23, 1885. [Google Scholar] [CrossRef]
- Klein, S.E.; Rumpf, J.; Kusch, P.; Albach, R.; Rehahn, M.; Witzleben, S.; Schulze, M. Utilization of Unmodified Kraft Lignin for the Preparation of Highly Flexible and Transparent Polyurethane Coatings. RSC Adv. 2018, 8, 40765. [Google Scholar] [CrossRef]
- Klein, S.E.; Rumpf, J.; Rehahn, M.; Witzleben, S.; Schulze, M. Biobased Flexible Polyurethane Coatings Prepared from Kraft Lignin: One-Pot Synthesis and Antioxidant Activity. J. Coat. Technol. Res. 2019, in press. [Google Scholar]
- Guo, M.; Jin, T.; Nghiem, N.P.; Fan, X.; Qi, P.X.; Jang, C.H.; Shao, L.; Wu, C. Assessment of Antioxidant and Antimicrobial Properties of Lignin from Corn Stover Residue Pretreated with Low-Moisture Anhydrous Ammonia and Enzymatic Hydrolysis Process. Appl. Biochem. Biotechnol. 2018, 184, 350–365. [Google Scholar] [CrossRef]
- Lupoi, J.S.; Singh, S.; Parthasarathi, R.; Simmons, B.A.; Henry, R.J. Recent innovations in analytical methods for the qualitative and quantitative assessment of lignin. Renew. Sustain. Energy Rev. 2015, 49, 871–906. [Google Scholar] [CrossRef]
- Alzagameem, A.; El Khaldi-Hansen, B.; Kamm, B.; Schulze, M. Lignocellulosic biomass for energy, biofuels, biomaterials, and chemicals. In Biomass and Green Chemistry, 1st ed.; Vaz, S., Jr., Ed.; Springer International Publishing: Basel, Switzerland, 2018; pp. 95–132. ISBN 978-3-319-66736-2. [Google Scholar]
- Hansen, B.; Kamm, B.; Schulze, M. Qualitative and quantitative analysis of lignins from different sources and isolation methods for an application as a biobased chemical resource and polymeric material. In Analytical Techniques and Methods for Biomass Products; Vaz, S., Jr., Seidl, P., Eds.; Springer: Berlin, Germany, 2017; pp. 15–44. ISBN 978-3-319-41414-0. [Google Scholar]
- Bergs, M. Einfluss von Miscanthus-Genotyp und Erntezeit auf Gehalt und Struktur von Lignin aus Organosolv-Verfahren. Ph.D. Thesis, Rheinische Friedrich-Wilhelms-University, Bonn, Germany, 2018. [Google Scholar]
- Bergs, M.; Völkering, G.; Kraska, T.; Do, X.T.; Kusch, P.; Monakhova, Y.; Konow, C.; Pude, R.; Schulze, M. Miscanthus X giganteus Stem versus Leave-derived Lignins Differing in Monolignol Ratio and Linkage. Int. J. Mol. Sci. 2019, 20, 1200. [Google Scholar] [CrossRef]
- Sadeghifar, H.; Venditti, R.; Jur, J.; Gorga, R.E.; Pawlak, J.J. Cellulose-Lignin Biodegradable and Flexible UV Protection Film. ACS Sustain. Chem. Eng. 2017, 5, 625–631. [Google Scholar] [CrossRef]
- Kaur, R.; Uppal, S.K.; Sharma, P. Antioxidant and Antibacterial Activities of Sugarcane Bagasse Lignin and Chemically Modified Lignins. Sugar Tech. 2017, 19, 675–680. [Google Scholar] [CrossRef]
- Hambardzumyan, A.; Foulon, L.; Bercu, N.B.; Pernes, M.; Maigret, J.E.; Molinari, M.; Chabbert, B.; Aguié-Béghin, V. Organosolv lignin as natural grafting additive to improve the water resistance of films using cellulose nanocrystals. Chem. Eng. J. 2015, 264, 780–788. [Google Scholar] [CrossRef]
- Yang, W.; Fortunati, E.; Dominici, F.; Giovanale, G.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Puglia, D. Synergic effect of cellulose and lignin nanostructures in PLA based systems for food antibacterial packaging. Eur. Polym. J. 2016, 79, 1–12. [Google Scholar] [CrossRef]
- Rhimi, W.; Boulila, A.; Gheribia, R.; Khwaldia, K. Development, characterization and application of hydroxypropylmethylcellulose films enriched with cypress seed extract. RCS Adv. 2018, 8, 23615–23622. [Google Scholar] [CrossRef]
- Yang, W.; Owczarek, J.S.; Fortunati, E.; Kozanecki, M.; Mazzaglia, A.; Balestra, G.M.; Kenny, J.M.; Torre, L.; Puglia, D. Antioxidant and antibacterial lignin nanoparticles in polyvinyl alcohol/chitosan films for active packaging. Ind. Crops Prod. 2016, 94, 800–811. [Google Scholar] [CrossRef]
- Hansen, B.; Kusch, P.; Schulze, M.; Kamm, B. Qualitative and quantitative analysis of lignin produced from beech wood by different conditions of the Organosolv process. J. Polym. Environ. 2016, 24, 85–97. [Google Scholar] [CrossRef]
- Determination of Structural Carbohydrates and Lignin in Biomass. Available online: https://www.nrel.gov/docs/gen/fy13/42618.pdf (accessed on 10 October 2018).
