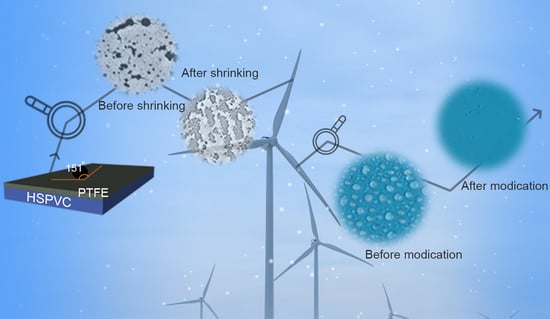
Superhydrophobic Polytetrafluoroethylene/Heat-Shrinkable Polyvinyl Chloride Composite Film with Super Anti-Icing Property
Abstract
:1. Introduction
2. Experimental
2.1. Materials
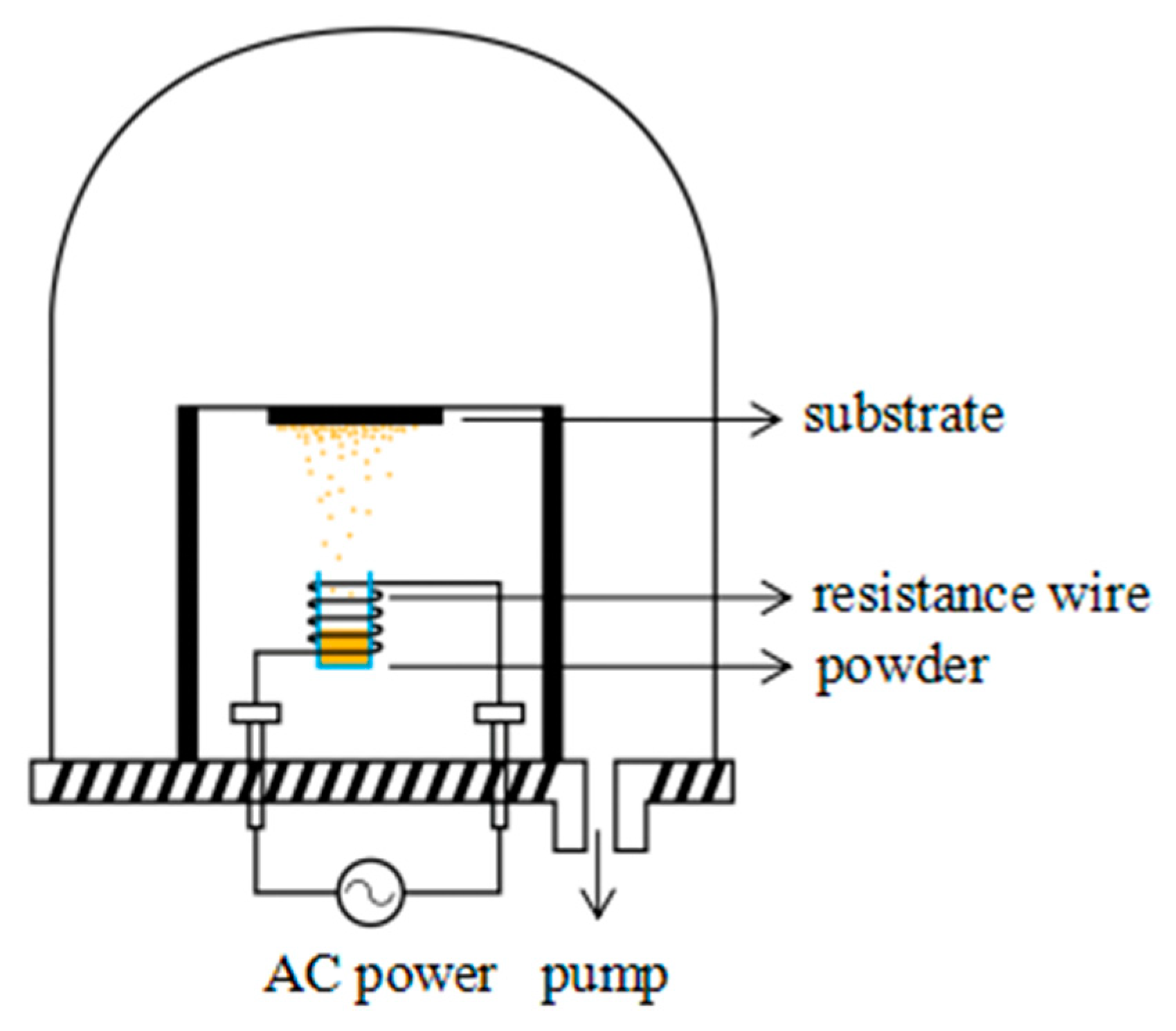
2.2. Sample Preparation
2.3. Anti-Icing Testing of Samples
2.4. Characterization
3. Results and Discussion
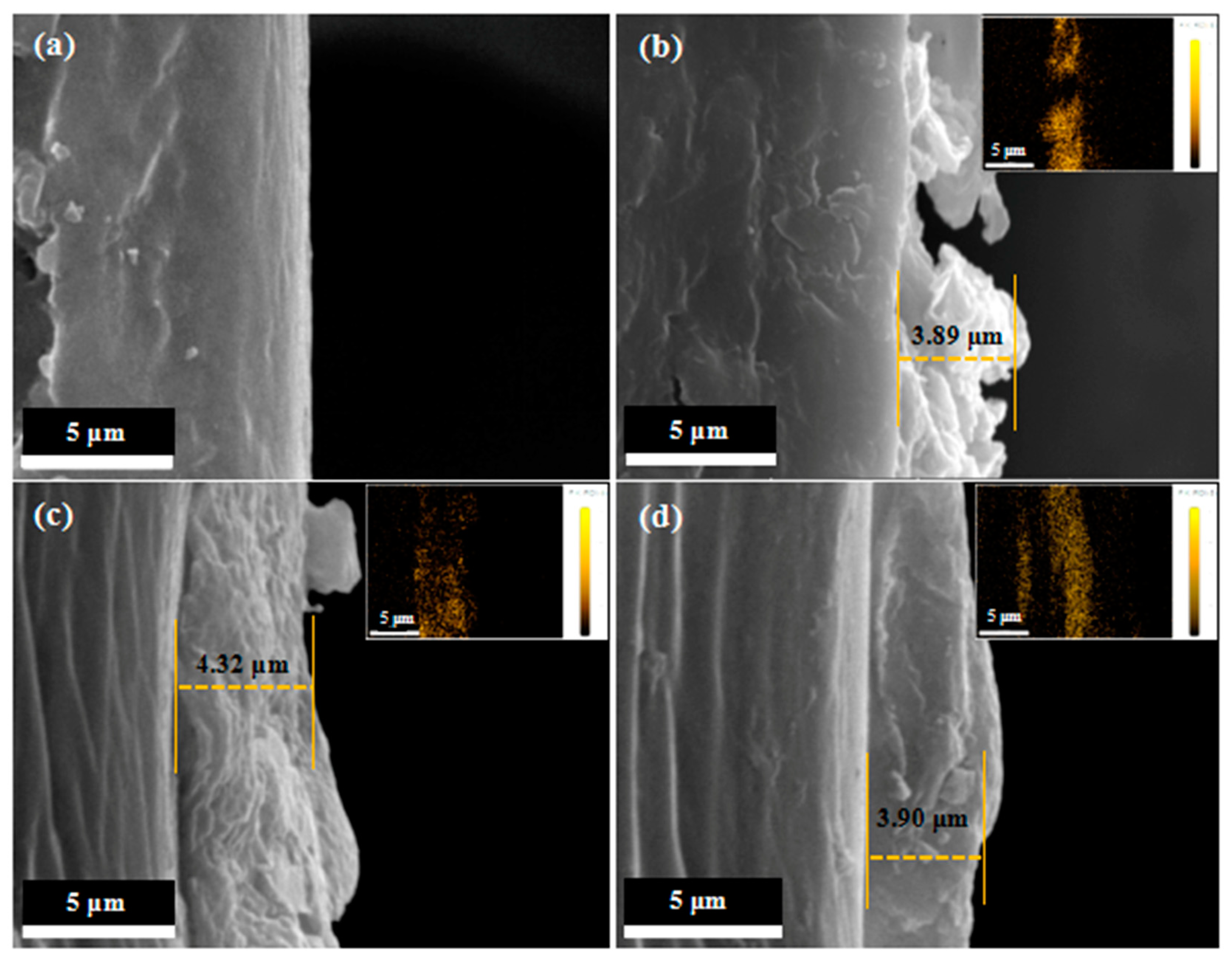
3.1. Surface Morphology
3.2. Chemical Composition of PTFE Coatings
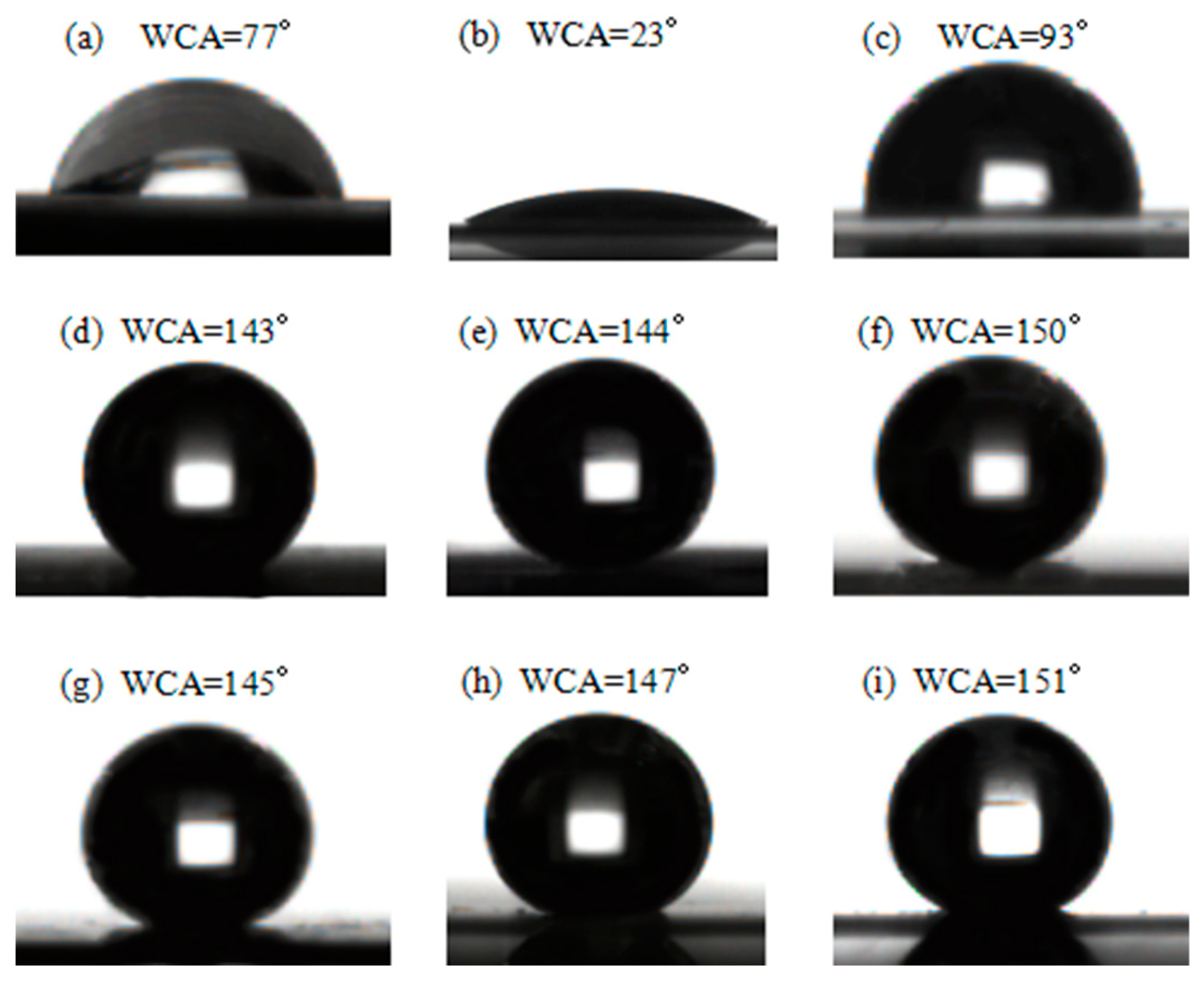
3.3. AWCA of Modified HSPVC Films
3.4. UV Resistance of PTFE/HSPVC Composite Films
3.5. Adhesive Strength of PTFE/HSPVC Composite Films
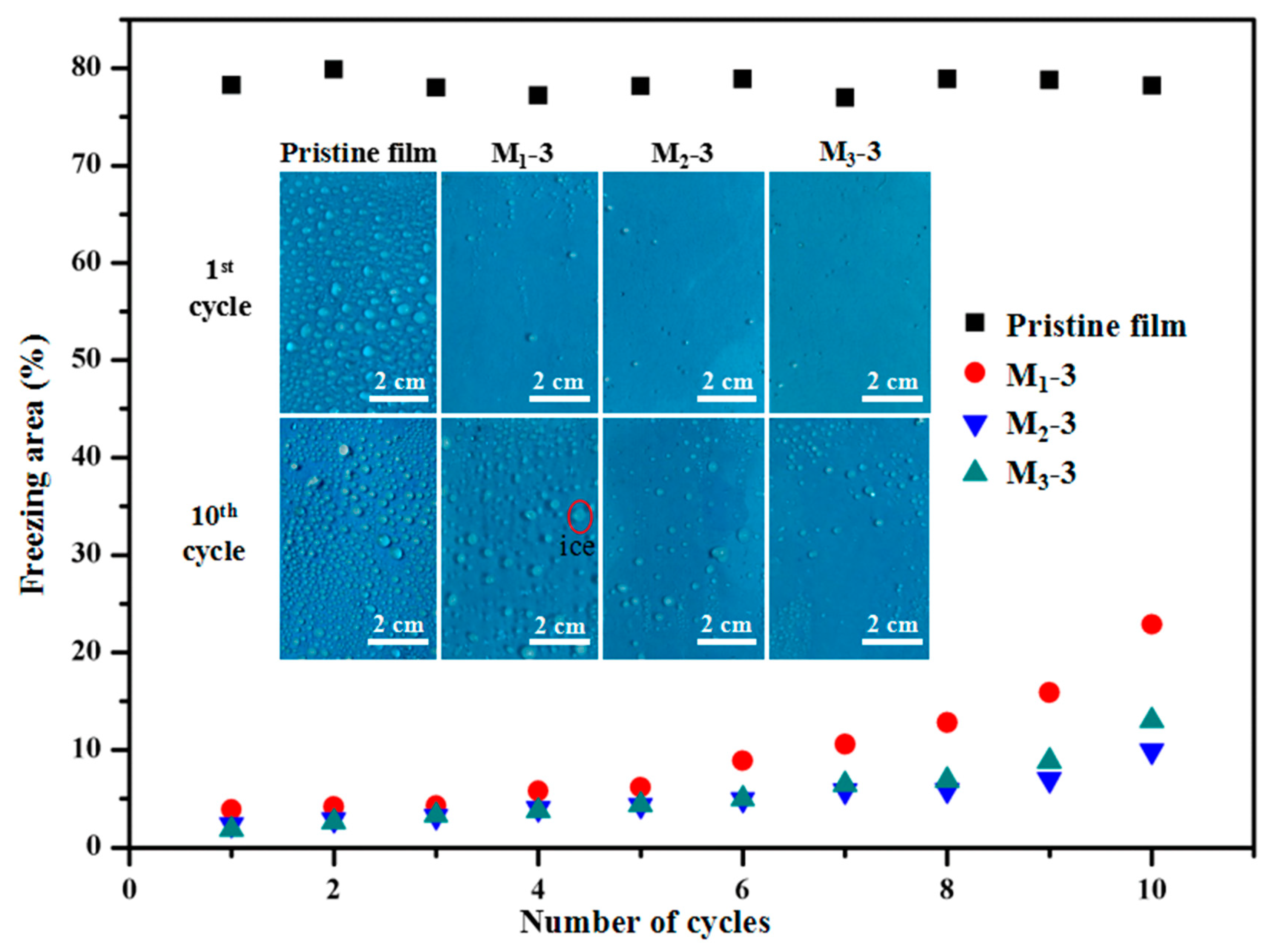
3.6. Anti-Icing Property of PTFE/HSPVC Composite Films
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wang, Y.; Xu, Y.; Huang, Q. Progress on ultrasonic guided waves de-icing techniques in improving aviation energy efficiency. Renew. Sustain. Energy Rev. 2017, 79, 638–645. [Google Scholar] [CrossRef]
- Zuo, Z.; Liao, R.; Guo, C.; Yuan, Y.; Zhang, A. Fabrication and anti-icing property of coral-like superhydrophobic aluminum surface. Appl. Surf. Sci. 2015, 331, 132–139. [Google Scholar] [CrossRef]
- Muhaimin; Kandasamy, R.; Hashim, I. Effect of chemical reaction, heat and mass transfer on nonlinear boundary layer past a porous shrinking sheet in the presence of suction. Nucl. Eng. Des. 2010, 240, 933–939. [Google Scholar] [CrossRef]
- Kondratov, A.P.; Volinsky, A.A.; Chen, J. Macro-mechanism of polyvinyl chloride shrink sleeves embossed marking. J. Appl. Polym. Sci. 2016, 133, 43691–43695. [Google Scholar] [CrossRef]
