Study on Crystallization Behaviors and Properties of F-III Fibers during Hot Drawing in Supercritical Carbon Dioxide
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Hot-Drawing Process of F-III Fibers in Sc-CO2 Reactor
2.3. Characterizations
2.3.1. Mechanical Performance Test
2.3.2. Wide Angle X-ray Scattering (WAXS) Measurement
2.3.3. Small Angle X-ray Scattering (SAXS) Measurement
2.3.4. Thermogravimetric Analysis (TGA)
3. Results and Discussion
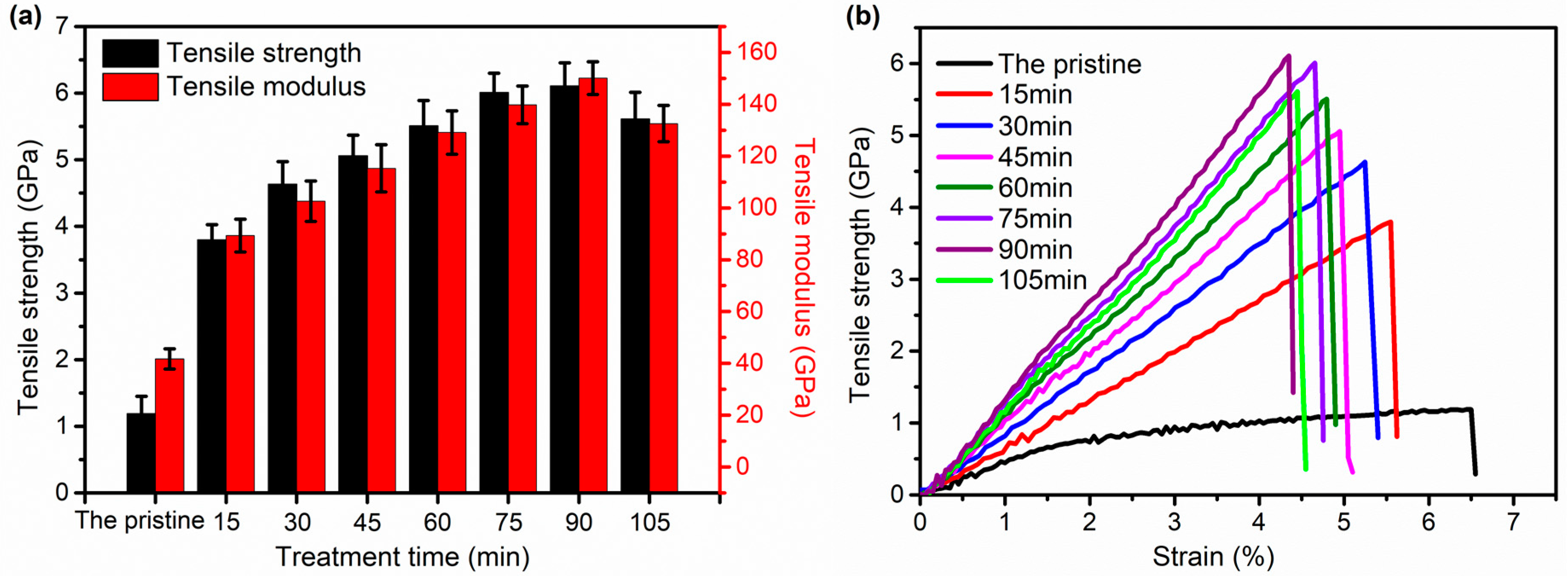
3.1. Mechanical Performance Analysis
3.2. Wide Angle X-ray Scattering (WAXS) Analysis
3.3. Small Angle X-ray Scattering (SAXS) Analysis
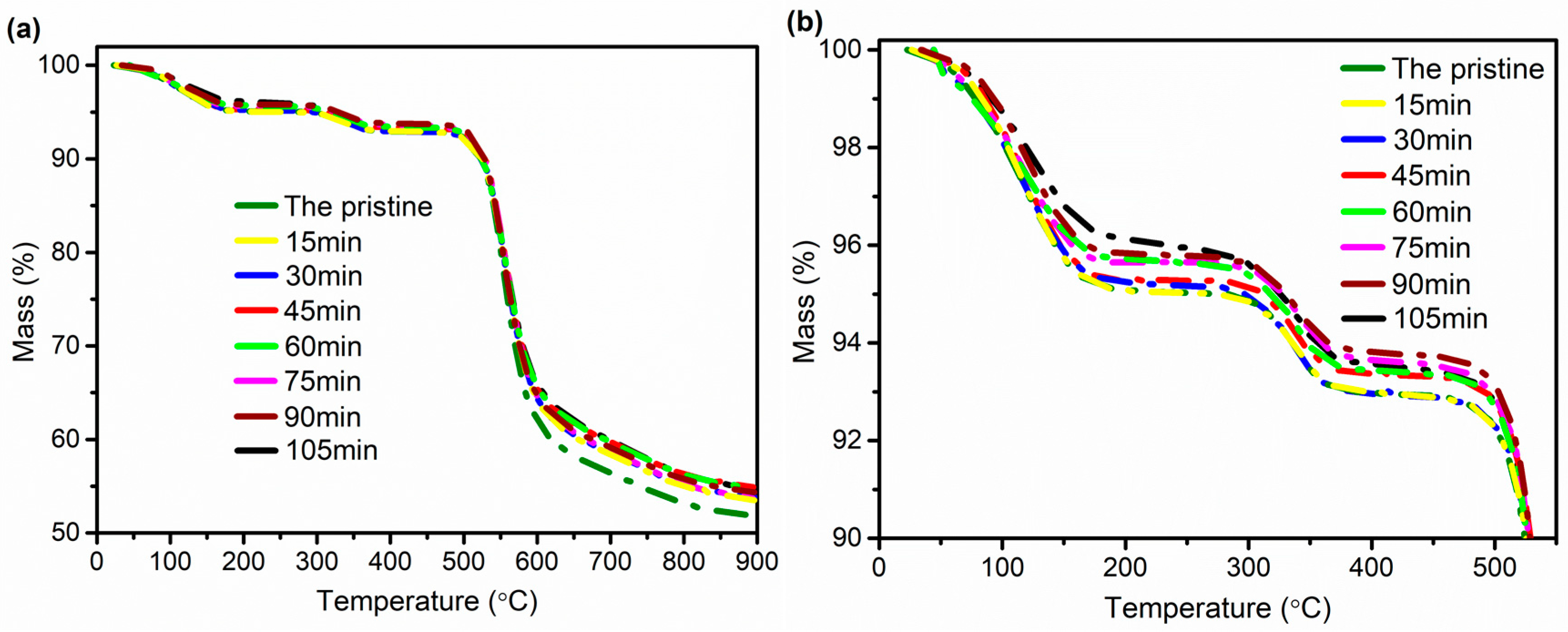
3.4. Thermogravimetric Analysis (TGA)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Minglin, Q.; Haijuan, K.; Kang, Z.; Cuiqing, T.; Muhuo, Y.; Yaozu, L. Simple Synthesis of Hydroxyl and Ethylene Functionalized Aromatic Polyamides as Sizing Agents to Improve Adhesion Properties of Aramid Fiber/Vinyl Epoxy Composites. Polymers 2017, 9, 143. [Google Scholar] [CrossRef]
- Hao, Y.; Wang, W.; Yang, D.; Zhou, X.; Zhao, Z.; Li, Z.; Wang, S.; Jing, F. Hydrophilicity modification of aramid fiber using a linear shape plasma excited by nanosecond pulse. Surf. Coat. Technol. 2018, 344, S0257897218303086. [Google Scholar]
- Zheng, C.; Zhang, L.; Chan, J.; Yu, D.; Meng, C.; Luo, L.; Liu, X. Aramid fiber with excellent interfacial properties suitable for resin composite in a wide polarity range. Chem. Eng. J. 2018, 347, 483–492. [Google Scholar]
- Gao, J.; Yang, X.; Huang, L.H. Numerical prediction of mechanical properties of rubber composites reinforced by aramid fiber under large deformation. Compos. Struct. 2018, S0263822318311292. [Google Scholar] [CrossRef]
