Compressive Alginate Sponge Derived from Seaweed Biomass Resources for Methylene Blue Removal from Wastewater
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Extraction of Seaweed
2.3. Preparation of AS
2.4. Characterizations
2.5. Adsorption Measurement
3. Results and Discussions
3.1. Material Properties
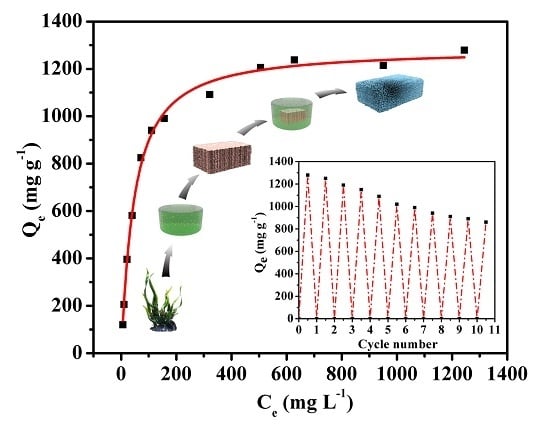
3.2. Absorption Properties
3.3. Compressive Properties and Recyclability
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Adegoke, K.A.; Bello, O.S. Dye sequestration using agricultural wastes as adsorbents. Water Resour. Ind. 2015, 12, 8–24. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, S.M.; El-Dib, F.I.; El-Gendy, N.S.; Sayed, W.M.; El-Khodary, M. A kinetic study for the removal of anionic sulphonated dye from aqueous solution using nano-polyaniline and Baker’s yeast. Arab. J. Chem. 2016, 9, S1721–S1728. [Google Scholar] [CrossRef]
- Aljeboree, A.M.; Alshirifi, A.N.; Alkaim, A.F. Kinetics and equilibrium study for the adsorption of textile dyes on coconut shell activated carbon. Arab. J. Chem. 2017, 10, S3381–S3393. [Google Scholar] [CrossRef] [Green Version]
- Aboelmagd, A.; El-Safty, S.A.; Shenashen, M.A.; Elshehy, E.A.; Khairy, M.; Sakaic, M.; Yamaguchi, H. Nanomembrane canister architectures for the visualization and filtration of oxyanion toxins with one-step processing. Chem. Asian J. 2015, 10, 2467–2478. [Google Scholar] [CrossRef] [PubMed]
- Shenashen, M.A.; El-Safty, S.A.; Elshehy, E.A. Monolithic scaffolds for highly selective ion sensing/removal of Co(II), Cu(II), and Cd(II) ions in water. Analyst 2014, 139, 6393–6405. [Google Scholar] [CrossRef] [PubMed]
- Gomaa, H.; Shenashen, M.A.; Yamaguchi, H.; Alamoudi, A.S.; El-Safty, S.A. Extraction and recovery of Co2+ ions from spent lithium-ion batteries using hierarchical mesosponge γ-Al2O3 monolith extractors. Green Chem. 2018, 20, 1841–1857. [Google Scholar] [CrossRef]
- El-Sewify, I.M.; Shenashen, M.A.; Shahat, A.; Yamaguchi, H.; Selim, M.M.; Khalil, M.M.H.; El-Safty, S.A. Ratiometric fluorescent chemosensor for Zn2+ ions in environmental samples using supermicroporous organic-inorganic structures as potential platforms. Chem. Sel. 2017, 2, 11083–11090. [Google Scholar] [CrossRef]
- Derbalah, A.; El-Safty, S.A.; Shenashen, M.A.; Abdel Ghany, N.A. Mesoporous alumina nanoparticles as host tunnel-like pores for removal and recovery of insecticides from environmental samples. ChemPlusChem 2015, 80, 1119–1126. [Google Scholar] [CrossRef]
- El Qada, E.N.; Allen, S.J.; Walker, G.M. Adsorption of basic dyes from aqueous solution onto activated carbons. Chem. Eng. J. 2008, 135, 174–184. [Google Scholar] [CrossRef]
- Han, R.; Ding, D.; Xu, Y.; Zou, W.; Wang, Y.; Li, Y.; Zou, L. Use of rice husk for the adsorption of congo red from aqueous solution in column mode. Bioresour. Technol. 2008, 99, 2938–2946. [Google Scholar] [CrossRef]
- Pearce, C.I.; Lloyd, J.R.; Guthrie, J.T. The removal of colour from textile wastewater using whole bacterial cells: A review. Dyes Pigments 2003, 58, 179–196. [Google Scholar] [CrossRef]
- Gómez, J.M.; Galán, J.; Rodríguez, A.; Walker, G.M. Dye adsorption onto mesoporous materials: PH influence, kinetics and equilibrium in buffered and saline media. J. Environ. Manag. 2014, 146, 355–361. [Google Scholar] [CrossRef] [PubMed]
- Wan, X.; Zhan, Y.; Long, Z.; Zeng, G.; He, Y. Core@double-shell structured magnetic halloysite nanotube nano-hybrid as efficient recyclable adsorbent for methylene blue removal. Chem. Eng. J. 2017, 330, 491–504. [Google Scholar] [CrossRef]
- He, X.; Male, K.B.; Nesterenko, P.N.; Brabazon, D.; Paull, B.; Luong, J.H.T. Adsorption and desorption of methylene blue on porous carbon monoliths and nanocrystalline cellulose. ACS Appl. Mater. Interfaces 2013, 5, 8796–8804. [Google Scholar] [CrossRef]
