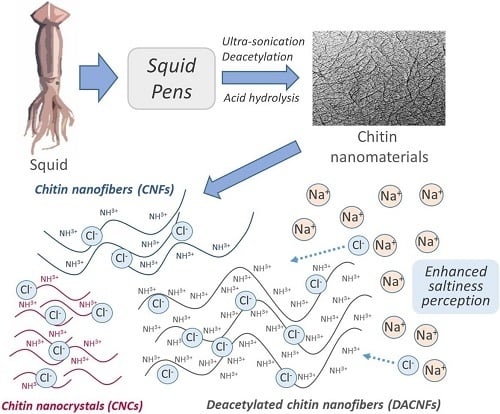
Enhancing Saltiness Perception Using Chitin Nanomaterials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of β-Chitin and Deacetylated Chitin
2.3. Preparation of CNF and Deacetylated Chitin Nanofiber (DACNF)
2.4. Preparation of CNC
2.5. Physicochemical Properties
2.5.1. Transmission Electron Microscopy
2.5.2. Fourier-Transform Infrared Spectroscopy
2.5.3. X-ray Diffraction
2.6. Properties of Chitin Nanomaterials Suspensions
2.6.1. Zeta Potential
2.6.2. Sensory Evaluation
2.7. Statistical Analysis
3. Results and Discussion
3.1. Yields of Chitin Nanomaterials
3.2. TEM Morphology Study
3.3. Functional Groups Identification and DD Determination of Chitins and its Nanomaterials
3.4. X-ray Diffraction
3.5. Properties of Chitin Nanomaterials/NaCl Suspensions
3.5.1. Zeta Potential
3.5.2. Sensory Evaluation
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Israr, T.; Rakha, A.; Sohail, M.; Rashid, S.; Shehzad, A. Salt reduction in baked products: Strategies and constraints. Trends Food Sci. Technol. 2016, 51, 98–105. [Google Scholar] [CrossRef]
- Liem, D.G.; Miremadi, F.; Keast, R.S. Reducing sodium in foods: The effect on flavor. Nutrients 2011, 3, 694–711. [Google Scholar] [CrossRef] [PubMed]
- Webster, J.L.; Dunford, E.K.; Hawkes, C.; Neal, B.C. Salt reduction initiatives around the world. J. Hypertens. 2011, 29, 1043–1050. [Google Scholar] [CrossRef] [PubMed]
- Hoppu, U.; Hopia, A.; Pohjanheimo, T.; Rotola-Pukkila, M.; Mäkinen, S.; Pihlanto, A.; Sandell, M. Effect of salt reduction on consumer acceptance and sensory quality of food. Foods 2017, 6, 103. [Google Scholar] [CrossRef]
- Brown, I.J.; Tzoulaki, I.; Candeias, V.; Elliott, P. Salt intakes around the world: Implications for public health. Int. J. Epidemiol. 2009, 38, 791–813. [Google Scholar] [CrossRef] [PubMed]
- Quilaqueo, M.; Duizer, L.; Aguilera, J.M. The morphology of salt crystals affects the perception of saltiness. Food Res. Int. 2015, 76, 675–681. [Google Scholar] [CrossRef] [PubMed]
- Desmond, E. Reducing salt: A challenge for the meat industry. Meat Sci. 2006, 74, 188–196. [Google Scholar] [CrossRef] [PubMed]
- Campagnol, P.C.B.; dos Santos, B.A.; Terra, N.N.; Pollonio, M.A.R. Lysine, disodium guanylate and disodium inosinate as flavor enhancers in low-sodium fermented sausages. Meat Sci. 2012, 91, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Kilcast, D.; Den Ridder, C. Sensory issues in reducing salt in food products. In Reducing Salt in Foods; Kilcast, D., Angus, F., Eds.; Woodhead Publishing: Sawston, UK, 2007; Volume 10, pp. 201–220. [Google Scholar]
- Koliandris, A.-L.; Morris, C.; Hewson, L.; Hort, J.; Taylor, A.J.; Wolf, B. Correlation between saltiness perception and shear flow behaviour for viscous solutions. Food Hydrocol. 2010, 24, 792–799. [Google Scholar] [CrossRef]
- Phan, V.A.; Yven, C.; Lawrence, G.; Chabanet, C.; Reparet, J.M.; Salles, C. In vivo sodium release related to salty perception during eating model cheeses of different textures. Int. Dairy J. 2008, 18, 956–963. [Google Scholar] [CrossRef]
- Mosca, A.C.; Andriot, I.; Guichard, E.; Salles, C. Binding of Na+ ions to proteins: Effect on taste perception. Food Hydrocoll. 2015, 51, 33–40. [Google Scholar] [CrossRef]
- Jiang, W.J.; Tsai, M.L.; Liu, T. Chitin nanofiber as a promising candidate for improved salty taste. LWT-Food Sci. Technol. 2017, 75, 65–71. [Google Scholar] [CrossRef]
- Kumirska, J.; Weinhold, M.X.; Thöming, J.; Stepnowski, P. Biomedical activity of chitin/chitosan based materials—influence of physicochemical properties apart from molecular weight and degree of N-acetylation. Polymers 2011, 3, 1875–1901. [Google Scholar] [CrossRef]