- Ding, X.; Kong, H.; Qiao, M.; Hu, Z.; Yu, M. Effect of Different Pressures on Microstructure and Mechanical Performance of F-III Fibers in Supercritical Carbon Dioxide Fluid. Materials 2019, 12, 690. [Google Scholar] [CrossRef]
- Nalawade, S.P.; Picchioni, F.; Janssen, L.P.B.M. Supercritical carbon dioxide as a green solvent for processing polymer melts: Processing aspects and applications. Prog. Polym. Sci. 2006, 31, 19–43. [Google Scholar] [CrossRef] [Green Version]
- Marubayashi, H.; Akaishi, S.; Akasaka, S.; Asai, S.; Sumita, M. Crystalline Structure and Morphology of Poly(L-lactide) Formed under High-Pressure CO2. Macromolecules 2008, 41, 9192–9203. [Google Scholar] [CrossRef]
- Li, B.; Zhu, X.; Hu, G.H.; Liu, T.; Cao, G.; Zhao, L.; Yuan, W. Supercritical carbon dioxide-induced melting temperature depression and crystallization of syndiotactic polypropylene. Polym. Eng. Sci. 2010, 48, 1608–1614. [Google Scholar] [CrossRef]
- Zheng, H.; Zhang, J.; Bing, D.; Wei, Q.; Zheng, L. An investigation for the performance of meta-aramid fiber blends treated in supercritical carbon dioxide fluid. Fibers Polym. 2015, 16, 1134–1141. [Google Scholar] [CrossRef]
- Garcia-Leiner, M.; Song, J.; Lesser, A.J. Drawing of ultrahigh molecular weight polyethylene fibers in the presence of supercritical carbon dioxide. J. Polym. Sci. Part B Polym. Phys. 2003, 41, 1375–1383. [Google Scholar] [CrossRef]
- Amaral, G.V.; Silva, E.K.; Cavalcanti, R.N.; Cappato, L.P.; Guimaraes, J.T.; Alvarenga, V.O.; Esmerino, E.A.; Portela, J.B.; Ana, A.S.S.; Freitas, M.Q. Dairy processing using supercritical carbon dioxide technology: Theoretical fundamentals, quality and safety aspects. Trends Food Sci. Technol. 2017, 64, 94–101. [Google Scholar] [CrossRef]
