Some Important Issues of the Commercial Production of 1-D Nano-PANI
Abstract
:1. Introduction
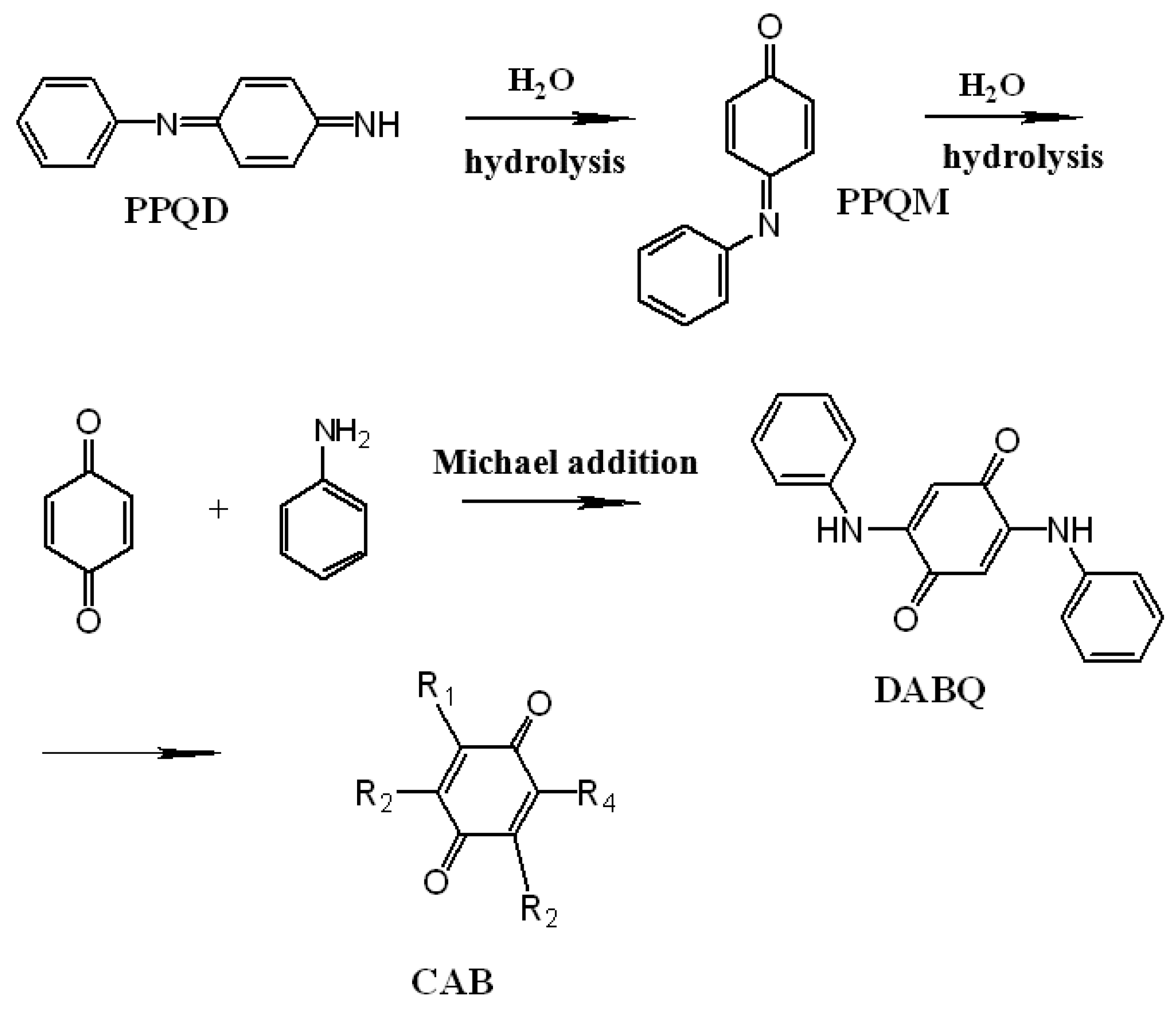
2. Complexity of the Polymerization Process of PANI
3. Characters of the Various Synthesis Methods of 1D Nano-PANI
3.1. Hard Template Methods
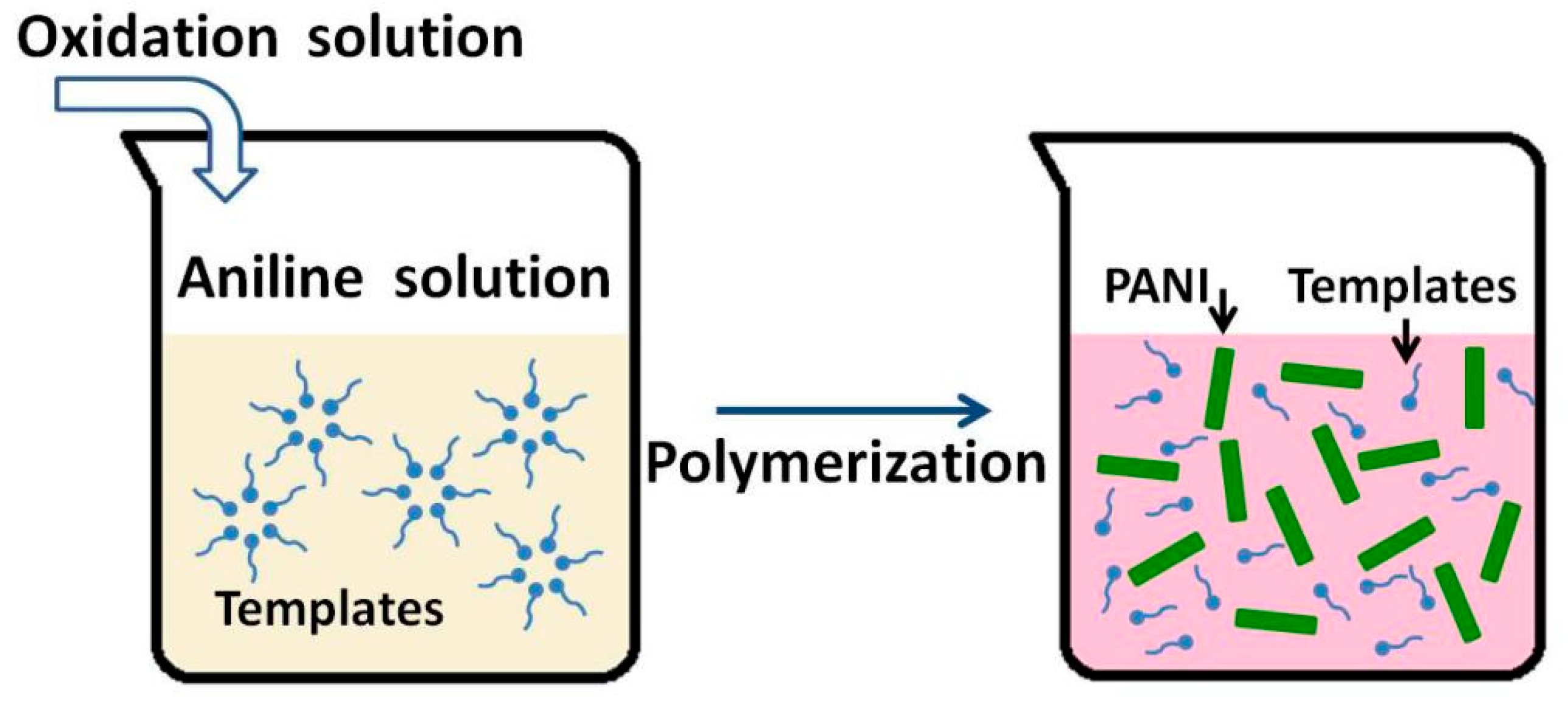
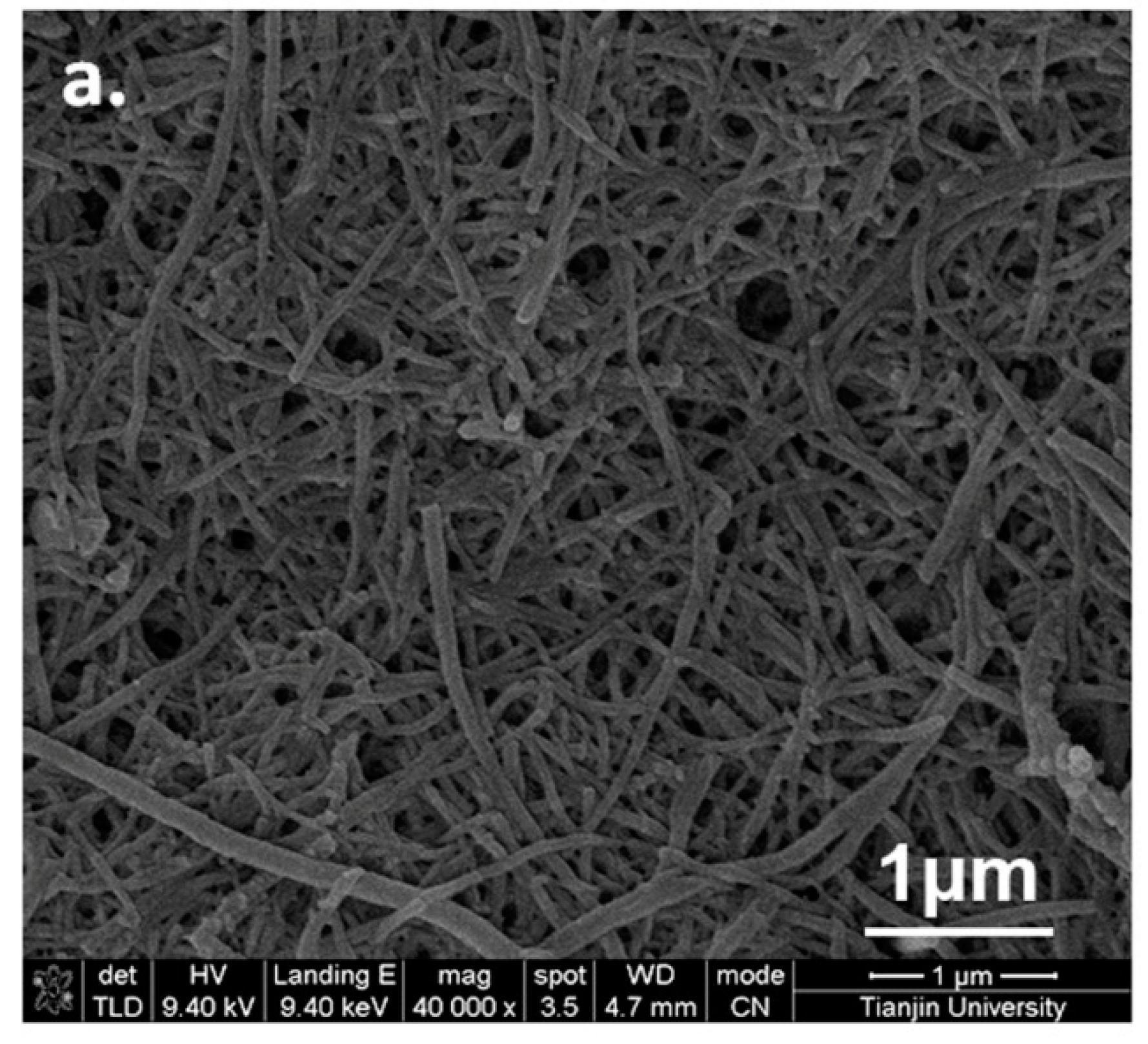
3.2. Soft Template Methods
3.3. Chemical Polymerization with Template-Free Approaches
3.3.1. Interfacial Polymerization
3.3.2. Dilute Polymerization
3.3.3. Rapid Mixing Methods
3.3.4. Seeding Polymerization
3.4. Electrochemical Approaches
3.5. Other Methods
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 1-D nano-PANI | One-dimensional nano-polyaniline materials |
| PANI | Polyaniline |
| BQ | Benzoquinone |
| CAB | Co-oligomers of aniline and p-benzoquinone; |
| HQ | Hydroquinone |
| HPLC–DAD | High-performance liquid chromatography coupled to photodiode diode array detection |
| UV–vis | Ultraviolet and visible |
| UV | Ultraviolet |
| PBQD | N-phenyl-1,4-benzoquinonediimine |
| PBQM | N-phenyl-1,4-benzoquinonemonoimine |
| PPD | p-aminodiphenylamine |
| DABQ | 2,5-dianilino-p-benzoquinone |
| SS | Stainless steel |
| AAO | Anodic aluminum oxide |
| CNTs | Carbon nanotubes |
| NSA | Naphthalene sulfonic acids |
| p-TSA | p-toluenesulfonic acid |
| SAN | Aminobenzenesulfonic acid |
| DBSA | Dodecylbenzenesulfonic acid |
| CSA | Camphorsulfonic acid |
| PSA | Pyrene sulfonic acid |
| CTAB | Cetyltrimethylammonium bromide |
| C60(OSO3H)6 | Hydrogensulfated fullerenol of six -(O)SO3H groups |
| PAMAM4.0[naphthyl(SO3H)2]24 | Sulfonated dendrimer with 24 terminal groups of 3,6-disulfonaphthylthiourea |
| PS-b-P4VP | Poly(styrene-block-4-vinylpyridine) |
| [bmim]Cl | 1-butyl-3-methylimidazolium chloride |
| MA | Malic acid |
| HOOC(CH2)nCOOH | Dicarboxylic acids |
| MO | Methyl orange |
| APS | Oxidant ammonium persulfate |
| NHE | Normal hydrogen electrode |
| EB | Emeraldine |
| HGCOP | High-gravity chemical oxidative polymerization |
| STR | Smaller diameter than those produced in a stirred tank reactor |
| SEM | Scanning electron microscope |
References
- Kang, E.T.; Neoh, K.G.; Tan, K.L. Polyaniline: A polymer with many interesting intrinsic redox states. Prog. Polym. Sci. 1998, 23, 277–324. [Google Scholar] [CrossRef]
- Pellegrino, J. The use of conducting polymers in membrane-based separations—A review and recent developments. Ann. N. Y. Acad. Sci. 2003, 984, 289–305. [Google Scholar] [CrossRef]
- Yang, X.; Li, B.; Wang, H.; Hou, B. Anticorrosion performance of polyaniline nanostructures on mild steel. Prog. Org. Coat. 2010, 69, 267–271. [Google Scholar] [CrossRef]
- Jiao, S.; Tu, J.; Fan, C.; Hou, J.; Fray, D.J. Electrochemically assembling of a porous nano-polyaniline network in a reverse micelle and its application in a supercapacitor. J. Mater. Chem. 2011, 21, 9027–9030. [Google Scholar] [CrossRef]
- Hosoda, M.; Hino, T.; Kurarnoto, N. Facile preparation of conductive paint made with polyaniline/dodecylbenzenesulfonic acid dispersion and poly(methyl methacrylate). Polym. Int. 2007, 56, 1448–1455. [Google Scholar] [CrossRef]
- Li, Y.; Ying, B.; Hong, L.; Yang, M. Water-soluble polyaniline and its composite with poly(vinyl alcohol) for humidity sensing. Synth. Met. 2010, 160, 455–461. [Google Scholar] [CrossRef]
- Zhou, Y.K.; He, B.L.; Zhou, W.J.; Huang, J.; Li, X.H.; Wu, B.; Li, H.I. Electrochemical capacitance of well-coated single-walled carbon nanotube with polyaniline composites. Electrochim. Acta 2004, 49, 257–262. [Google Scholar] [CrossRef]
- Kim, B.R.; Lee, H.K.; Park, S.H.; Kim, H.K. Electromagnetic interference shielding characteristics and shielding effectiveness of polyaniline-coated films. Thin Solid Films 2011, 519, 3492–3496. [Google Scholar] [CrossRef]
- Guo, B.; Zhao, Y.; Wu, W.; Meng, H.; Zou, H.; Chen, J.; Chu, G. Research on the preparation technology of polyaniline nanofiber based on high gravity chemical oxidative polymerization. Chem. Eng. Process. 2013, 70, 1–8. [Google Scholar] [CrossRef]
- Quoc Minh, P.; Kim, S. High surface area polyaniline nanofiber synthesized in compressed CO2 and its application to a hydrogen sensor. Korean J. Chem. Eng. 2016, 33, 290–298. [Google Scholar]
- Yin, C.; Gao, L.; Zhou, F.; Duan, G. Facile Synthesis of Polyaniline Nanotubes Using Self-Assembly Method Based on the Hydrogen Bonding: Mechanism and Application in Gas Sensing. Polymers 2017, 9, 544. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Ye, Y.; Shen, Y.; Wang, F.; Lu, X.; Xie, Y.; Chen, S.; Tan, H.; Xu, F.; Song, Y. Hierarchical nanocomposites of Co3O4/polyaniline nanowire arrays/reduced graphene oxide sheets for amino acid detection. Sens. Actuators B Chem. 2014, 203, 864–872. [Google Scholar] [CrossRef]
- Shivani, T.; Ja-An Annie, H. Green Synthesis of Novel Polyaniline Nanofibers: Application in pH Sensing. Molecules 2015, 20, 18585–18596. [Google Scholar] [Green Version]
