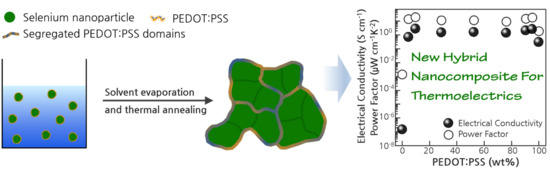
Synthesis and Thermoelectric Properties of Selenium Nanoparticles Coated with PEDOT:PSS
Abstract
:1. Introduction
2. Results and Discussion
3. Conclusions
4. Materials and Methods
4.1. Materials
4.2. Synthesis of PEDOT:PSS/Se, PSS/Se, Se Particles Aqueous Solution
4.3. Fabrication of the Films (or Pellets)
4.3.1. PEDOT:PSS/Se Films
4.3.2. PEDOT:PSS Films
4.3.3. Se Pellets
4.4. Characterization
4.5. Thermoelectric Property
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Bubnova, O.; Khan, Z.U.; Malti, A.; Braun, S.; Fahlman, M.; Berggren, M.; Crispin, X. Optimization of the thermoelectric figure of merit in the conducting polymer poly(3,4-ethylenedioxythiophene). Nat. Mater. 2011, 10, 429. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Park, C.; Kim, B.; Shin, H.; Kim, E. Flexible PEDOT electrodes with large thermoelectric power factors to generate electricity by the touch of fingertips. Energy Environ. Sci. 2013, 6, 788–792. [Google Scholar] [CrossRef]
- Kim, G.H.; Shao, L.; Zhang, K.; Pipe, K.P. Engineered doping of organic semiconductors for enhanced thermoelectric efficiency. Nat. Mater. 2013, 12, 719. [Google Scholar] [CrossRef] [PubMed]
- Yi, C.; Wilhite, A.; Zhang, L.; Hu, R.; Chuang, S.S.C.; Zheng, J.; Gong, X. Enhanced thermoelectric properties of poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) by binary secondary dopants. ACS Appl. Mater. Interfaces 2015, 7, 8984–8989. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Li, P.; Du, D.; Ouyang, J. Significantly enhanced thermoelectric properties of PEDOT: PSS films through sequential post-treatments with common acids and bases. Adv. Energy Mater. 2017, 7, 1602116. [Google Scholar] [CrossRef]
- See, K.C.; Feser, J.P.; Chen, C.E.; Majumdar, A.; Urban, J.J.; Segalman, R.A. Water-processable polymer−nanocrystal hybrids for thermoelectrics. Nano Lett. 2010, 10, 4664–4667. [Google Scholar] [CrossRef] [PubMed]
- Sahu, A.; Russ, B.; Su, N.C.; Forster, J.D.; Zhou, P.; Cho, E.S.; Ercius, P.; Coates, N.E.; Segalman, R.A.; Urban, J.J. Bottom-up design of de novo thermoelectric hybrid materials using chalcogenide resurfacing. J. Mater. Chem. A 2017, 5, 3346–3357. [Google Scholar] [CrossRef] [Green Version]
- Ju, H.; Kim, J. Chemically exfoliated SnSe nanosheets and their SnSe/Poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate) composite films for polymer based thermoelectric applications. ACS Nano 2016, 10, 5730–5739. [Google Scholar] [CrossRef]
- Xiong, J.; Jiang, F.; Shi, H.; Xu, J.; Liu, C.; Zhou, W.; Jiang, Q.; Zhu, Z.; Hu, Y. Liquid exfoliated graphene as dopant for improving the thermoelectric power factor of conductive PEDOT: PSS nanofilm with hydrazine treatment. ACS Appl. Mater. Interfaces 2015, 7, 14917–14925. [Google Scholar] [CrossRef]
- Yee, S.K.; Coates, N.E.; Majumdar, A.; Urban, J.J.; Segalman, R.A. Thermoelectric power factor optimization in PEDOT: PSS tellurium nanowire hybrid composites. Phys. Chem. Chem. Phys. 2013, 15, 4024–4032. [Google Scholar] [CrossRef]