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Crocker, D. Determination of structural carbohydrates and lignin in biomass. Lab. Anal. Proced. 2008, 1617, 1–16. [Google Scholar]
- Sluiter, A.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of Extractives in Biomass (NREL/TP-510-42619). Natl. Renew. Energy Lab. Gold. 2008, 2008, 42619. [Google Scholar]
- Sluiter, A.; Hames, B.; Hyman, D.; Payne, C.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D.; Wolfe, J. Determination of Total Solids in Biomass and Total Dissolved Solids in Liquid Process Samples (NREL/TP-510-42621). Natl. Renew. Energy Lab. Gold. 2008, 2008, 42621. [Google Scholar]
- Sluiter, A.; Hames, B.; Ruiz, R.; Scarlata, C.; Sluiter, J.; Templeton, D. Determination of ash in Biomass (NREL/TP-510-42622). Natl. Renew. Energy Lab. Gold. 2008, 2008, 42622. [Google Scholar]
- Sebti, I.; Chollet, E.; Degraeve, P.; Noel, C.; Peyrol, E. Water Sensitivity, Antimicrobial, and Physicochemical Analyses of Edible Films Based on HPMC and/or Chitosan. J. Agric. Food Chem. 2007, 55, 693–699. [Google Scholar] [CrossRef]
- Dohlen, S.; Braun, C.; Brodkorb, F.; Fischer, B.; Ilg, Y.; Kalbfleisch, K.; Kreyenschmidt, M.; Lorenz, R.; Kreyenschmidt, J. Effect of different packaging materials containing poly-[2-(tert-butylamino) methylstyrene] on the growth of spoilage and pathogenic bacteria on fresh meat. Int. J. Food Microbiol. 2017, 257, 91–100. [Google Scholar] [CrossRef]
- Hüwe, C.; Schmeichel, J.; Brodkorb, F.; Dohlen, S.; Kalbfleisch, K.; Kreyenschmidt, M.; Lorenz, R.; Kreyenschmidt, J. Potential of antimicrobial treatment of linear low-density polyethylene with poly((tert-butyl-amino)-methyl-styrene) to reduce biofilm Formation in the Food industry. Biofouling 2018, 34, 378–387. [Google Scholar] [CrossRef]
- Dohlen, S.; Braun, C.; Brodkorb, F.; Fischer, B.; Ilg, Y.; Kalbfleisch, K.; Kreyenschmidt, M.; Lorenz, R.; Robers, O.; Kreyenschmidt, J. Potential of the polymer poly-[2-(tert-butylamino) methylstyrene] as antimicrobial packaging material for meat products. J. Appl. Microbiol. 2016, 4, 1059–1070. [Google Scholar] [CrossRef]
- Braun, C.; Dohlen, S.; Ilg, Y.; Brodkorb, F.; Fischer, B.; Heindirk, P.; Kalbfleisch, K.; Richter, T.; Robers, O.; Kreyenschmidt, M.; et al. Antimicrobial Activity of Intrinsic Antimicrobial Polymers Based on Poly((tertbutyl-amino)-methyl-styrene) Against Selected Pathogenic and Spoilage Microorganisms Relevant in Meat Processing Facilities. J. Antimicrob Agents 2017, 3, 1000136. [Google Scholar] [CrossRef]
- Strotmann, C.; Göbel, C.; Friedrich, S.; Kreyenschmidt, J.; Ritter, G.; Teitscheid, P. A Participatory Approach to Minimizing Food Waste in the Food Industry—A Manual for Managers. Sustainability 2017, 9, 66. [Google Scholar] [CrossRef]
- Rocca, D.M.; Vanegas, J.P.; Fournier, K.; Becerra, M.C.; Scaiano, J.C.; Lanterna, A.E. Biocompatibility and photo-induced antibacterial activity of lignin-stabilized noble metal nanoparticles. RSC Adv. 2018, 8, 40454–40463. [Google Scholar] [CrossRef]