- Ma, D.; Shi, G.; Wang, H.; Zhang, Q.; Li, Y. Controllable growth of high-quality metal oxide/conducting polymer hierarchical nanoarrays with outstanding electrochromic properties and solar-heat shielding ability. J. Mater. Chem. A 2014, 2, 13541–13549. [Google Scholar] [CrossRef]
- Erro, E.M.; Baruzzi, A.M.; Iglesias, R.A. Fast electrochromic response of ultraporous polyaniline nanofibers. Polymer 2014, 55, 2440–2444. [Google Scholar] [CrossRef]
- Olejnik, P.; Gniadek, M.; Echegoyen, L.; Plonska-Brzezinska, M.E. Nanoforest: Polyaniline Nanotubes Modified with Carbon Nano-Onions as a Nanocomposite Material for Easy-to-Miniaturize High-Performance Solid-State Supercapacitors. Polymers 2018, 10, 1408. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wang, J.; Gao, X.; Wang, Z.; Wang, S. The electrochemical activity of polyaniline: An important issue on its use in electrochemical energy storage devices. Synth. Met. 2014, 187, 46–51. [Google Scholar] [CrossRef]
- Xie, Y.; Xia, C.; Du, H.; Wang, W. Enhanced electrochemical performance of polyaniline/carbon/titanium nitride nanowire array for flexible supercapacitor. J. Power Sources 2015, 286, 561–570. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Liu, X.; Wang, Z.; Wang, S. High Performance Self-Healing Epoxy/Polyamide Protective Coating Containing Epoxy Microcapsules and Polyaniline Nanofibers for Mild Carbon Steel. J. Power Sources 2013, 52, 10172–10180. [Google Scholar] [CrossRef]
- Chu, D.; Wang, J.; Han, Y.; Ma, Q.; Wang, Z. High performance epoxy protective coatings incorporated with polyaniline nanowires using cardanol-based phenalkamine as the curing agent. RSC Adv. 2015, 5, 11378–11384. [Google Scholar] [CrossRef]
- Wanekaya, A.K.; Bangar, M.A.; Yun, M.; Chen, W.; Myung, N.V.; Mulchandani, A. Field-effect transistors based on single nanowires of conducting polymers. J. Phys. Chem. C 2007, 111, 5218–5221. [Google Scholar] [CrossRef]
- Vlad, A.; Dutu, C.A.; Jedrasik, P.; Sodervall, U.; Gohy, J.F.; Melinte, S. Vertical single nanowire devices based on conducting polymers. Nanotechnology 2012, 23, 025302. [Google Scholar] [CrossRef]
- Masomboon, N.; Ratanatamskul, C.; Lu, M.C. Chemical oxidation of 2,6-dimethylaniline in the Fenton process. Environ. Sci. Technol. 2009, 43, 8629–8634. [Google Scholar] [CrossRef]
- Liu, J.; Guan, J.; Lu, M.; Kan, Q.; Li, Z. Hemoglobin immobilized with modified “fish-in-net” approach for the catalytic removal of aniline. J. Hazard. Mater. 2012, 217–218, 156–163. [Google Scholar] [CrossRef]
- Sun, R.; Yong, W.; Ni, Y.; Kokot, S. Spectrophotometric analysis of phenols, which involves a hemin–graphene hybrid nanoparticles with peroxidase-like activity. J. Hazard. Mater. 2014, 266, 60–67. [Google Scholar] [CrossRef]
- Beadle, P.M.; Nicolau, Y.F.; Banka, E.; Rannou, P.; Djurado, D. Controlled polymerization of aniline at sub-zero temperatures. Synth. Met. 1998, 95, 29–45. [Google Scholar] [CrossRef]
- Zhao, Y.; Tomsik, E.; Wang, J.; Moravkova, Z.; Zhigunov, A.; Stejskal, J.; Trchova, M. Self-Assembly of Aniline Oligomers. Chem. Asian J. 2013, 8, 129–137. [Google Scholar] [CrossRef]
- Zhao, Y.; Stejskal, J.; Wang, J. Towards directional assembly of hierarchical structures: Aniline oligomers as the model precursors. Nanoscale 2013, 5, 2620–2626. [Google Scholar] [CrossRef]
- Olinga, T.E.; Fraysse, J.; Travers, J.P.; Dufresne, A.; Pron, A. Highly Conducting and Solution-Processable Polyaniline Obtained via Protonation with a New Sulfonic Acid Containing Plasticizing Functional Groups. Macromolecules 2000, 33, 2107–2113. [Google Scholar] [CrossRef]
- Gospodinova, N.; Terlemezyan, L. Conducting polymers prepared by oxidative polymerization: Polyaniline. Prog. Polym. Sci. 1998, 23, 1443–1484. [Google Scholar] [CrossRef]
- Tandon, V.K.; Maurya, H.K. ‘On water’: Unprecedented nucleophilic substitution and addition reactions with 1,4-quinones in aqueous suspension. Tetrahedron Lett. 2009, 50, 5896–5902. [Google Scholar] [CrossRef]
- Wu, Y.; Wang, J.; Ou, B.; Zhao, S.; Wang, Z.; Wang, S. Electrochemical Preparation of Polyaniline Nanowires with the Used Electrolyte Solution Treated with the Extraction Process and Their Electrochemical Performance. Nanomaterials 2018, 8, 103. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.; Park, S.M. Electrochemistry of Conductive Polymers: XX. Early Stages of Aniline Polymerization Studied by Spectroelectrochemical and Rotating Ring Disk Electrode Techniques. J. Electrochem. Soc. 1996, 143, 1277–1282. [Google Scholar] [CrossRef]
- Hong, S.-Y.; Jung, Y.M.; Kim, S.B.; Park, S.-M. Electrochemistry of Conductive Polymers. 34. Two-Dimensional Correlation Analysis of Real-Time Spectroelectrochemical Data for Aniline Polymerization. J. Phys. Chem. B 2005, 109, 3844–3850. [Google Scholar] [CrossRef]