- Kuang, T.; Feng, C.; Chang, L.; Zhao, Y.; Fu, D.; Xiao, G.; Peng, X. Facile Preparation of Open-cellular Porous Poly (L-lactic acid) Scaffold by Supercritical Carbon Dioxide Foaming for Potential Tissue Engineering Applications. Chem. Eng. J. 2017, 307, 1017–1025. [Google Scholar] [CrossRef]
- Benito-Román, O.; Rodríguez-Perrino, M.; Sanz, M.T.; Melgosa, R.; Beltrán, S. Supercritical carbon dioxide extraction of quinoa oil: Study of the influence of process parameters on the extraction yield and oil quality. J. Supercrit. Fluids 2018, 139, 62–71. [Google Scholar] [CrossRef]
- Zhang, M.; Dou, M.; Wang, M.; Yu, Y. Study on the solubility parameter of supercritical carbon dioxide system by molecular dynamics simulation. J. Mol. Liq. 2017, 248, 322–329. [Google Scholar] [CrossRef]
- Cooper, A.I.; Desimone, J.M. Polymer synthesis and characterization in liquid / supercritical carbon dioxide. Curr. Opin. Solid State Mater. Sci. 1996, 1, 761–768. [Google Scholar] [CrossRef]
- Asai, S.; Shimada, Y.; Tominaga, Y.; Sumita, M. Characterization of higher-order structure of poly(ethylene-2,6-naphthalate) treated with supercritical carbon dioxide. Macromolecules 2005, 38, 6544–6550. [Google Scholar] [CrossRef]
- Hobbs, T.; Lesser, A.J. Drawing in high pressure CO2—A new route to high performance fibers—In memory of the late Roger S. Porter. Polym. Eng. Sci. 2001, 41, 135–144. [Google Scholar] [CrossRef]
- Qiao, M.; Kong, H.; Ding, X.; Hu, Z.; Zhang, L.; Cao, Y.; Yu, M. Effect of Different Pressures of Supercritical Carbon Dioxide on the Microstructure of PAN Fibers during the Hot-Drawing Process. Polymers 2019, 11, 403. [Google Scholar] [CrossRef]
- Hotokezaka, K.; Kiuchi, K.; Shibata, M.; Nakar, E.; Piran, T. Synchrotron radiation from the fast tail of dynamical ejecta of neutron star mergers. Astrophys. J. 2018, 867, 95. [Google Scholar] [CrossRef]