- Monakhova, Y.; Diehl, B.W.K.; Do, X.T.; Witzleben, S.; Schulze, M. Novel method for the determination of average molecular weight of natural polymers based on 2D DOSY NMR and chemometrics: Example of heparin. J. Pharm. Biomed. Anal. 2018, 149, 128–132. [Google Scholar] [CrossRef]
- Gilca, I.A.; Ghitescu, R.E.; Puitel, A.C.; Popa, V.I. Preparation of lignin nanoparticles by chemical modification. Iran. Polym. J. 2014, 23, 355–363. [Google Scholar] [CrossRef]
- Argyropoulos, D.S. Quantitative phosphorus 31 NMR analysis of lignins: A new tool for the lignin chemist. J. Wood Chem. Technol. 1994, 14, 45–63. [Google Scholar] [CrossRef]
- Sun, S.-N.; Cao, X.-F.; Xu, F.; Sun, R.-C.; Jones, G.L. Structural Features and Antioxidant Activities of Lignins from Steam-Exploded Bamboo (Phyllostachys pubescens). J. Agric. Food Chem. 2014, 62, 5939–5947. [Google Scholar] [CrossRef]
- Do, X.T.; Nöster, J.; Weber, M.; Nietsch, A.; Jung, C.; Witzleben, S.; Schulze, M. Comparative Studies of Lignin Depolymerisation: Photolysis versus Ozonolysis in Alkaline Medium. In Proceedings of the Annual Conference of the GDCh Division Sustainable Chemistry, Aachen, Germany, 17–19 September 2018. [Google Scholar]
- Do, X.T.; Nietzsch, A.; Jung, C.; Witzleben, S.; Schulze, M. Lignin-Depolymerisation via UV-Photolysis and Titanium Dioxide Photocatalysis. Preprints 2017, 2017, 100128. [Google Scholar] [CrossRef]
- Ponomarenko, J.; Lauberts, M.; Dizhbite, T.; Lauberte, L.; Jurkjane, V.; Telysheva, G. Antioxidant activity of various lignins and lignin-related phenylpropanoid units with high and low molecular weight. Holzforschung 2015, 69, 795–805. [Google Scholar] [CrossRef]
- Rajalakshmi, A.; Krithiga, N.; Jayachitr, A. Antioxidant Activity of the Chitosan Extracted from Shrimp Exoskeleton. Middle-East J. Sci. Res. 2013, 16, 1446–1451. [Google Scholar] [CrossRef]
- Shen, D.K.; Gu, S.; Luo, K.H.; Wang, S.R.; Fang, M.X. The pyrolytic degradation of wood-derived lignin from pulping process. Bioresour. Technol. 2010, 101, 6136–6146. [Google Scholar] [CrossRef]
- Dizhbite, T.; Telysheva, G.; Jurkjane, V.; Viesturs, U. Characterization of the radical scavenging activity of lignins natural antioxidants. Bioresour. Technol. 2004, 95, 309–317. [Google Scholar] [CrossRef]
- El Mansouri, N.-E.; Salvadó, J. Structural characterization of technical lignins for the production of adhesives: Application to lignosulfonate, kraft, soda-anthraquinone, organosolv and ethanol process lignins. Ind. Crops Prod. 2006, 24, 8–16. [Google Scholar] [CrossRef]
- Witzleben, S.T.; Walbrück, K.; Klein, S.E.; Schulze, M. Investigation of Temperature Dependency of Morphological Properties of Thermoplastic Polyurethane Using WAXS and SAXS Monitoring. J. Chem. Chem. Eng. 2016, 9, 494–499. [Google Scholar]
- Stanborough, T.; Fegan, N.; Powell, S.M.; Tamplin, M.; Chandry, P.S. Insight into the Genome of Brochothrix thermosphacta, a Problematic Meat Spoilage Bacterium. Appl. Environ. Microbiol. 2017, 83, 1–20. [Google Scholar] [CrossRef]
- Garrido-Maestu, A.; Ma, Z.; Paik, S.-Y.-R.; Chen, N.; Ko, S.; Tong, Z.; Jeong, K.C.C. Engineering of chitosan-derived nanoparticles to enhance antimicrobial activity against foodborne pathogen Escherichia coli O157:H7. Carbohydr. Polym. 2018, 197, 623–630. [Google Scholar] [CrossRef]