- Balón, M.; Guardado, P.; Carmona, C.; Hidalgo, J.; Munoz, M.A. Influence of the acidity on the kinetics of diphenylamine oxidation by peroxodisulfate anions. Can. J. Chem. 1993, 71, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Nayaki, S.K.; Swaminathan, M. Excited state solvatochromic and prototropic behaviour of 4-aminodiphenylamine and 4,4′-diaminodiphenylamine—A comparative study by electronic spectra. Spectrochim. Acta Part A 2006, 64, 631–636. [Google Scholar] [CrossRef]
- Stejskal, J.; Bober, P.; Trchova, M.; Horsky, J.; Pilar, J.; Walterova, Z. The oxidation of aniline with p-benzoquinone and its impact on the preparation of the conducting polymer, polyaniline. Synth. Met. 2014, 192, 66–73. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Zhou, Z.; Wang, Z.; Zhang, F.; Wang, S. Preparation of nanostructured polyaniline and its super-amphiphilic behavior. Macromol. Rapid Commun. 2008, 29, 68–73. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, L.; Qi, B.; Yang, G.; Gong, J. Synthesis of vertical aligned TiO2@polyaniline core-shell nanorods for high-performance supercapacitors. RSC Adv. 2015, 5, 1680–1683. [Google Scholar] [CrossRef]
- Sim, B.; Choi, H.J. Facile synthesis of polyaniline nanotubes and their enhanced stimuli-response under electric fields. RSC Adv. 2015, 5, 11905–11912. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Qin, Z.Y.; Li, T.; Li, Z.Z.; Zhou, Z.; Zhu, M.F. Influence of acetone on nanostructure and electrochemical properties of interfacial synthesized polyaniline nanofibers. Prog. Nat. Sci. 2015, 25, 316–322. [Google Scholar] [CrossRef] [Green Version]
- Abdolahi, A.; Hamzah, E.; Ibrahim, Z.; Hashim, S. Synthesis of Uniform Polyaniline Nanofibers through Interfacial Polymerization. Materials 2012, 5, 1487–1494. [Google Scholar] [CrossRef] [Green Version]
- Qiang, J.; Yu, Z.; Wu, H.; Yun, D. Polyaniline nanofibers synthesized by rapid mixing polymerization. Synth. Met. 2008, 158, 544–547. [Google Scholar] [CrossRef]
- Chiou, N.R.; Epstein, A.J. Polyaniline nanofibers prepared by dilute polymerization. Adv. Mater. 2005, 17, 1679–1683. [Google Scholar] [CrossRef]
- Thanpitcha, T.; Sirivat, A.; Jamieson, A.M.; Rujiravanit, R. Synthesis of polyaniline nanofibrils using an in situ seeding technique. Synth. Met. 2008, 158, 695–703. [Google Scholar] [CrossRef]
- Zhao, Y.; Wei, H.; Arowo, M.; Yan, X.; Wu, W.; Chen, J.; Wang, Y.; Guo, Z. Electrochemical energy storage by polyaniline nanofibers: High gravity assisted oxidative polymerization vs. rapid mixing chemical oxidative polymerization. Phys. Chem. Chem. Phys. 2015, 17, 1498–1502. [Google Scholar] [CrossRef]
- Manuel, J.; Kim, M.; Fapyane, D.; Chang, I.S.; Ahn, H.-J.; Ahn, J.-H. Preparation and electrochemical properties of polyaniline nanofibers using ultrasonication. Mater. Res. Bull. 2014, 58, 213–217. [Google Scholar] [CrossRef]
- Li, Y.; Wang, Y.; Wu, D.; Jing, X. Effects of Ultrasonic Irradiation on the Morphology of Chemically Prepared Polyaniline Nanofibers. J. Appl. Polym. Sci. 2009, 113, 868–875. [Google Scholar] [CrossRef]
- Bhandari, S.; Khastgir, D. Template-free solid state synthesis of ultra-long hairy polyaniline nanowire supercapacitor. Mater. Lett. 2014, 135, 202–205. [Google Scholar] [CrossRef]
- Du, X.S.; Zhou, C.F.; Wang, G.T.; Mai, Y.W. Novel solid-state and template-free synthesis of branched polyaniline nanofibers. Chem. Mater. 2008, 20, 3806–3808. [Google Scholar] [CrossRef]
- Li, Z.-F.; Blum, F.D.; Bertino, M.F.; Kim, C.-S.; Pillalamarri, S.K. One-step fabrication of a polyaniline nanofiber vapor sensor. Sens. Actuators B 2008, 134, 31–35. [Google Scholar] [CrossRef]
- Pillalamarri, S.K.; Blum, F.D.; Tokuhiro, A.T.; Bertino, M.F. One-pot synthesis of polyaniline—Metal nanocomposites. Chem. Mater. 2005, 17, 5941–5944. [Google Scholar] [CrossRef]
- Tsukuda, S.; Seki, S.; Sugimoto, M.; Tagawa, S. Formation of nanowires based on pi-conjugated polymers by high-energy ion beam irradiation. Jpn. J. Appl. Phys. Part 1 2005, 44, 5839–5842. [Google Scholar] [CrossRef]
- Gizdavic-Nikolaidis, M.R.; Jevremovic, M.M.; Allison, M.C.; Stanisavljev, D.R.; Bowmaker, G.A.; Zujovic, Z.D. Self-assembly of nanostructures obtained in a microwave-assisted oxidative polymerization of aniline. Exp. Polym. Lett. 2014, 8, 745–755. [Google Scholar] [CrossRef] [Green Version]
- Tiwari, A.; Kumar, R.; Prabaharan, M.; Pandey, R.R.; Kumari, P.; Chaturvedi, A.; Mishra, A.K. Nanofibrous polyaniline thin film prepared by plasma-induced polymerization technique for detection of NO2 gas. Polym. Adv. Technol. 2010, 21, 615–620. [Google Scholar] [CrossRef]
- Cheng, J.; Zhao, B.; Zheng, S.; Yang, J.; Zhang, D.; Cao, M. Enhanced microwave absorption performance of polyaniline-coated CNT hybrids by plasma-induced graft polymerization. Appl. Phys. A 2015, 119, 379–386. [Google Scholar] [CrossRef]
- Low, K.; Chartuprayoon, N.; Echeverria, C.; Li, C.; Bosze, W.; Myung, N.V.; Nam, J. Polyaniline/poly(epsilon-caprolactone) composite electrospun nanofiber-based gas sensors: Optimization of sensing properties by dopants and doping concentration. Nanotechnology 2014, 25, 115501. [Google Scholar] [CrossRef]