- Deepthi, S.; Viha, C.; Thitirat, C.; Furuike, T.; Tamura, H.; Jayakumar, R. Fabrication of chitin/poly (butylene succinate)/chondroitin sulfate nanoparticles ternary composite hydrogel scaffold for skin tissue engineering. Polymers 2016, 6, 2974–2984. [Google Scholar] [CrossRef]
- Aklog, Y.F.; Nagae, T.; Izawa, H.; Morimoto, M.; Saimoto, H.; Ifuku, S. Preparation of chitin nanofibers by surface esterification of chitin with maleic anhydride and mechanical treatment. Carbohydr. Polym. 2016, 153, 55–59. [Google Scholar] [CrossRef]
- Ifuku, S.; Nogi, M.; Yoshioka, M.; Morimoto, M.; Yano, H.; Saimoto, H. Fibrillation of dried chitin into 10-20 nm nanofibers by a simple grinding method under acidic conditions. Carbohydr. Polym. 2010, 81, 134–139. [Google Scholar] [CrossRef]
- Lu, Y.; Sun, Q.; She, X.; Xia, Y.; Liu, Y.; Li, J.; Yang, D. Fabrication and characterisation of alpha-chitin nanofibers and highly transparent chitin films by pulsed ultrasonication. Carbohydr. Polym. 2013, 98, 1497–1504. [Google Scholar] [CrossRef]
- Mushi, N.E.; Butchosa, N.; Salajkova, M.; Zhou, Q.; Berglund, L.A. Nanostructured membranes based on native chitin nanofibers prepared by mild process. Carbohydr. Polym. 2014, 112, 255–263. [Google Scholar] [CrossRef]
- Ifuku, S.; Yamada, K.; Morimoto, M.; Saimoto, H. Nanofibrillation of dry chitin powder by star burst system. J. Nanomater. 2012, 2012, 645624. [Google Scholar] [CrossRef]
- Salaberria, A.M.; Labidi, J.; Fernandes, S.C.M. Chitin nanocrystals and nanofibers as nano-sized fillers into thermoplastic starch-based biocomposites processed by melt-mixing. Chem. Eng. J. 2014, 256, 356–364. [Google Scholar] [CrossRef]
- Tsai, M.L.; Chen, R.H. Method of Preparing Chitin Nanofibers. U.S. Patent 9,644,084B2, 9 May 2017. [Google Scholar]
- Fan, Y.; Saito, T.; Isogai, A. Chitin nanocrystals prepared by TEMPO-mediated oxidation of α-chitin. Biomacromolecules 2008, 9, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Saito, T.; Isogai, A. Preparation of chitin nanofibers from squid pen β-chitin by simple mechanical treatment under acid conditions. Biomacromolecules 2008, 9, 1919–1923. [Google Scholar] [CrossRef] [PubMed]
- Hsueh, C.Y.; Tsai, M.L.; Liu, T. Enhancing saltiness perception using chitin nanofibers when curing tilapia fillets. LWT-Food Sci. Technol. 2017, 86, 93–98. [Google Scholar] [CrossRef]
- Hsieh, Y.Y.; Chin, H.Y.; Tsai, M.L. Enrichment desired quality chitosan fraction and advance yield by sequential static and static–dynamic supercritical CO2. Carbohydr. Polym. 2015, 133, 313–319. [Google Scholar] [CrossRef]
- Fan, Y.; Saito, T.; Isogai, A. Individual chitin nano-whiskers prepared from partially deacetylated α-chitin by fibril surface cationization. Carbohydr. Polym. 2010, 79, 1046–1051. [Google Scholar] [CrossRef]
- Tzoumaki, M.V.; Moschakis, T.; Biliaderis, C.G. Metastability of nematic gels made of aqueous chitin nanocrystal dispersions. Biomacromolecules 2010, 11, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Pereira, A.G.; Muniz, E.C.; Hsieh, Y.L. Chitosan-sheath and chitin-core nanowhiskers. Carbohydr. Polym. 2014, 107, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Tan, T.S.; Chin, H.Y.; Tsai, M.L.; Liu, C.L. Structural alterations, pore generation, and deacetylation of α- and β-chitin submitted to steam explosion. Carbohydr. Polym. 2015, 122, 321–328. [Google Scholar] [CrossRef] [PubMed]
- Singh-Ackbarali, D.; Maharaj, R. Sensory evaluation as a tool in determining acceptability of innovative products developed by undergraduate students in food science and technology at the university of Trinidad and Tobago. J. Curric. Teach. 2014, 3, 10–27. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Noishiki, Y.; Wada, M. X-ray structure of anhydrous β-chitin at 1 Å resolution. Macromolecules 2011, 44, 950–957. [Google Scholar] [CrossRef]