- Chang, K.H.; Maeng, H.; Song, K.; Kaang, S. Thermal behaviors of heat shrinkable poly(vinyl chloride) film. J. Appl. Polym. Sci. 2009, 112, 886–895. [Google Scholar]
- Xu, R. Application of Heat Stabilizer–Methyl Stannum Mercaptide in Poly (vinyl chloride). China Plast. 2000, 14, 76–78. [Google Scholar]
- Alizadeh, A.; Yamada, M.; Li, R.; Shang, W.; Otta, S. Dynamics of ice nucleation on water repellent surfaces. Langmuir 2012, 28, 3180–3186. [Google Scholar] [CrossRef]
- Liao, R.; Zuo, Z.; Guo, C.; Zhang, A.; Zhao, X.; Yuan, Y. Anti-icing performance in glaze ice of nanostructured film prepared by RF magnetron sputtering. Appl. Surf. Sci. 2015, 356, 539–545. [Google Scholar] [CrossRef]
- Gao, Q.; Hu, J.; Li, R.; Pang, L.; Xing, Z.; Xu, L.; Wang, M.; Guo, X.; Wu, G. Preparation and characterization of superhydrophobic organic-inorganic hybrid cotton fabrics via γ-radiation-induced graft polymerization. Carbohyd. Polym. 2016, 149, 308–316. [Google Scholar] [CrossRef]
- Anna Maria, C.; Yujun, S.; Gleason, K.K. Grafted crystalline poly-perfluoroacrylate structures for superhydrophobic and oleophobic functional coatings. Adv. Mater. 2012, 24, 4534–4539. [Google Scholar]
- Liu, X.; Ye, Q.; Song, X.; Zhu, Y.; Cao, X.; Liang, Y.; Zhou, F. Responsive wetting transition on superhydrophobic surfaces with sparsely grafted polymer brushes. Soft Matter 2011, 7, 515–523. [Google Scholar] [CrossRef]