- Martin, C.R. Template Synthesis of Electronically Conductive Polymer Nanostructures. Acc. Chem. Res. 1995, 28, 61–68. [Google Scholar] [CrossRef]
- Martin, C.R.; Van Dyke, L.S.; Cai, Z.; Liang, W. Template synthesis of organic microtubules. J. Am. Chem. Soc. 1990, 112, 8976–8977. [Google Scholar] [CrossRef]
- Martin, C.R. Membrane-Based Synthesis of Nanomaterials. Chem. Mater. 1996, 8, 1739–1746. [Google Scholar] [CrossRef]
- Parthasarathy, R.V.; Martin, C.R. Template-Synthesized Polyaniline Microtubules. Chem. Mater. 1994, 6, 1627–1632. [Google Scholar] [CrossRef]
- Wu, C.-G.; Bein, T. Conducting Polyaniline Filaments in a Mesoporous Channel Host. Science 1994, 264, 1757–1759. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.W.; Wang, Z.; Li, M.K.; Li, H.L. Well-aligned polyaniline nano-fibril array membrane and its field emission property. Chem. Phys. Lett. 2001, 341, 431–434. [Google Scholar] [CrossRef]
- Xiong, S.X.; Wang, Q.; Xia, H.S. Preparation of polyaniline nanotubes array based on anodic aluminum oxide template. Mater. Res. Bull. 2004, 39, 1569–1580. [Google Scholar] [CrossRef]
- Jang, J.; Bae, J. Formation of polyaniline nanorod/liquid crystalline epoxy composite nanowires using a temperature-gradient method. Adv. Funct. Mater. 2005, 15, 1877–1882. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Kalantar-zadeh, K. Synthesis and electrochemical properties of template-based polyaniline nanowires and template-free nanofibril arrays: Two potential nanostructures for gas sensors. Sens. Actuators B 2009, 136, 1–7. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, G.; Li, Y.; Zhang, X.; Qiao, N.; Wang, J.; Zhou, J.; Liu, Z.; Hao, Z. A new type of ordered mesoporous carbon/polyaniline composites prepared by a two-step nanocasting method for high performance supercapacitor applications. J. Mater. Chem. A 2014, 2, 16715–16722. [Google Scholar] [CrossRef]
- Kim, T.H.; Kim, Y.; Lee, S.J.; Han, W.S.; Jung, J.H. Fabrication of polyaniline silica nanotubes and closed polyaniline nanotubes using a template of silica nanotube. Chem. Lett. 2008, 37, 598–599. [Google Scholar] [CrossRef]
- Wang, P.; Du, M.; Zhang, M.; Zhu, H.; Bao, S.; Zou, M.; Yang, T. Facile fabrication of AuNPs/PANI/HNTs nanostructures for high-performance electrochemical sensors towards hydrogen peroxide. Chem. Eng. J. 2014, 248, 307–314. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, P. Facile fabrication of uniform polyaniline nanotubes with tubular aluminosilicates as templates. Nanoscale Res. Lett. 2008, 3, 299–302. [Google Scholar] [CrossRef]
- Chen, W.; Rakhi, R.B.; Alshareef, H.N. Facile synthesis of polyaniline nanotubes using reactive oxide templates for high energy density pseudocapacitors. J. Mater. Chem. A 2013, 1, 3315–3324. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, F.; Gong, J.; Su, Z.; Qu, L. Polyaniline Nanorods and Hollow-Microspheres Prepared by Using Copper Wires or Rings as Template. J. Nanosci. Nanotech. 2008, 8, 5972–5976. [Google Scholar] [CrossRef]
- Fu, J.; Pang, Z.; Yang, J.; Huang, F.; Cai, Y.; Wei, Q. Fabrication of polyaniline/carboxymethyl cellulose/cellulose nanofibrous mats and their biosensing application. Appl. Surf. Sci. 2015, 349, 35–42. [Google Scholar] [CrossRef]
- Jeevananda, T.; Siddaramaiah; Kim, N.H.; Heo, S.-B.; Lee, J.H. Synthesis and characterization of polyaniline-multiwalled carbon nanotube nanocomposites in the presence of sodium dodecyl sulfate. Polym. Adv. Technol. 2008, 19, 1754–1762. [Google Scholar] [CrossRef]
- Meier, C.; Lifincev, I.; Welland, M.E. Conducting Core-Shell Nanowires by Amyloid Nanofiber Templated Polymerization. Biomacromolecules 2015, 16, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Yao, S.; Gong, J.; Qu, L. Preparation of polyaniline nanotubes via “thin glass tubes template” approach and its gas response. Macromol. Rapid Commun. 2007, 28, 286–291. [Google Scholar] [CrossRef]
- Lee, S.-Y.; Lim, H.; Choi, G.-R.; Kim, J.-D.; Suh, E.-K.; Lee, S.-K. Metal-to-Insulating Transition of Single Polyaniline (PANI) Nanowire: A Dedoping Effect. J. Phys. Chem. C 2010, 114, 11936–11939. [Google Scholar] [CrossRef]
- Drury, A.; Chaure, S.; Kroell, M.; Nicolosi, V.; Chaure, N.; Blau, W.J. Fabrication and characterization of silver/polyaniline composite nanowires in porous anodic alumina. Chem. Mater. 2007, 19, 4252–4258. [Google Scholar] [CrossRef]
- Lahav, M.; Weiss, E.A.; Xu, Q.; Whitesides, G.M. Core-shell and segmented polymer-metal composite nanostructures. Nano Lett. 2006, 6, 2166–2171. [Google Scholar] [CrossRef]
- Wei, Z.X.; Zhang, Z.M.; Wan, M.X. Formation mechanism of self-assembled polyaniline micro/nanotubes. Langmuir 2002, 18, 917–921. [Google Scholar] [CrossRef]