- Chen, X.; Fei, L.; Su, F.; Ji, Y.; Meng, L.; Wan, C.; Lin, Y.; Li, X.; Li, L. Deformation mechanism of iPP under uniaxial stretching over a wide temperature range: An in-situ synchrotron radiation SAXS/WAXS study. Polymer 2017, 118, 12–21. [Google Scholar] [CrossRef]
- Zhang, R.; Zhang, Q.; Ji, Y.; Su, F.; Meng, L.; Qi, Z.; Lin, Y.; Li, X.; Chen, X.; Lv, F. Stretch-induced complexation reaction between poly(vinyl alcohol) and iodine: An in situ synchrotron radiation small- and wide-angle X-ray scattering study. Soft Matter 2018, 14, 2535–2546. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Rui, Z.; Meng, L.; Ji, Y.; Su, F.; Lin, Y.; Li, X.; Chen, X.; Fei, L.; Li, L. Stretch-induced structural evolution of poly (vinyl alcohol) film in water at different temperatures: An in-situ synchrotron radiation small- and wide-angle X-ray scattering study. Polymer 2018, 142, 233–243. [Google Scholar] [CrossRef]
- Ran, S.; Fang, D.; Zong, X.; Hsiao, B.S.; Chu, B.; Cunniff, P.M. Structural changes during deformation of Kevlar fibers via on-line synchrotron SAXS/WAXD techniques. Polymer 2001, 42, 1601–1612. [Google Scholar] [CrossRef]
- Zhu, C.; Liu, X.; Guo, J.; Zhao, N.; Li, C.; Wang, J.; Liu, J.; Xu, J. Relationship between performance and microvoids of aramid fibers revealed by two-dimensional small-angle X-ray scattering. J. Appl. Crystallogr. 2013, 46, 1178–1186. [Google Scholar] [CrossRef]
- Yue, C.Y.; Sui, G.X.; Looi, H.C. Effects of heat treatment on the mechanical properties of Kevlar-29 fibre. Compos. Sci. Technol. 2000, 60, 421–427. [Google Scholar] [CrossRef]
- Rao, Y.; Waddon, A.J.; Farris, R.J. Structure–property relation in poly(p-phenylene terephthalamide) (PPTA) fibers. Polymer 2001, 42, 5937–5946. [Google Scholar] [CrossRef]
- Uppal, R.; Ramaswamy, G.N.; Loughin, T. A novel method to assess degree of crystallinity of aramid filament yarns. J. Ind. Text. 2013, 43, 3–19. [Google Scholar] [CrossRef]