- Zhang, Y.-P.; Yang, J.-H.; Li, L.-L.; Cui, C.-X.; Li, Y.; Liu, S.-Q.; Zhou, X.-M.; Qu, L.-B. Facile fabrication of superhydrophobic copper-foam and electrospinning polystyrene fiber for combinational oil–water separation. Polymers 2019, 11, 97. [Google Scholar] [CrossRef]
- Liu, S.; Liu, X.; Latthe, S.S.; Gao, L.; An, S.; Yoon, S.S.; Liu, B.; Xing, R. Self-cleaning transparent superhydrophobic coatings through simple sol–gel processing of fluoroalkylsilane. Appl. Surf. Sci. 2015, 351, 897–903. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Q.; Zhan, X.; Chen, F. Superhydrophobic and anti-icing properties at overcooled temperature of a fluorinated hybrid surface prepared via a sol-gel process. Soft Matter 2015, 11, 4540–4550. [Google Scholar] [CrossRef]
- Tripathi, S.; Haque, S.M.; Rao, K.D.; De, R.; Shripathi, T.; Deshpande, U.; Ganesan, V.; Sahoo, N.K. Investigation of optical and microstructural properties of RF magnetron sputtered PTFE films for hydrophobic applications. Appl. Surf. Sci. 2016, 385, 289–298. [Google Scholar] [CrossRef]
- Kylián, O.; Petr, M.; Serov, A.; Solař, P.; Polonskyi, O.; Hanuš, J.; Choukourov, A.; Biederman, H. Hydrophobic and super-hydrophobic coatings based on nanoparticles overcoated by fluorocarbon plasma polymer. Vacuum 2014, 100, 57–60. [Google Scholar] [CrossRef]
- Bayat, A.; Ebrahimi, M.; Nourmohammadi, A.; Moshfegh, A.Z. Wettability properties of PTFE/ZnO nanorods thin film exhibiting UV-resilient superhydrophobicity. Appl. Surf. Sci. 2015, 341 (Suppl. C), 92–99. [Google Scholar] [CrossRef]