- Han, Y.-G.; Kusunose, T.; Sekino, T. Facile One-Pot Synthesis and Characterization of Novel Nanostructured Organic Dispersible Polyaniline. J. Polym. Sci. Part B Polym. Phys. 2009, 47, 1024–1029. [Google Scholar] [CrossRef]
- Mallick, K.; Witcomb, M.; Erasmus, R.; Strydom, A. Electrical and Optical Properties of Polyaniline with a Weblike Morphology. J. Appl. Polym. Sci. 2010, 116, 1587–1592. [Google Scholar] [CrossRef]
- Zhang, C.; Li, G.; Peng, H. Large-scale synthesis of self-doped polyaniline nanofibers. Mater. Lett. 2009, 63, 592–594. [Google Scholar] [CrossRef]
- Pahovnik, D.; Zagar, E.; Vohlidal, J.; Zigon, M. Ionic liquid-induced formation of polyaniline nanostructures during the chemical polymerization of aniline in an acidic aqueous medium. Synth. Met. 2010, 160, 1761–1766. [Google Scholar] [CrossRef]
- Li, G.C.; Zhang, Z.K. Synthesis of dendritic polyaniline nanofibers in a surfactant gel. Macromolecules 2004, 37, 2683–2685. [Google Scholar] [CrossRef]
- Meng, L.; Lu, Y.; Wang, X.; Zhang, J.; Duan, Y.; Li, C. Facile synthesis of straight polyaniline nanostick in hydrogel. Macromolecules 2007, 40, 2981–2983. [Google Scholar] [CrossRef]
- Hsieh, B.-Z.; Chuang, H.-Y.; Chao, L.; Li, Y.-J.; Huang, Y.-J.; Tseng, P.-H.; Hsieh, T.-H.; Ho, K.-S. Formation mechanism of a nanotubular polyanilines prepared by an emulsion polymerization without organic solvent. Polymer 2008, 49, 4218–4225. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Wei, Z.X.; Wan, M.X. Nanostructures of polyaniline doped with inorganic acids. Macromolecules 2002, 35, 5937–5942. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Wei, Z.X.; Zhang, L.J.; Wan, M.X. Polyaniline nanotubes and their dendrites doped with different naphthalene sulfonic acids. Acta Mater. 2005, 53, 1373–1379. [Google Scholar] [CrossRef]
- Zhang, L.J.; Wan, M.X.; Wei, Y. Nanoscaled polyaniline fibers prepared by ferric chloride as an oxidant. Macromol. Rapid Commun. 2006, 27, 366–371. [Google Scholar] [CrossRef]
- Tavandashti, N.P.; Ghorbani, M.; Shojaei, A. Morphology transition control of polyaniline from nanotubes to nanospheres in a soft template method. Polym. Int. 2015, 64, 88–95. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Yang, Z.; Wang, Z.; Zhang, F.; Wang, S. A novel strategy for the synthesis of polyaniline nanostructures with controlled morphology. React. Funct. Polym. 2008, 68, 1435–1440. [Google Scholar] [CrossRef]
- Yang, C.H.; Chih, Y.K.; Tsai, M.S.; Chen, C.H. Self-doped polyaniline nanostructures for casting metal nanorods. Electrochem. Solid State Lett. 2006, 9, G49–G52. [Google Scholar] [CrossRef]
- Zhang, L.; Peng, H.; Zujovic, Z.D.; Kilmartin, P.A.; Travas-Sejdic, J. Characterization of polyaniline nanotubes formed in the presence of amino acids. Macromol. Chem. Phys. 2007, 208, 1210–1217. [Google Scholar] [CrossRef]
- Wang, F.; Wang, Z.; Tana, M.B.H.; He, C. Uniform Polyaniline Nanotubes Formation via Frozen Polymerization and Application for Oxygen Reduction Reactions. Macromol. Chem. Phys. 2015, 216, 977–984. [Google Scholar] [CrossRef]
- Janosevic, A.; Ciric-Marjanovic, G.; Marjanovic, B.; Trchova, M.; Stejskal, J. 3,5-Dinitrosalicylic acid-assisted synthesis of self-assembled polyaniline nanorods. Mater. Lett. 2010, 64, 2337–2340. [Google Scholar] [CrossRef]
- Yang, Y.S.; Wan, M.X. Chiral nanotubes of polyaniline synthesized by a template-free method. J. Mater. Chem. 2002, 12, 897–901. [Google Scholar] [CrossRef]
- Shinde, S.D.; Jayakannan, M. Probing the Molecular Interactions at the Conducting Polyaniline Nanomaterial Surface via a Pyrene Fluorophore. J. Phys. Chem. C 2010, 114, 15491–15498. [Google Scholar] [CrossRef]
- Zhang, L.X.; Zhang, L.J.; Wan, M.X.; Wei, Y. Polyaniline micro/nanofibers doped with saturation fatty acids. Synth. Met. 2006, 156, 454–458. [Google Scholar] [CrossRef]
- Qiu, H.J.; Wan, M.X.; Matthews, B.; Dai, L.M. Conducting polyaniline nanotubes by template-free polymerization. Macromolecules 2001, 34, 675–677. [Google Scholar] [CrossRef]
- Sarno, D.M.; Manohar, S.K.; MacDiarmid, A.G. Controlled interconversion of semiconducting and metallic forms of polyaniline nanofibers. Synth. Met. 2005, 148, 237–243. [Google Scholar] [CrossRef]
- Zu, X.H.; Zhang, Z.; Yi, G.B.; Deng, Y.L.; Huang, H.L.; Zhong, B.B.; Wang, C.; Luo, H.S.; Wang, H. A novel density-controlled growth of vertical polyaniline nanowire arrays using diblock copolymer as a template. Mater. Exp. 2016, 6, 83–87. [Google Scholar] [CrossRef]
- Qiu, H.; Qi, S.; Wang, D.; Wang, J.; Wu, X. Synthesis of polyaniline nanostructures via soft template of sucrose octaacetate. Synth. Met. 2010, 160, 1179–1183. [Google Scholar] [CrossRef]
- Yu, Y.J.; Si, Z.H.; Chen, S.J.; Bian, C.Q.; Chen, W.; Xue, G. Facile synthesis of polyaniline-sodium alginate nanofibers. Langmuir 2006, 22, 3899–3905. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhao, X.; Xu, Q.; Zhang, Q.; Chen, D. Facile Preparation and Enhanced Capacitance of the Polyaniline/Sodium Alginate Nanofiber Network for Supercapacitors. Langmuir 2011, 27, 6458–6463. [Google Scholar] [CrossRef]