- Lee, K.G.; Jr, R.B.; Schultz, J.M. Structure and property development in poly(p-phenylene terephthalamide) during heat treatment under tension. J. Polym. Sci. Part B Polym. Phys. 1995, 33, 1–14. [Google Scholar] [CrossRef]
- Kong, H.; Teng, C.; Liu, X.; Zhou, J.; Yu, M. Simultaneously improving the tensile strength and modulus of aramid fiber by enhancing amorphous phase in supercritical carbon dioxide. RSC Adv. 2014, 4, 20599–20604. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Dai, Y.; Yuan, Y.; Meng, C.; Cheng, Z.; Wang, X.; Liu, X. The introduction of asymmetric heterocyclic units into poly(p-phenylene terephthalamide) and its effect on microstructure, interactions and properties. J. Mater. Sci. 2018, 53, 13291–13303. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Huang, J.; Hong, D.; Wang, X.; Liu, X. Pre-drawing induced evolution of phase, microstructure and property in para-aramid fibres containing benzimidazole moiety. RSC Adv. 2016, 6, 62695–62704. [Google Scholar] [CrossRef]
- Yu, J.; Tian, F.; Chen, S.; Wang, X.; Zhang, Y.; Wang, H. Structure and property development of aromatic copolysulfonamide fibers during wet spinning process. J. Appl. Polym. Sci. 2015, 132. [Google Scholar] [CrossRef]
- Rao, Y.; Waddon, A.J.; Farris, R.J. The evolution of structure and properties in poly(p-phenylene terephthalamide) fibers. Polymer 2001, 42, 5925–5935. [Google Scholar] [CrossRef]
- Cheng, Y.; Dong, J.; Yang, C.; Wu, T.; Zhao, X.; Zhang, Q. Synthesis of poly(benzobisoxazole-co-imide) and fabrication of high-performance fibers. Polymer 2017, 133, 50–59. [Google Scholar] [CrossRef]
- Dong, J.; Yin, C.; Zhao, X.; Li, Y.; Zhang, Q. High strength polyimide fibers with functionalized graphene. Polymer 2013, 54, 6415–6424. [Google Scholar] [CrossRef]