- Park, B.J.; Furst, E.M. Fabrication of unusual asymmetric colloids at an oil−water interface. Langmuir 2010, 26, 10406–10410. [Google Scholar] [CrossRef]
- Kim, D.-K.; Lee, S.-B.; Doh, K.-S. Surface Properties of Fluorosilicone copolymers and their surface modification effects on PVC film. J. Colloid. Interf. Sci. 1998, 205, 417–422. [Google Scholar] [CrossRef]
- Marmur, A.; Della Volpe, C.; Siboni, S.; Amirfazli, A.; Drelich, J.W. Contact angles and wettability: Towards common and accurate terminology. Surf. Innov. 2017, 1, 3–8. [Google Scholar] [CrossRef]
- Marmur, A. A guide to the equilibrium contact angles maze. In Contact Angle Wettability and Adhesion; K.L. Mittal: Leiden, The Netherlands, 2009; Volume 6, pp. 3–8. [Google Scholar]
- Wang, K.; Hou, D.; Wang, J.; Wang, Z.; Tian, B.; Liang, P. Hydrophilic surface coating on hydrophobic PTFE membrane for robust anti-oil-fouling membrane distillation. Appl. Surf. Sci. 2018, 450, 57–65. [Google Scholar] [CrossRef]
- Huang, Q.; Huang, Y.; Gao, S.; Zhang, M.; Xiao, C. Novel Ultrafine Fibrous Poly(tetrafluoroethylene) hollow fiber membrane fabricated by electrospinning. Polymers 2018, 10, 464. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Z.; Jiang, L.; Jia, H.; Zhou, Y.; Ma, J.; Chen, S. Modification of polytetrafluoroethylene-fiberglass composite film using polydopamine deposition with improved hydrophilicity. Fiber Polym. 2018, 19, 1760–1766. [Google Scholar] [CrossRef]