- Gu, Z.J.; Ye, J.R.; Song, W.; Shen, Q. Synthesis of polyaniline nanotubes with controlled rectangular or square pore shape. Mater. Lett. 2014, 121, 12–14. [Google Scholar] [CrossRef]
- Gu, Z.J.; Wang, J.T.; Li, L.L.; Chen, L.F.; Shen, Q. Formation of polyaniline nanotubes with different pore shapes using α-, β- and γ-cyclodextrins as templates. Mater. Lett. 2014, 117, 66–68. [Google Scholar] [CrossRef]
- Zou, F.; Li, Y.; Yu, X.; Zhang, J.; Huang, X.; Qu, Y. β-Cyclodextrin improves the linearity of polyaniline synthesized enzymatically in AOT micellar solution. J. Mol. Catal. B Enzym. 2014, 104, 35–41. [Google Scholar] [CrossRef]
- Sk, M.M.; Yue, C.Y.; Jena, R.K. Facile growth of heparin-controlled porous polyaniline nanofiber networks and their application in supercapacitors. RSC Adv. 2014, 4, 5188–5197. [Google Scholar] [CrossRef]
- Sk, M.M.; Yue, C.Y. Synthesis of polyaniline nanotubes using the self-assembly behavior of vitamin C: A mechanistic study and application in electrochemical supercapacitors. J. Mater. Chem. A 2014, 2, 2830–2838. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, C.; Li, G.; Peng, H.; Chen, K. Self-assembled necklace-like polyaniline nanochains from elliptical nanoparticles. Synth. Met. 2010, 160, 1204–1209. [Google Scholar] [CrossRef]
- Ma, Y.F.; Zhang, J.M.; Zhang, G.J.; He, H.X. Polyaniline nanowires on Si surfaces fabricated with DNA templates. J. Am. Chem. Soc. 2004, 126, 7097–7101. [Google Scholar] [CrossRef] [PubMed]
- Nickels, P.; Dittmer, W.U.; Beyer, S.; Kotthaus, J.P.; Simmel, F.C. Polyaniline nanowire synthesis templated by DNA. Nanotechnology 2004, 15, 1524–1529. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Wan, M.X.; Li, X.N.; Zhao, G.L. The role of DNA in PANI-DNA hybrid: Template and dopant. Polymer 2009, 50, 4529–4534. [Google Scholar] [CrossRef]
- Niu, Z.; Bruckman, M.; Kotakadi, V.S.; He, J.; Emrick, T.; Russell, T.P.; Yang, L.; Wang, Q. Study and characterization of tobacco mosaic virus head-to-tail assembly assisted by aniline polymerization. Chem. Commun. 2006, 28, 3019–3021. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Bruckman, M.A.; Li, S.; Lee, L.A.; Lee, B.; Pingali, S.V.; Thiyagarajan, P.; Wang, Q. Assembly of tobacco mosaic virus into fibrous and macroscopic bundled arrays mediated by surface aniline polymerization. Langmuir 2007, 23, 6719–6724. [Google Scholar] [CrossRef]
- Niu, Z.; Liu, J.; Lee, L.A.; Bruckman, M.A.; Zhao, D.; Koley, G.; Wang, Q. Biological templated synthesis of water-soluble conductive polymeric nanowires. Nano Lett. 2007, 7, 3729–3733. [Google Scholar] [CrossRef]
- Long, Y.Z.; Chen, Z.J.; Zheng, P.; Wang, N.L.; Zhang, Z.M.; Wan, M.X. Low-temperature resistivities of nanotubular polyaniline doped with H3PO4 and beta-naphthalene sulfonic acid. J. Appl. Phys. 2003, 93, 2962–2965. [Google Scholar] [CrossRef]
- Xia, H.B.; Narayanan, J.; Cheng, D.M.; Xiao, C.Y.; Liu, X.Y.; Chan, H.S.O. Formation of ordered arrays of oriented polyaniline nanoparticle nanorods. J. Phys. Chem. B 2005, 109, 12677–12684. [Google Scholar] [CrossRef]
- Li, G.C.; Pang, S.P.; Xie, G.W.; Wang, Z.B.; Peng, H.R.; Zhang, Z.K. Synthesis of radially aligned polyaniline dendrites. Polymer 2006, 47, 1456–1459. [Google Scholar] [CrossRef]
- Dai, T.; Lu, Y. Water-soluble methyl orange fibrils as versatile templates for the fabrication of conducting polymer microtubules. Macromol. Rapid Commun. 2007, 28, 629–633. [Google Scholar] [CrossRef]
- Zhang, Z.M.; Wan, M.X.; Wei, Y. Highly crystalline polyaniline nanostructures doped with dicarhoxylic acids. Adv. Funct. Mater. 2006, 16, 1100–1104. [Google Scholar] [CrossRef]
- Gu, Z.-J.; Zhang, Q.-C.; Shen, Q. Synthesis and comparison of polyaniline nanofibers templated by alpha-, beta- and gamma-cyclodextrin. J. Poly. Res. 2015, 22, 7. [Google Scholar] [CrossRef]
- Anilkumar, P.; Jayakannan, M. Single-molecular-system-based selective micellar templates for polyaniline nanomaterials: Control of shape, size, solid state ordering, and expanded chain to coillike conformation. Macromolecules 2007, 40, 7311–7319. [Google Scholar] [CrossRef]
- Stejskal, J.; Sapurina, I.; Trchova, M. Polyaniline nanostructures and the role of aniline oligomers in their formation. Prog. Polym. Sci. 2010, 35, 1420–1481. [Google Scholar] [CrossRef]
- Tran, H.D.; Wang, Y.; D’Arcy, J.M.; Kaner, R.B. Toward an understanding of the formation of conducting polymer nanofibers. ACS Nano 2008, 2, 1841–1848. [Google Scholar] [CrossRef]
- Huang, J.X.; Kaner, R.B. Nanofiber formation in the chemical polymerization of aniline: A mechanistic study. Angew. Chem. Int. Ed. 2004, 43, 5817–5821. [Google Scholar] [CrossRef]
- Huang, J.X.; Virji, S.; Weiller, B.H.; Kaner, R.B. Polyaniline nanofibers: Facile synthesis and chemical sensors. J. Am. Chem. Soc. 2003, 125, 314–315. [Google Scholar] [CrossRef]
