Influence of Hydrophobicity of Backbone Polymer in Thermo-Responsive Hydrogel with Immobilized Amine on Cycle Capacity for Absorption and Recovery of CO2
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of the Gels
2.3. IR Measurement
2.4. Swelling Behaviour of the Gels
2.5. Influence of the Gels on the Slurry pH
2.6. CO2 Adsorption/Desorption Cycle Test
3. Results
3.1. FTIR Spectra
3.2. Swelling Behaviour of the Gels
3.3. Influence of the Gel on the pH of the Slurry
3.4. CO2 Adsorption/Desorption Behaviour in the Cycle Test
4. Discussion
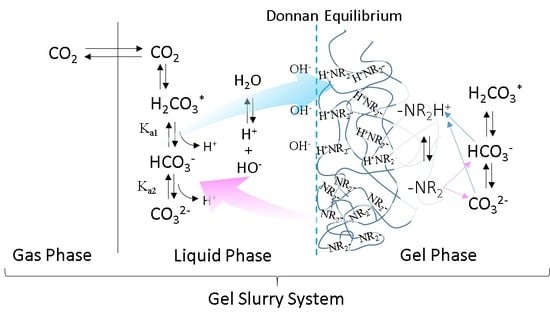
Theoretical Consideration
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
Surface Charge Contribution


References
- Buijs, W.; Flart, S. Direct air capture of CO2 with an amine resin: A molecular modeling study of the CO2 capturing process. Ind. Eng. Chem. Res. 2017, 56, 12297–12304. [Google Scholar] [CrossRef] [PubMed]
- SanzPerez, E.S.; Murdock, C.R.; Didas, S.A.; Jones, C.W. Direct capture of CO2 from ambient air. Chem. Rev. 2016, 116, 11840–11876. [Google Scholar] [CrossRef] [PubMed]
- Bajamundi, C.J.E.; Koponen, J.; Ruuskanen, V.; Elfving, J.; Kosonen, A.; Kauppinen, J.; Ahola, J. Capturing CO2 from air: Technical performance and process control improvement. J. CO2 Util. 2019, 30, 232–239. [Google Scholar] [CrossRef]
- Rao, A.B.; Rubin, E.S. A Technical, economic, and environmental assessment of amine-based CO2 capture technology for power plant greenhouse gas control. Environ. Sci. Technol. 2002, 36, 4467–4475. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, T.; Tanaka, K.; Arase, T.; Sakohara, S. A Novel thermosensitive gel adsorbent for phosphate ions. Macromol. Symp. 2010, 295, 81–87. [Google Scholar] [CrossRef]
- Hoshino, Y.; Imamura, K.; Yue, M.; Inoue, G.; Miura, Y. Reversible absorption of CO2 triggered by phase transition of amine-containig micro- and nanogel particles. J. Am. Chem. Soc. 2012, 134, 18177–18180. [Google Scholar] [CrossRef] [PubMed]
- Werz, P.D.L.; Kainz, J.; Rieger, B. Thermo- and pH-responsive nanogel particles bearing secondary amine functionalities for reversible carbon dioxide capture and release. Macromolecules 2015, 48, 6433–6439. [Google Scholar] [CrossRef]
- Feil, H.; Bae, Y.H.; Feijen, J.; Kim, S.W. Mutual influence of pH and temperature on the swelling of ionizable and thermosensitive hydrogels. Macromolecules 1992, 25, 5528–5530. [Google Scholar] [CrossRef]
- Yang, Y.; Mijalis, A.J.; Fu, H.; Agosto, C.; Tan, K.J.; Batteas, J.D.; Bergbreiter, D.E. Reversible changes in solution pH resulting from changes in thermoresponsive polymer solubility. J. Am. Chem. Soc. 2012, 134, 7378–7383. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Peiton, R. pH-dependence of the properties of hydrophobically modified polyvinylamine. Langmuir 2005, 21, 11673–11677. [Google Scholar] [CrossRef]
- Kainz, J.; Werz, P.D.L.; Troll, C.; Rieger, B. Temperature and CO2 responsive polyethylenimine for highly efficient carbon dioxide release. RSC Adv. 2015, 5, 9556–9560. [Google Scholar] [CrossRef]