- Jin Bae, E.; Hun Kang, Y.; Jang, K.-S.; Yun Cho, S. Enhancement of thermoelectric properties of PEDOT: PSS and tellurium-PEDOT: PSS hybrid composites by simple chemical treatment. Sci. Rep. 2016, 6, 18805. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Qiu, G.; Jian, J.; Zhou, H.; Yang, L.; Charnas, A.; Zemlyanov, D.Y.; Xu, C.-Y.; Xu, X.; Wu, W.; et al. Controlled growth of a large-size 2D selenium nanosheet and its electronic and optoelectronic applications. ACS Nano 2017, 11, 10222–10229. [Google Scholar] [CrossRef] [PubMed]
- Henkels, H.W. Thermoelectric power and mobility of carriers in selenium. Phys. Rev. 1950, 77, 734–736. [Google Scholar] [CrossRef]
- Park, S.H.; Choi, J.Y.; Lee, Y.H.; Park, J.T.; Song, H. Formation of metal selenide and metal–selenium nanoparticles using distinct reactivity between selenium and noble metals. Chem. Asian J. 2015, 10, 1452–1456. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Zhao, J.; Zhang, S.; Niu, H.; Jin, B.; Tian, Y. Synthesis and electrochemical properties of PbSe nanotubes. J. Phys. Chem. C 2009, 113, 18091–18096. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Lee, Y.H.; Jeong, U.; Zou, Z.; Xia, Y. Cation exchange: A simple and versatile route to inorganic colloidal spheres with the same size but different compositions and properties. Langmuir 2007, 23, 2985–2992. [Google Scholar] [CrossRef] [PubMed]
- Gates, B.; Wu, Y.; Yin, Y.; Yang, P.; Xia, Y. Single-crystalline nanowires of Ag2Se can be synthesized by templating against nanowires of trigonal se. J. Am. Chem. Soc. 2001, 123, 11500–11501. [Google Scholar] [CrossRef] [PubMed]
- Sinha, S.; Chatterjee, S.K.; Ghosh, J.; Meikap, A.K. Semiconducting selenium nanoparticles: Structural, electrical characterization, and formation of a back-to-back Schottky diode device. J. Appl. Phys. 2013, 113, 123704. [Google Scholar] [CrossRef]
- Mezzenga, R.; Ruokolainen, J.; Fredrickson, G.H.; Kramer, E.J.; Moses, D.; Heeger, A.J.; Ikkala, O. Templating organic semiconductors via self-assembly of polymer colloids. Science 2003, 299, 1872. [Google Scholar] [CrossRef]
- Kang, D.J.; Kang, H.; Kim, K.-H.; Kim, B.J. Nanosphere templated continuous PEDOT: PSS films with low percolation threshold for application in efficient polymer solar cells. ACS Nano 2012, 6, 7902–7909. [Google Scholar] [CrossRef]
- Gates, B.; Yin, Y.; Xia, Y. A solution-phase approach to the synthesis of uniform nanowires of crystalline selenium with lateral dimensions in the range of 10−30 nm. J. Am. Chem. Soc. 2000, 122, 12582–12583. [Google Scholar] [CrossRef]
- Xia, Y.; Ouyang, J. Significant Different conductivities of the two grades of Poly(3,4-ethylenedioxythiophene): Poly(styrenesulfonate), Clevios P and Clevios PH1000, arising from different molecular weights. ACS Appl. Mater. Interfaces 2012, 4, 4131–4140. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Chu, C.-W.; Chen, F.-C.; Xu, Q.; Yang, Y. High-conductivity Poly(3,4-ethylenedioxythiophene): Poly(styrene sulfonate) film and its application in polymer optoelectronic devices. Adv. Funct. Mater. 2005, 15, 203–208. [Google Scholar] [CrossRef]