- Yin, C.; Dong, J.; Tan, W.; Lin, J.; Chen, D.; Zhang, Q. Strain-induced crystallization of polyimide fibers containing 2-(4-aminophenyl)-5-aminobenzimidazole moiety. Polymer 2015, 75, 178–186. [Google Scholar] [CrossRef]
- Yang, X.; Yu, J.; Tian, F.; Chen, S.; Wang, F.; Zhang, Y.; Wang, H. The combined effect of heat-draw ratios and residence time on the morphology and property of aromatic copolysulfonamide fibers. RSC Adv. 2015, 5, 27163–27167. [Google Scholar] [CrossRef]
- Kikic, I.; Vecchione, F.; Alessi, P.; Cortesi, A.; Eva, F.; Elvassore, N. Polymer Plasticization Using Supercritical Carbon Dioxide: Experiment and Modeling. Ind. Eng. Chem. Res. 2003, 42, 3022–3029. [Google Scholar] [CrossRef]
- Mizoguchi, K.; Hirose, T.; Naito, Y.; Kamiya, Y. CO2-induced crystallization of poly(ethylene terephthalate). Polymer 1987, 28, 1298–1302. [Google Scholar] [CrossRef]
- Rindfleisch, F.; Dinoia, T.P.; Mchugh, M.A. Solubility of Polymers and Copolymers in Supercritical CO2. J. Phys. Chem 1996, 100, 15581–15587. [Google Scholar] [CrossRef]
- Schaefer, D.J.; Schadt, R.J.; Gardner, K.H.; Gabara, V.; Allen, S.R.; English, A.D. Microscopic Dynamics and Macroscopic Mechanical Deformation of Poly(p-phenyleneterephthalamide) Fibers. Macromolecules 1995, 28, 1152–1158. [Google Scholar] [CrossRef]
- Schadt, R.J.; Cain, E.J.; Gardner, K.H.; Gabara, V.; Allen, S.R.; English, A.D. Terephthalamide ring dynamics of poly(p-phenyleneterephthalamide). Macromolecules 2002, 26, 6503–6508. [Google Scholar] [CrossRef]
- Jackson, C.L.; Schadt, R.J.; Gardner, K.H.; Chase, D.B.; Allen, S.R.; Gabara, V.; English, A.D. Dynamic structure and aqueous accessibility of poly(p-phenylene terephthalamide) crystallites. Polymer 1994, 35, 1123–1131. [Google Scholar] [CrossRef]