- Prelipceanu, M.; Tudose, O.-G.; Prelipceanu, O.-S.; Schrader, S.; Grytsenko, K. Study of oriented growth of oligofluorene–thiophene films onto aligned vacuum-deposited polytetrafluoroethylene layers. Mat. Sci. Semicon. Proc. 2007, 10, 24–35. [Google Scholar] [CrossRef]
- Kim, H.-M.; Sohn, S.; Ahn, J.S. Transparent and super-hydrophobic properties of PTFE films coated on glass substrate using RF-magnetron sputtering and Cat-CVD methods. Surf. Coat. Technol. 2013, 228, S389–S392. [Google Scholar] [CrossRef]
- Zhan, Y.L.; Ruan, M.; Li, W.; Li, H.; Hu, L.Y.; Ma, F.M.; Yu, Z.L.; Feng, W. Fabrication of anisotropic PTFE superhydrophobic surfaces using laser microprocessing and their self-cleaning and anti-icing behavior. Colloid. Surf. A 2017, 535, 8–15. [Google Scholar] [CrossRef]
- Zhuang, A.; Liao, R.; Dixon, S.C.; Lu, Y.; Sathasivam, S.; Parkin, I.P.; Carmalt, C.J. Transparent superhydrophobic PTFE films via one-step aerosol assisted chemical vapor deposition. RSC Adv. 2017, 7, 29275–29283. [Google Scholar] [CrossRef] [Green Version]
- Alawajji, R.A.; Kannarpady, G.K.; Biris, A.S. Fabrication of transparent superhydrophobic polytetrafluoroethylene coating. Appl. Surf. Sci. 2018, 444, 208–215. [Google Scholar] [CrossRef]
- Yi, N.; Bao, S.; Zhou, H.; Xin, Y.; Huang, A.; Ma, Y.; Li, R.; Jin, P. Preparation of microstructure-controllable superhydrophobic polytetrafluoroethylene porous thin film by vacuum thermal-evaporation. Front. Mater. Sci. 2016, 10, 320–327. [Google Scholar] [CrossRef]
- Wang, J.M.; Wang, L.D.; Feng, L. One-step fabrication of fluoropolymer transparent films with superhydrophobicity by dry method. J. Appl. Polym. Sci. 2011, 120, 524–529. [Google Scholar] [CrossRef]