- Lee, H.J.; Anoop, G.; Lee, H.J.; Kim, C.; Park, J.-W.; Choi, J.; Kim, H.; Kim, Y.-J.; Lee, E.; Lee, S.-G.; et al. Enhanced thermoelectric performance of PEDOT: PSS/PANI–CSA polymer multilayer structures. Energy Environ. Sci. 2016, 9, 2806–2811. [Google Scholar] [CrossRef]
- Özenbas, M.; Kalebozan, H. Crystallization of amorphous selenium thin films. J. Cryst. Growth 1986, 78, 523–527. [Google Scholar] [CrossRef]
- Gates, B.; Mayers, B.; Cattle, B.; Xia, Y. Synthesis and characterization of uniform nanowires of trigonal selenium. Adv. Funct. Mater. 2002, 12, 219–227. [Google Scholar] [CrossRef]
- Champness, C.H.; Hoffmann, R.H. Conductivity changes associated with the crystallization of amorphous selenium. J. Non-Cryst. Solids 1970, 4, 138–148. [Google Scholar] [CrossRef]
- Huang, J.; Miller, P.F.; Wilson, J.S.; de Mello, A.J.; de Mello, J.C.; Bradley, D.D.C. Investigation of the effects of doping and post-deposition treatments on the conductivity, morphology, and work function of Poly(3,4-ethylenedioxythiophene)/Poly(styrene sulfonate) films. Adv. Funct. Mater. 2005, 15, 290–296. [Google Scholar] [CrossRef]
- Friedel, B.; Keivanidis, P.E.; Brenner, T.J.K.; Abrusci, A.; McNeill, C.R.; Friend, R.H.; Greenham, N.C. Effects of layer thickness and annealing of PEDOT: PSS layers in organic photodetectors. Macromolecules 2009, 42, 6741–6747. [Google Scholar] [CrossRef]
- Pingree, L.S.C.; MacLeod, B.A.; Ginger, D.S. The changing face of PEDOT: PSS films: Substrate, bias, and processing effects on vertical charge transport. J. Phys. Chem. C 2008, 112, 7922–7927. [Google Scholar] [CrossRef]
- Montes, J.M.; Cuevas, F.G.; Cintas, J. Porosity effect on the electrical conductivity of sintered powder compacts. Appl. Phys. A 2008, 92, 375–380. [Google Scholar] [CrossRef]
- Probst, N.; Grivei, E. Structure and electrical properties of carbon black. Carbon 2002, 40, 201–205. [Google Scholar] [CrossRef]
- Cuevas, F.G.; Montes, J.M.; Cintas, J.; Urban, P. Electrical conductivity and porosity relationship in metal foams. J. Porous Mater. 2008, 16, 675. [Google Scholar] [CrossRef]




| System | Electrical Conductivity (σ) / S cm−1 | Seebeck Coefficient (S) / μV K−1 | Power Factor (S2σ) / μW cm−1K−2 |
|---|---|---|---|
| Se a | 1.4 x 10−7 | ~1000 b | ~0.0014 |
| PEDOT:PSS | 0.29 (±0.17) | 24.9 (±0.9) | 1.7 |
| PEDOT:PSS/Se | 0.37 (±0.19) | 45.5 (±3.5) | 9.5 |
| PEDOT:PSS/Se c | 0.71 (±0.10) | 44.5 (±6.7) | 15.0 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, C.; Hong, J.; Park, J.-W. Synthesis and Thermoelectric Properties of Selenium Nanoparticles Coated with PEDOT:PSS. Polymers 2019, 11, 1052. https://doi.org/10.3390/polym11061052
Kim C, Hong J, Park J-W. Synthesis and Thermoelectric Properties of Selenium Nanoparticles Coated with PEDOT:PSS. Polymers. 2019; 11(6):1052. https://doi.org/10.3390/polym11061052
Chicago/Turabian StyleKim, Chingu, Jiyeon Hong, and Ji-Woong Park. 2019. "Synthesis and Thermoelectric Properties of Selenium Nanoparticles Coated with PEDOT:PSS" Polymers 11, no. 6: 1052. https://doi.org/10.3390/polym11061052