- Seida, Y.; Ichida, H.; Nakano, Y. Surface properties of temperature sensitive N-isopropylacrylamide-copolymer gels. Kagaku Kogaku Ronbunshu 1992, 18, 346–352. [Google Scholar] [CrossRef]
- Melekaslan, D.; Okay, O. Temperature dependent swelling behavior of ionic poly(N-isopropylacrylamide) gels in PEG solutions. Polym. Bull. 2002, 49, 181–188. [Google Scholar] [CrossRef]
- Seida, Y.; Nakano, Y. Concept to control the phase behavior of stimuli-sensitive polymer gel. J. Chem. Eng. Jpn 1995, 28, 425–428. [Google Scholar] [CrossRef]
- Katayama, S.; Hirokawa, Y.; Tanaka, T. Reentrant phase transition in acrylamide-derivative copolymer gel. Macromolecules 1984, 17, 2641–2643. [Google Scholar] [CrossRef]
- Jiang, X.; Feng, C.; Lu, G.; Huang, X. Thermoresponsive homopolymer tunable by pH and CO2. ACS Macro Lett. 2014, 3, 1121–1125. [Google Scholar] [CrossRef]
- Inomata, H.; Goto, S.; Otake, K.; Saito, S. Effect of additives on phase transition of N-Isopropylacrylamide gels. Langmuir 1992, 8, 687–690. [Google Scholar] [CrossRef]
- Tanaka, T.; Fillmore, D.; Sun, S.-T.; Nishio, I.; Swislow, G.; Shah, A. Phase transitions in ionic gels. Phys. Rev. Lett. 1980, 45, 1636–1639. [Google Scholar] [CrossRef]
- Kariznovi, M.; Nourozieh, H.; Abedi, J. Experimental measurements and predictions of density, viscosity, and carbon dioxide solubility in methanol, ethanol, and 1-propanol. J. Chem. Thermodyn. 2013, 57, 408–415. [Google Scholar] [CrossRef]
- Gui, X.; Tang, Z.G.; Fei, W.Y. Solubility of CO2 in alcohols, glycols, ethers, and ketones at high pressures from (288.15 to 318.15) K. J. Chem. Eng. Data 2011, 56, 2420–2429. [Google Scholar] [CrossRef]
- Dalmolin, I.; Skovroinski, E.; Biasi, A.; Corazza, M.L.; Dariva, C.; Oliveira, J.V. Solubility of carbon dioxie in binary and ternary mixtures with ethanol and water. Fluid Phase Equilibria 2006, 245, 193–200. [Google Scholar] [CrossRef]
- Koschel, D.; Coxam, J.Y.; Rodier, L.; Majer, V. Enthalpy and solubility data of CO2 in water and NaCl(aq) at conditions of interest for geological sequestration. Fluid Phase Equilibria 2006, 247, 107–120. [Google Scholar] [CrossRef]
- Amano, Y.; Nagasawa, Y.; Seida, Y.; Gotoh, T.; Furuya, E. Recovery of CO2 using temperature-responsive amine gel slurry. Macromol. Symp. 2019, 385, 1800165. [Google Scholar] [CrossRef]
- Seida, Y.; Nakano, Y. Effect of pH on the phase transition of N-isopropylacrylamide-sodium acrylate copolymer gel. J. Chem. Eng. Jpn. 1993, 26, 328–330. [Google Scholar] [CrossRef]
- Amano, Y.; Seida, Y.; Furuya, E. Influence of phase transition temperature on CO2 adsorption/desorption behavior of thermo-responsive gel slurry. AIChE Proc. 2016, 446a. Available online: https://www.aiche.org/conferences/aiche-annual-meeting/2016/proceeding/paper/446a-influence-phase-transition-temperature-on-co2-adsorptiondesorption-behavior-thermo-responsive (accessed on 30 March 2019).