- Huang, J.X.; Kaner, R.B. A general chemical route to polyaniline nanofibers. J. Am. Chem. Soc. 2004, 126, 851–855. [Google Scholar] [CrossRef]
- Liu, Y.D.; Kim, H.Y.; Kim, J.E.; Kim, I.G.; Choi, H.J.; Park, S.-J. Enhanced effect of dopant on polyaniline nanofiber based electrorheological response. Mater. Chem. Phys. 2014, 147, 843–849. [Google Scholar] [CrossRef]
- Pramanik, S.; Karak, N.; Banerjee, S.; Kumar, A. Effects of solvent interactions on the structure and properties of prepared PAni nanofibers. J. Appl. Polym. Sci. 2012, 126, 830–836. [Google Scholar] [CrossRef]
- Chiou, N.-R.; Lee, L.J.; Epstein, A.J. Porous membrane controlled polymerization of nanofibers of polyaniline and its derivatives. J. Mater. Chem. 2008, 18, 2085–2089. [Google Scholar] [CrossRef]
- Pham, Q.M.; Kim, J.-S.; Kim, S. Polyaniline nanofibers synthesized in compressed CO2. Synth. Met. 2010, 160, 394–399. [Google Scholar] [CrossRef]
- Bhadra, S.; Lee, J.H. Synthesis of Higher Soluble Nanostructured Polyaniline by Vapor-Phase Polymerization and Determination of its Crystal Structure. J. Appl. Polym. Sci. 2009, 114, 331–340. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, V.; Aggarwal, S.; Mandal, U.K. Synthesis of 1-Dimensional polyaniline nanofibers by reverse microemulsion. Colloid Polym. Sci. 2009, 287, 1107–1110. [Google Scholar] [CrossRef]
- Su, C.; Wang, G.; Huang, F.; Li, X. Effects of synthetic conditions on the structure and electrical properties of polyaniline nanofibers. J. Mater. Sci. 2008, 43, 197–202. [Google Scholar] [CrossRef]
- Ding, H.; Shen, J.; Wan, M.; Chen, Z. Formation mechanism of polyaniline nanotubes by a simplified template-free method. Macromol. Chem. Phys. 2008, 209, 864–871. [Google Scholar] [CrossRef]
- Wang, P.-C.; Dan, Y.; Liu, L.-H. Effect of thermal treatment on conductometric response of hydrogen gas sensors integrated with HCl-doped polyaniline nanofibers. Mater. Chem. Phys. 2014, 144, 155–161. [Google Scholar] [CrossRef]
- Hu, Z.; Xu, J.; Tian, Y.; Peng, R.; Xian, Y.; Ran, Q.; Jin, L. Layer-by-layer assembly of poly(sodium 4-styrenesulfonate) wrapped multiwalled carbon nanotubes with polyaniline nanofibers and its electrochemistry. Carbon 2010, 48, 3729–3736. [Google Scholar] [CrossRef]
- Huang, J.X.; Kaner, R.B. The intrinsic nanofibrillar morphology of polyaniline. Chem. Commun. 2006, 367–376. [Google Scholar] [CrossRef]
- Rezaei, F.; Tavandashti, N.P.; Zahedi, A.R. Morphology of polyaniline nanofibers synthesized under different conditions. Res. Chem. Intermed. 2014, 40, 1233–1247. [Google Scholar] [CrossRef]
- Chiou, N.R.; Epstein, A.J. A simple approach to control the growth of polyaniline nanofibers. Synth. Met. 2005, 153, 69–72. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, X.; Li, J.; Wang, F. Facile synthesis of polyaniline nanofibers using pseudo-high dilution technique. Synth. Met. 2009, 159, 1508–1511. [Google Scholar] [CrossRef]
- Zhao, Y.; Arowo, M.; Wu, W.; Chen, J. Effect of Additives on the Properties of Polyaniline Nanofibers Prepared by High Gravity Chemical Oxidative Polymerization. Langmuir 2015, 31, 5155–5163. [Google Scholar] [CrossRef]
- Zhang, X.Y.; Goux, W.J.; Manohar, S.K. Synthesis of polyaniline nanofibers by “nanofiber seeding”. J. Am. Chem. Soc. 2004, 126, 4502–4503. [Google Scholar] [CrossRef]
- Wang, D.; Ma, F.; Qi, S.; Song, B. Synthesis and electromagnetic characterization of polyaniline nanorods using Schiff base through ‘seeding’ polymerization. Synth. Met. 2010, 160, 2077–2084. [Google Scholar] [CrossRef]
- Wu, W.; Pan, D.; Li, Y.; Zhao, G.; Jing, L.; Chen, S. Facile fabrication of polyaniline nanotubes using the self-assembly behavior based on the hydrogen bonding: A mechanistic study and application in high-performance electrochemical supercapacitor electrode. Electrochim. Acta 2015, 152, 126–134. [Google Scholar] [CrossRef]
- Hatano, T.; Takeuchi, M.; Ikeda, A.; Shinkai, S. New morphology-controlled poly(aniline) synthesis using anionic porphyrin aggregate as a template. Chem. Lett. 2003, 32, 314–315. [Google Scholar] [CrossRef]
- Kuila, B.K.; Stamm, M. Fabrication of oriented polyaniline nanostructures using block copolymer nanotemplates and their optical, electrochemical and electric properties. J. Mater. Chem. 2010, 20, 6086–6094. [Google Scholar] [CrossRef]
- Li, X.; Tian, S.J.; Ping, Y.; Kim, D.H.; Knoll, W. One-step route to the fabrication of highly porous polyaniline nanofiber films by using PS-b-PVP diblock copolymers as templates. Langmuir 2005, 21, 9393–9397. [Google Scholar] [CrossRef]
- Cao, Y.; Mallouk, T.E. Morphology of Template-Grown Polyaniline Nanowires and Its Effect on the Electrochemical Capacitance of Nanowire Arrays. Chem. Mater. 2008, 20, 5260–5265. [Google Scholar] [CrossRef]
- Liu, J.; Lin, Y.H.; Liang, L.; Voigt, J.A.; Huber, D.L.; Tian, Z.R.; Coker, E.; McKenzie, B.; McDermott, M.J. Templateless assembly of molecularly aligned conductive polymer nanowires: A new approach for oriented nanostructures. Chem. Eur. J. 2003, 9, 604–611. [Google Scholar] [CrossRef]