- Zhu, F.-L.; Feng, Q.-Q.; Xin, Q.; Zhou, Y. Thermal Degradation Process of Polysulfone Aramid Fiber. Therm. Sci. 2014, 18, 1637–1641. [Google Scholar] [CrossRef]
- Hu, C.; Chen, L.; Gu, R.; Yu, J.; Zhu, J.; Hu, Z. Thermal Decomposition Behavior of a Heterocyclic Aramid Fiber. J. Macromol. Sci. Part B-Phys. 2013, 52, 726–737. [Google Scholar] [CrossRef]


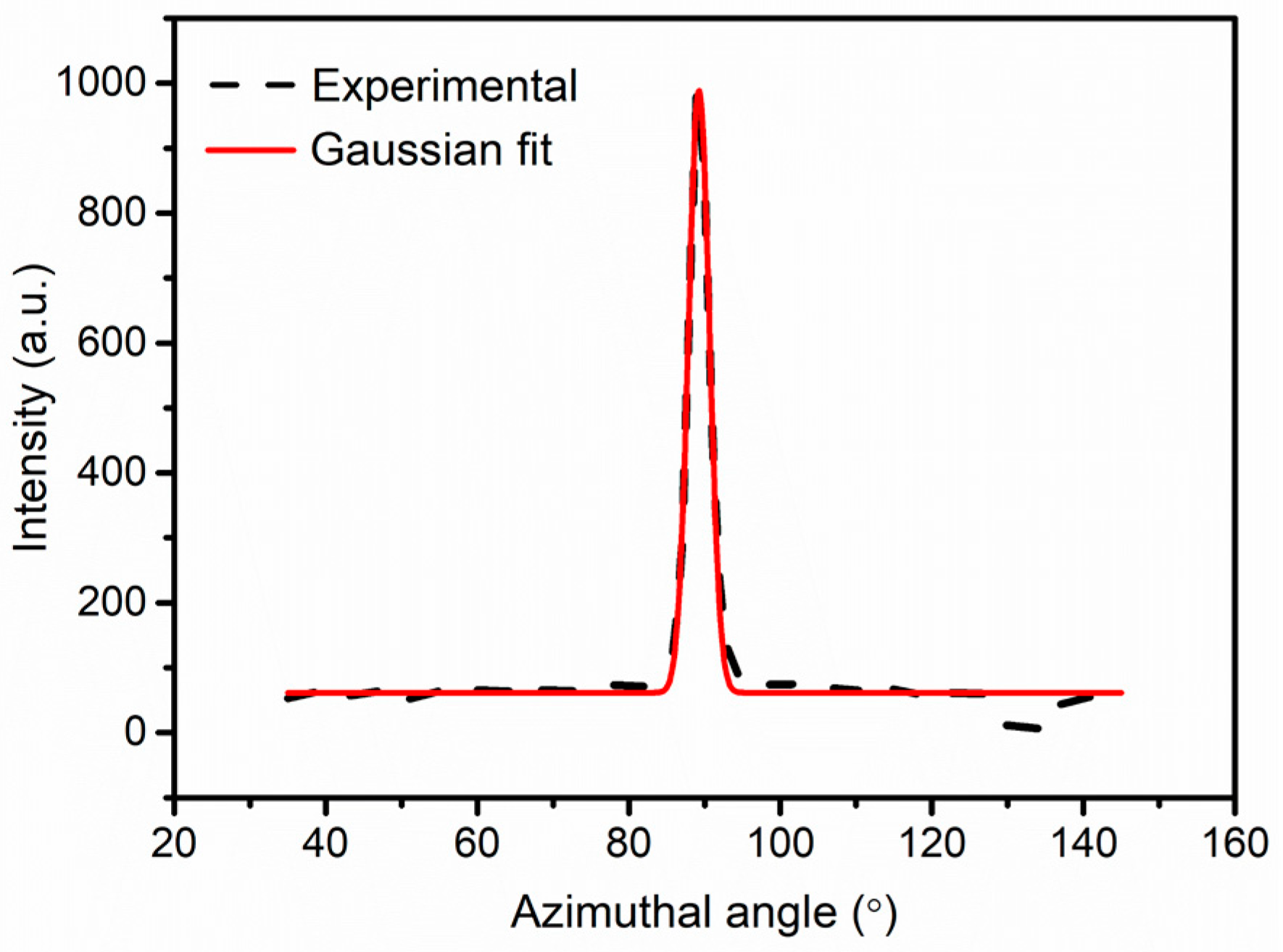
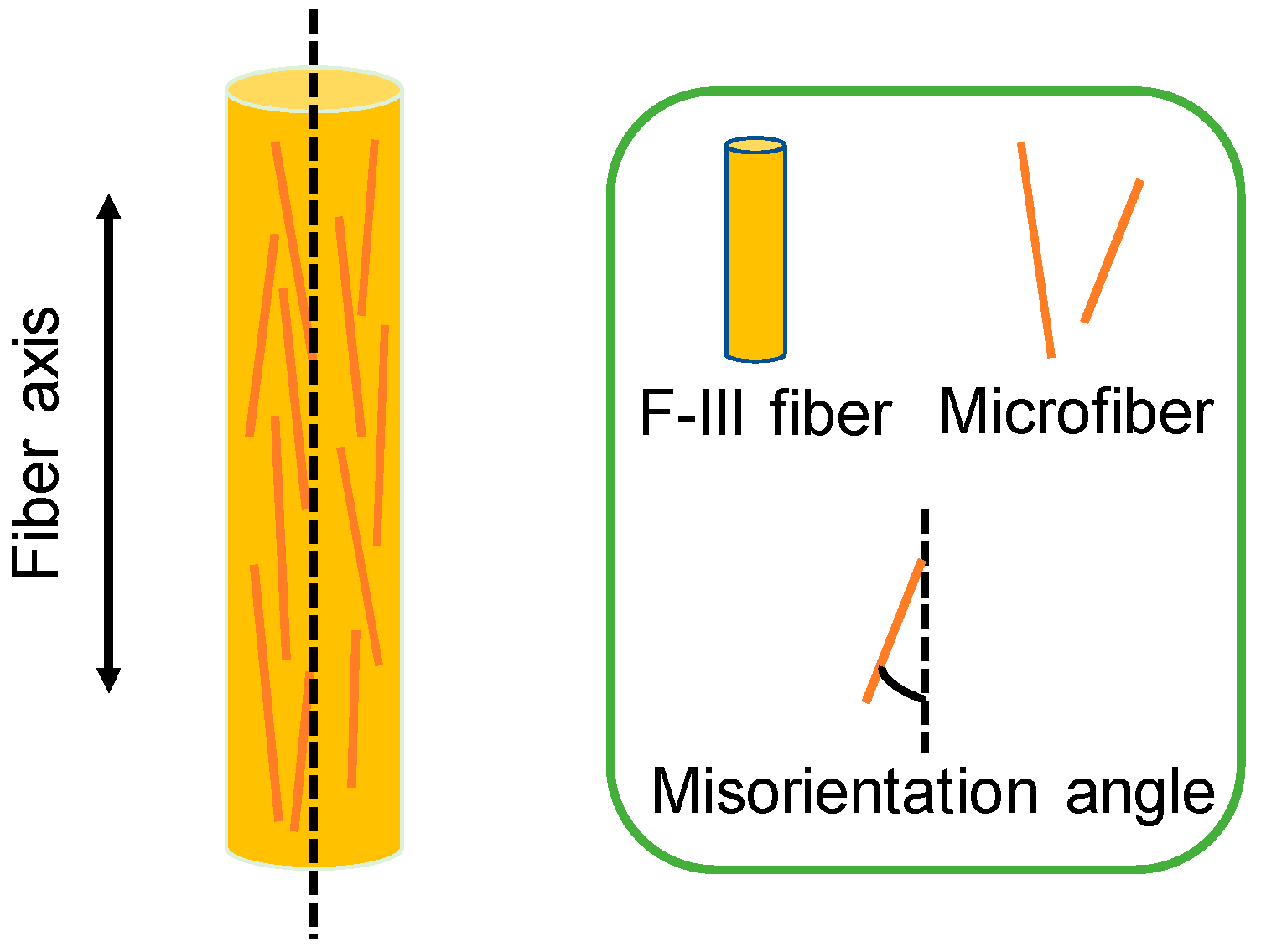






| Samples | Fibril Length lf (nm) | Misorientation Angle Bφ (°) |
|---|---|---|
| The pristine | 92.16 | 15.78 |
| 15 min | 95.87 | 13.26 |
| 30 min | 97.35 | 11.38 |
| 45 min | 99.49 | 9.76 |
| 60 min | 103.56 | 8.01 |
| 75 min | 107.12 | 7.37 |
| 90 min | 109.46 | 6.64 |
| 105 min | 94.26 | 9.54 |
| Samples | Temperature (°C, at Mass of 95%) | Residual Mass (%) |
|---|---|---|
| The pristine | 281.32 | 51.82 |
| 15 min | 282.84 | 53.52 |
| 30 min | 296.49 | 53.69 |
| 45 min | 307.31 | 54.86 |
| 60 min | 316.36 | 54.72 |
| 75 min | 324.36 | 54.35 |
| 90 min | 328.82 | 54.16 |
| 105 min | 326.86 | 54.44 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, X.; Kong, H.; Qiao, M.; Hu, Z.; Yu, M. Study on Crystallization Behaviors and Properties of F-III Fibers during Hot Drawing in Supercritical Carbon Dioxide. Polymers 2019, 11, 856. https://doi.org/10.3390/polym11050856
Ding X, Kong H, Qiao M, Hu Z, Yu M. Study on Crystallization Behaviors and Properties of F-III Fibers during Hot Drawing in Supercritical Carbon Dioxide. Polymers. 2019; 11(5):856. https://doi.org/10.3390/polym11050856
Chicago/Turabian StyleDing, Xiaoma, Haijuan Kong, Mengmeng Qiao, Zhifeng Hu, and Muhuo Yu. 2019. "Study on Crystallization Behaviors and Properties of F-III Fibers during Hot Drawing in Supercritical Carbon Dioxide" Polymers 11, no. 5: 856. https://doi.org/10.3390/polym11050856