- Ma, B.; Qin, A.; Li, X.; Zhao, X.; He, C. Structure and properties of chitin whisker reinforced chitosan membranes. Int. J. Biol. Macromol. 2014, 64, 341–346. [Google Scholar] [CrossRef]
- Paillet, M.; Dufresne, A. Chitin whisker reinforced thermoplastic nanocomposites. Macromolecules 2001, 34, 6527–6530. [Google Scholar] [CrossRef]
- Rubentheren, V.; Ward, T.A.; Chee, C.Y.; Tang, C.K. Processing and analysis of chitosan nanocomposites reinforced with chitin whiskers and tannic acid as a crosslinker. Carbohydr. Polym. 2015, 115, 379–387. [Google Scholar] [CrossRef]
- Goodrich, J.D.; Winter, W.T. α-Chitin nanocrystals prepared from shrimp shells and their specific surface area measurement. Biomacromolecules 2007, 8, 252–257. [Google Scholar] [CrossRef]
- Sriupayo, J.; Supaphol, P.; Blackwell, J.; Rujiravanit, R. Preparation and characterization of α-chitin whisker-reinforced chitosan nanocomposite films with or without heat treatment. Carbohydr. Polym. 2005, 62, 130–136. [Google Scholar] [CrossRef]
- Suenaga, S.; Nikaido, N.; Totani, K.; Kawasaki, K.; Ito, Y.; Yamashita, K.; Osada, M. Effect of purification method of beta-chitin from squid pen on the properties of beta-chitin nanofibers. Int. J. Biol. Macromol. 2016, 91, 987–993. [Google Scholar] [CrossRef]
- Fiamingo, A.; Delezuk, J.A.D.M.; Trombotto, S.; David, L.; Campana-Filho, S.P. Extensively deacetylated high molecular weight chitosan from the multistep ultrasound-assisted deacetylation of beta-chitin. Ultrason. Sonochem. 2016, 32, 79–85. [Google Scholar] [CrossRef]
- Abdou, E.S.; Nagy, K.S.; Elsabee, M.Z. Extraction and characterization of chitin and chitosan from local sources. Bioresour. Technol. 2008, 99, 1359–1367. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, H.T.; Wu, G.J.; Tsai, G.J. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef]
- Rezaei, F.; Richard, T.L.; Logan, B.E. Analysis of chitin particle size on maximum power generation, power longevity, and Coulombic efficiency in solid–substrate microbial fuel cells. J. Power Sources 2009, 192, 304–309. [Google Scholar] [CrossRef]






| Sample | Method | Yield (%) | DD (%) | Crystalline Index (%) | |
|---|---|---|---|---|---|
| CrI110 | CrI020 | ||||
| Chitin | 22.84 ± 1.97 a | 81.40 | 73.33 | ||
| CNF | Ultrasonication | 50.67 ± 4.93 b | 22.89 ± 1.05 a | 69.23 | 66.67 |
| CNC | Acid hydrolysis | 44.47 ± 3.44 b | 23.43 ± 1.41 a | 77.14 | 70.37 |
| DAChitin | 48.45 ± 0.91 b | 70.37 | 42.86 | ||
| DACNF | Ultrasonication | 19.00 ± 0.04 a | 53.36 ± 2.41 b | 57.14 | 34.07 |
| Item | CNF | DACNF | CNC |
|---|---|---|---|
| Average diameter (nm) | 17.24 ± 2.02 a | 15.01 ± 3.57 c | 16.05 ± 1.10 b |
| Average length (nm) | 1725.05 ± 591.49 a | 1806.60 ± 496.71 a | 116.91 ± 2.06 b |
| Aspect ratio | 100.35 | 120.59 | 7.28 |
| Volume (nm3)/CNF or CNC | 4.038 × 105 | 3.203 × 105 | 2.365 × 104 |
| Surface area (nm2)/CNF or CNC | 9.417 × 104 | 8.571 × 104 | 6.300 × 103 |
| Number/mL of CNF or CNC * | 1.390 × 1011 | 1.753 × 1011 | 2.373 × 1012 |
| Total surface area (nm2/mL) | 1.309 × 1016 | 1.502 × 1016 | 1.495 × 1016 |
| Sample | 0.3% NaCl | CNF + 0.3% NaCl | DACNF + 0.3% NaCl | CNC + 0.3% NaCl |
|---|---|---|---|---|
| Saltiness | 3.17 ± 1.32 a | 4.07 ± 1.12 b | 4.83 ± 1.12 c | 4.83 ± 0.92 c |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsai, W.-C.; Wang, S.-T.; Chang, K.-L.B.; Tsai, M.-L. Enhancing Saltiness Perception Using Chitin Nanomaterials. Polymers 2019, 11, 719. https://doi.org/10.3390/polym11040719
Tsai W-C, Wang S-T, Chang K-LB, Tsai M-L. Enhancing Saltiness Perception Using Chitin Nanomaterials. Polymers. 2019; 11(4):719. https://doi.org/10.3390/polym11040719
Chicago/Turabian StyleTsai, Wan-Chen, Shang-Ta Wang, Ke-Liang Bruce Chang, and Min-Lang Tsai. 2019. "Enhancing Saltiness Perception Using Chitin Nanomaterials" Polymers 11, no. 4: 719. https://doi.org/10.3390/polym11040719