- Li, S.-Y.; Li, Y.; Wang, J.; Nan, Y.-G.; Ma, B.-H.; Liu, Z.-L.; Gu, J.-X. Fabrication of pinecone-like structure superhydrophobic surface on titanium substrate and its self-cleaning property. Chem. Eng. J. 2016, 290, 82–90. [Google Scholar] [CrossRef]
- Audic, J.-L.; Poncin, F.; Reyx, D.; Brosse, J.-C. Cold plasma surface modification of conventionally and nonconventionally plasticized poly(vinyl chloride)-based flexible films: Global and specific migration of additives into isooctane. J. Appl. Polym. Sci. 2001, 79, 1384–1393. [Google Scholar] [CrossRef]
- Sohbatzadeh, F.; Mirzanejhad, S.; Ghasemi, M.; Talebzadeh, M. Characterization of a non-thermal plasma torch in streamer mode and its effect on polyvinyl chloride and silicone rubber surfaces. J. Electrostat. 2013, 71, 875–881. [Google Scholar] [CrossRef]
- Tan, C.; Cai, P.; Xu, L.; Yang, N.; Xi, Z.; Li, Q. Fabrication of superhydrophobic surface with controlled adhesion by designing heterogeneous chemical composition. Appl. Surf. Sci. 2015, 349, 516–523. [Google Scholar] [CrossRef]
- Kamegawa, T.; Shimizu, Y.; Yamashita, H. Superhydrophobic surfaces with photocatalytic self-cleaning properties by nanocomposite coating of TiO(2) and polytetrafluoroethylene. Adv. Mater. 2012, 24, 3697–3700. [Google Scholar] [CrossRef] [PubMed]
- Ru, L.; Jie-rong, C. Studies on wettability of medical poly(vinyl chloride) by remote argon plasma. Appl. Surf. Sci. 2006, 252, 5076–5082. [Google Scholar] [CrossRef]