- Liu, Q.S.; Fukuda, K.; Matsuda, T. Study of solution process of carbon dioxide in seawater. J. Jpn. Inst. Mar. Eng. 2006, 41, 144–149. [Google Scholar] [CrossRef]
- Ishida, T.; Kawai, K.; Ichiba, D.; Sato, R. A model for dissolution of carbon dioxide gas in high pH solution based on chemical kinetics. Doboku Gakkai Ronbunshu E 2010, 66, 80–93. [Google Scholar] [CrossRef]
- Plummer, L.N.; Busenberg, E. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90 °C and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Liu, H.; Li, M.; Idem, R.; Tontiwachwuthikul, P.T.; Liang, Z. Analysis of solubility, absorption heat and kinetics of CO2 absorption into 1-(2-hydroxyethyl)pyrrolidine solvent. Chem. Eng. Sci. 2017, 162, 120–130. [Google Scholar] [CrossRef]
- Xiao, M.; Liu, H.; Gao, H.; Liang, Z. CO2 absorption with aqueous tertiary amine solutions: Equilibrium solubility and thermodynamic modeling. J. Chem. Thermodyn. 2018, 122, 170–182. [Google Scholar] [CrossRef]
- Tetens, O. Uber einige meteorologische begriffe. Z. Geophys. 1930, 6, 297–309. [Google Scholar]
- Cooperstein, M.A.; Canavan, H.E. Assessment of cytotoxicity of (N-isopropyl acrylamide) and Poly(N-isopropyl acrylamide)-coated surfaces. Biointerphases 2013, 8, 19. [Google Scholar] [CrossRef] [PubMed]









| Gel No. | Molar Ratio among NIPA, NTBA and DMAPAA (Total 700 mM) | BIS [mM] | mmol of DMAPAA /1 g Monomer Mixture | ||
|---|---|---|---|---|---|
| NIPA | NTBA | DMAPAA | |||
| ① | 9 | 0 | 1 | 35 | 0.80 |
| ② | 8 | 1 | 1 | 35 | 0.79 |
| ③ | 7 | 2 | 1 | 35 | 0.78 |
| ④ | 6 | 3 | 1 | 35 | 0.77 |
| ⑤ | 4 | 5 | 1 | 35 | 0.76 |
| ⑥ | 5 | 3 | 2 | 35 | 1.50 |
| ⑦ | 4 | 4 | 2 | 35 | 1.48 |
| ⑧ | 3 | 5 | 2 | 35 | 1.47 |
| ⑨ | 3 | 4 | 3 | 70 | 2.04 |
| ⑩ | 0 | 6 | 4 | 105 | 2.47 |
| ⑪ | 0 | 5 | 5 | 105 | 3.03 |
| ⑫ | 0 | 4 | 6 | 105 | 3.58 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nagasawa, Y.; Seida, Y.; Gotoh, T.; Furuya, E. Influence of Hydrophobicity of Backbone Polymer in Thermo-Responsive Hydrogel with Immobilized Amine on Cycle Capacity for Absorption and Recovery of CO2. Polymers 2019, 11, 1024. https://doi.org/10.3390/polym11061024
Nagasawa Y, Seida Y, Gotoh T, Furuya E. Influence of Hydrophobicity of Backbone Polymer in Thermo-Responsive Hydrogel with Immobilized Amine on Cycle Capacity for Absorption and Recovery of CO2. Polymers. 2019; 11(6):1024. https://doi.org/10.3390/polym11061024
Chicago/Turabian StyleNagasawa, Yuma, Yoshimi Seida, Takehiko Gotoh, and Eiji Furuya. 2019. "Influence of Hydrophobicity of Backbone Polymer in Thermo-Responsive Hydrogel with Immobilized Amine on Cycle Capacity for Absorption and Recovery of CO2" Polymers 11, no. 6: 1024. https://doi.org/10.3390/polym11061024