- Kemp, N.T.; Cochrane, J.W.; Newbury, R. Characteristics of the nucleation and growth of template-free polyaniline nanowires and fibrils. Synth. Met. 2009, 159, 435–444. [Google Scholar] [CrossRef]
- Wang, K.; Huang, J.; Wei, Z. Conducting Polyaniline Nanowire Arrays for High Performance Supercapacitors. J. Phys. Chem. C 2010, 114, 8062–8067. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Wang, Z.; Zhang, F.; Wang, S. Electrodeposition of polyaniline nanostructures: A lamellar structure. Synth. Met. 2009, 159, 277–281. [Google Scholar] [CrossRef]
- Zhang, H.; Li, H.; Zhang, F.; Wang, J.; Wang, Z.; Wang, S. Polyaniline nanofibers prepared by a facile electrochemical approach and their supercapacitor performance. J. Mater. Res. 2008, 23, 2326–2332. [Google Scholar] [CrossRef]
- Langer, J.J.; Czajkowski, I. Polyaniline Microrods. Adv. Mater. Opt. Electron. 1997, 7, 149–156. [Google Scholar] [CrossRef]
- Okamoto, H.; Okamoto, M.; Kotaka, T. Structure development in polyaniline films during electrochemical polymerization. II: Structure and properties of polyaniline films prepared via electrochemical polymerization. Polymer 1998, 39, 4359–4367. [Google Scholar] [CrossRef]
- Chen, X.; Tang, Q.; He, B. Efficient dye-sensitized solar cell from spiny polyaniline nanofiber counter electrode. Mater. Lett. 2014, 119, 28–31. [Google Scholar] [CrossRef]
- Liang, L.; Liu, J.; Windisch, C.F.; Exarhos, G.J.; Lin, Y.H. Direct assembly of large arrays of oriented conducting polymer nanowires. Angew. Chem. Int. Ed. 2002, 41, 3665–3668. [Google Scholar] [CrossRef]
- Kellenberger, A.; Plesu, N.; Mihali, M.T.-L.; Vaszilcsin, N. Synthesis of polyaniline nanostructures by electrochemical deposition on niobium. Polymer 2013, 54, 3166–3174. [Google Scholar] [CrossRef]
- Smith, J.A.; Josowicz, M.; Janata, J. Polyaniline-gold nanocomposite system. J. Electrochem. Soc. 2003, 150, E384–E388. [Google Scholar] [CrossRef]
- Jiang, H.-F.; Liu, X.-X. One-dimensional growth and electrochemical properties of polyaniline deposited by a pulse potentiostatic method. Electrochim. Acta 2010, 55, 7175–7181. [Google Scholar] [CrossRef]
- Yu, X.; Li, Y.; Zhu, N.; Yang, Q.; Kalantar-zadeh, K. A polyaniline nanofibre electrode and its application in a self-powered photoelectrochromic cell. Nanotechnology 2007, 18, 015201. [Google Scholar] [CrossRef]
- Wei, D.; Kvarnstrom, C.; Lindfors, T.; Ivaska, A. Polyaniline nanotubules obtained in room-temperature ionic liquids. Electrochem. Commun. 2006, 8, 1563–1566. [Google Scholar] [CrossRef]
- Ghanbari, K.; Mousavi, M.F.; Shamsipur, M. Preparation of polyaniline nanofibers and their use as a cathode of aqueous rechargeable batteries. Electrochim. Acta 2006, 52, 1514–1522. [Google Scholar] [CrossRef]
- Gupta, V.; Miura, N. Large-area network of polyaniline nanowires prepared by potentiostatic deposition process. Electrochem. Commun. 2005, 7, 995–999. [Google Scholar] [CrossRef]
- Yeager, E.; Bockris, J.O.M.; Conway, B.E.; Sarangapani, S. Comprehensive Treatise of Electrochemistry vol.6; Springer: Boston, MA, USA, 1983; pp. 242–277. [Google Scholar]
- Zhang, H. Preparation and Characterization of Polyaniline Nanomaterials. Ph.D. Thesis, Tianjin University, Tianjin, China, 2009. [Google Scholar]
- Zhou, C.F.; Du, X.S.; Liu, Z.; Ringer, S.P.; Mai, Y.W. Solid phase mechanochemical synthesis of polyaniline branched nanofibers. Synth. Met. 2009, 159, 1302–1307. [Google Scholar] [CrossRef]
- Bhadra, S.; Kim, N.H.; Rhee, K.Y.; Lee, J.H. Preparation of nanosize polyaniline by solid-state polymerization and determination of crystal structure. Polym. Int. 2009, 58, 1173–1180. [Google Scholar] [CrossRef]









| Synthesis Method | Composition Complexity of Discharged Waste Liquid | Volume of Waste Liquid | Waste Quantity | Recycle Complexity of Waste Liquid | Method Costs |
|---|---|---|---|---|---|
| Hard template | High | High | High | High | High |
| Soft template | High | Low | High | High | Relatively low |
| Interfacial polymerization | High | Low | High | High | Relatively low |
| Dilute polymerization | Relatively low | High | Relatively low | Low | Relatively low |
| Rapid mixing | Relatively low | Low | High | Low | Low |
| Electro-polymerization | Low | Low | High | Low | Low |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Wang, J.; Ou, B.; Zhao, S.; Wang, Z. Some Important Issues of the Commercial Production of 1-D Nano-PANI. Polymers 2019, 11, 681. https://doi.org/10.3390/polym11040681
Wu Y, Wang J, Ou B, Zhao S, Wang Z. Some Important Issues of the Commercial Production of 1-D Nano-PANI. Polymers. 2019; 11(4):681. https://doi.org/10.3390/polym11040681
Chicago/Turabian StyleWu, Ying, Jixiao Wang, Bin Ou, Song Zhao, and Zhi Wang. 2019. "Some Important Issues of the Commercial Production of 1-D Nano-PANI" Polymers 11, no. 4: 681. https://doi.org/10.3390/polym11040681