- Asadinezhad, A.; Lehocký, M.; Sáha, P.; Mozetič, M. Recent progress in surface modification of polyvinyl chloride. Materials 2012, 5, 2937–2959. [Google Scholar] [CrossRef]
- Wen, X.Q.; Liu, X.H.; Liu, G.S. Improvement in the hydrophilic property of inner surface of polyvinyl chloride tube by DC glow discharge plasma. Vacuum 2010, 85, 406–410. [Google Scholar] [CrossRef]
- Fresnais, J.; Chapel, J.P.; Poncin-Epaillard, F. Synthesis of transparent superhydrophobic polyethylene surfaces. Surf. Coat. Technol. 2006, 200, 5296–5305. [Google Scholar] [CrossRef]
- Wei, X.; Zhao, B.; Li, X.M.; Wang, Z.; He, B.Q.; He, T.; Jiang, B. CF4 plasma surface modification of asymmetric hydrophilic polyethersulfone membranes for direct contact membrane distillation. J. Membr. Sci. 2012, 407, 164–175. [Google Scholar] [CrossRef]
- Tian, M.; Yin, Y.; Yang, C.; Zhao, B.; Song, J.; Liu, J.; Li, X.-M.; He, T. CF4 plasma modified highly interconnective porous polysulfone membranes for direct contact membrane distillation (DCMD). Desalination 2015, 369, 105–114. [Google Scholar] [CrossRef]
- Dreux, F.; Marais, S.; Poncin-Epaillard, F.; Métayer, M.; Labbé, M. Surface modification by low-pressure plasma of polyamide 12 (PA12). improvement of the water barrier properties. Langmuir 2002, 18, 10411–10420. [Google Scholar] [CrossRef]
- Park, S.-J.; Sohn, H.-J.; Hong, S.-K.; Shin, G.-S. Influence of atmospheric fluorine plasma treatment on thermal and dielectric properties of polyimide film. J. Colloid. Interf. Sci. 2009, 332, 246–250. [Google Scholar] [CrossRef]