- Kurniasih, M.; Dewi, R.S. Toxicity tests, antioxidant activity, and antimicrobial activity of chitosan. In Proceedings of the 12th Joint Conference on Chemistry, Semarang, Indonesia, 19–20 September 2017. [Google Scholar]
Sample Availability: Samples of the compounds are not available from the authors. |




| Lignin Platelets | Bacteria | |
|---|---|---|
| S. aureus | L. monocytogenes | |
| Reference DMSO | − −1x contaminated with yeast | −− |
| L1 | + (max 1–2 mm) | ++ (max 5 mm) |
| L2 | − | + (max 1 mm) |
| L3 | − | + (max 1 mm), slight growth in inhibition zone |
| L4 | − | − |
| L5 | + (max 1 mm) | + (max 1–2 mm) |
| L6 | ++ (max 2–3 mm) | + (max 7 mm) |
| L7 | − | − |
| Fraction | AIL [%] | ASL [%] | Total Lignin [%] | Ash [%] | Glucan [%] | Xylan [%] | Galactan [%] | Arabinan [%] | Rhamnan [%] | Mannan [%] | Sum [%] |
|---|---|---|---|---|---|---|---|---|---|---|---|
| L1 | 86.86 | 15.14 | 102.00 | 1.01 | 0.45 | 0.59 | 0.00 | 0.04 | 0.00 | 0.00 | 104.09 |
| L2 | 93.37 | 9.76 | 103.13 | 0.88 | 0.31 | 0.76 | 0.00 | 0.06 | 0.00 | 0.00 | 105.15 |
| L3 | 91.43 | 9.46 | 100.89 | 0.24 | 0.26 | 0.04 | 0.00 | 0.00 | 0.00 | 0.00 | 101.42 |
| L4 | 95.17 | 2.42 | 97.59 | 0.40 | 0.36 | 0.07 | 0.00 | 0.00 | 0.00 | 0.00 | 98.42 |
| PH2SK | 86.24 | 13.28 | 99.52 | 0.85 | 0.26 | 0.85 | 0.00 | 0.07 | 0.00 | 0.00 | 101.55 |
| Aliphatic OH | Condensed-OH | G and Dimethylated-OH | Carboxylic Acids-OH | |
|---|---|---|---|---|
| L1 | 8.70 | 5.04 | 6.30 | 0.59 |
| L2 | 8.52 | 0.28 | 0.71 | 0.00 |
| L3 | 3.81 | 2.99 | 2.91 | 0.16 |
| L4 | 3.17 | 0.48 | 1.79 | 0.15 |
| L5 * | 3.02 | 0.21 | 0.18 | 0.02 |
| PH2SK | 7.32 | 7.11 | 9.73 | 3.10 |
| L1 | L2 | L3 | L4 | L5 | L6 | L7 | |
|---|---|---|---|---|---|---|---|
| DPPH Inhibition (%) | 65.1 ± 3.7 | 66.8 ± 6.6 | 62.2 ± 9.5 | 68.2 ± 3.6 | 42 ± 1.9 | 64 ± 2.6 | 31 ± 1.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzagameem, A.; Klein, S.E.; Bergs, M.; Do, X.T.; Korte, I.; Dohlen, S.; Hüwe, C.; Kreyenschmidt, J.; Kamm, B.; Larkins, M.; et al. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers 2019, 11, 670. https://doi.org/10.3390/polym11040670
Alzagameem A, Klein SE, Bergs M, Do XT, Korte I, Dohlen S, Hüwe C, Kreyenschmidt J, Kamm B, Larkins M, et al. Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers. 2019; 11(4):670. https://doi.org/10.3390/polym11040670
Chicago/Turabian StyleAlzagameem, Abla, Stephanie Elisabeth Klein, Michel Bergs, Xuan Tung Do, Imke Korte, Sophia Dohlen, Carina Hüwe, Judith Kreyenschmidt, Birgit Kamm, Michael Larkins, and et al. 2019. "Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms" Polymers 11, no. 4: 670. https://doi.org/10.3390/polym11040670
APA StyleAlzagameem, A., Klein, S. E., Bergs, M., Do, X. T., Korte, I., Dohlen, S., Hüwe, C., Kreyenschmidt, J., Kamm, B., Larkins, M., & Schulze, M. (2019). Antimicrobial Activity of Lignin and Lignin-Derived Cellulose and Chitosan Composites against Selected Pathogenic and Spoilage Microorganisms. Polymers, 11(4), 670. https://doi.org/10.3390/polym11040670