- Wang, B.; Duan, Y.; Zhang, J. Titanium dioxide nanoparticles-coated aramid fiber showing enhanced interfacial strength and UV resistance properties. Mater. Des. 2016, 103, 330–338. [Google Scholar] [CrossRef]
- Gotoh, K.; Nakata, Y.; Tagawa, M.; Tagawa, M. Wettability of ultraviolet excimer-exposed PE, PI and PTFE films determined by the contact angle measurements. Colloid. Surf. A 2003, 224, 165–173. [Google Scholar] [CrossRef]






| Pretreatment Methods | Fluorine Content (at %) | Carbon Content (at %) | F/C |
|---|---|---|---|
| Nonplasma cleaning | 64.08 | 33.64 | 1.91 |
| Ar plasma cleaning | 67.47 | 31.7 | 2.13 |
| CF4 plasma cleaning | 68.82 | 30.54 | 2.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Z.; Wang, X.; Jia, H.; Zhou, Y.; Ma, J.; Liu, X.; Jiang, L.; Chen, S. Superhydrophobic Polytetrafluoroethylene/Heat-Shrinkable Polyvinyl Chloride Composite Film with Super Anti-Icing Property. Polymers 2019, 11, 805. https://doi.org/10.3390/polym11050805
Jiang Z, Wang X, Jia H, Zhou Y, Ma J, Liu X, Jiang L, Chen S. Superhydrophobic Polytetrafluoroethylene/Heat-Shrinkable Polyvinyl Chloride Composite Film with Super Anti-Icing Property. Polymers. 2019; 11(5):805. https://doi.org/10.3390/polym11050805
Chicago/Turabian StyleJiang, Zhiqing, Xueqin Wang, Huiying Jia, Yanfen Zhou, Jianwei Ma, Xinyu Liu, Liang Jiang, and Shaojuan Chen. 2019. "Superhydrophobic Polytetrafluoroethylene/Heat-Shrinkable Polyvinyl Chloride Composite Film with Super Anti-Icing Property" Polymers 11, no. 5: 805. https://doi.org/10.3390/polym11050805