- Lau, Y.-Y.; Wong, Y.-S.; Teng, T.-T.; Morad, N.; Rafatullah, M.; Ong, S.-A. Coagulation-flocculation of azo dye Acid Orange 7 with green refined laterite soil. Chem. Eng. J. 2014, 246, 383–390. [Google Scholar] [CrossRef]
- Kokabian, B.; Bonakdarpour, B.; Fazel, S. The effect of salt on the performance and characteristics of a combined anaerobic–aerobic biological process for the treatment of synthetic wastewaters containing Reactive Black 5. Chem. Eng. J. 2013, 221, 363–372. [Google Scholar] [CrossRef]
- Sakkas, V.A.; Islam, M.A.; Stalikas, C.; Albanis, T.A. Photocatalytic degradation using design of experiments: A review and example of the Congo red degradation. J. Hazard. Mater. 2010, 175, 33–44. [Google Scholar] [CrossRef]
- Qiu, W.-Z.; Yang, H.-C.; Wan, L.-S.; Xu, Z.-K. Co-deposition of catechol/polyethyleneimine on porous membranes for efficient decolorization of dye water. J. Mater. Chem. A 2015, 3, 14438–14444. [Google Scholar] [CrossRef]
- Martínez-Huitle, C.A.; Brillas, E. Decontamination of wastewaters containing synthetic organic dyes by electrochemical methods: A general review. Appl. Catal. B 2009, 87, 105–145. [Google Scholar] [CrossRef]
- Fernandez, M.E.; Nunell, G.V.; Bonelli, P.R.; Cukierman, A.L. Activated carbon developed from orange peels: Batch and dynamic competitive adsorption of basic dyes. Ind. Crops Prod. 2014, 62, 437–445. [Google Scholar] [CrossRef]
- Sun, L.; Chen, D.; Wan, S.; Yu, Z. Performance, kinetics, and equilibrium of methylene blue adsorption on biochar derived from eucalyptus saw dust modified with citric, tartaric, and acetic acids. Bioresour. Technol. 2015, 198, 300–308. [Google Scholar] [CrossRef] [PubMed]
- Li, G.; Zhu, W.; Zhang, C.; Zhang, S.; Liu, L.; Zhu, L.; Zhao, W. Effect of a magnetic field on the adsorptive removal of methylene blue onto wheat straw biochar. Bioresour. Technol. 2016, 206, 16–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, C.; Li, F.; Zhang, Y.; Wang, B.; Fan, Y.; Wang, X.; Sun, R. Compressive, ultralight and fire-resistant lignin-modified graphene aerogels as recyclable absorbents for oil and organic solvents. Chem. Eng. J. 2018, 350, 173–180. [Google Scholar] [CrossRef]
- Li, F.; Wang, X.; Yuan, T.; Sun, R. A lignosulfonate-modified graphene hydrogel with ultrahigh adsorption capacity for Pb(II) removal. J. Mater. Chem. A 2016, 4, 11888–11896. [Google Scholar] [CrossRef]
- Albadarin, A.B.; Collins, M.N.; Naushad, M.; Shirazian, S.; Walker, G.; Mangwandi, C. Activated lignin-chitosan extruded blends for efficient adsorption of methylene blue. Chem. Eng. J. 2017, 307, 264–272. [Google Scholar] [CrossRef] [Green Version]
- Fan, J.; Li, D.; Teng, W.; Yang, J.; Liu, Y.; Liu, L.; Elzatahry, A.A.; Alghamdi, A.; Deng, Y.; Li, G.; et al. Ordered mesoporous silica/polyvinylidene fluoride composite membranes for effective removal of water contaminants. J. Mater. Chem. A 2016, 4, 3850–3857. [Google Scholar] [CrossRef]
- Ge, H.; Wang, C.; Liu, S.; Huang, Z. Synthesis of citric acid functionalized magnetic graphene oxide coated corn straw for methylene blue adsorption. Bioresour. Technol. 2016, 221, 419–429. [Google Scholar] [CrossRef] [PubMed]
- Shu, Z.; Chen, Y.; Zhou, J.; Li, T.; Yu, D.; Wang, Y. Nanoporous-walled silica and alumina nanotubes derived from halloysite: Controllable preparation and their dye adsorption applications. Appl. Clay Sci. 2015, 112–113, 17–24. [Google Scholar] [CrossRef]
- Han, R.; Zhang, J.; Han, P.; Wang, Y.; Zhao, Z.; Tang, M. Study of equilibrium, kinetic and thermodynamic parameters about methylene blue adsorption onto natural zeolite. Chem. Eng. J. 2009, 145, 496–504. [Google Scholar] [CrossRef]
- Wang, L.; Cheng, C.; Tapas, S.; Lei, J.; Matsuoka, M.; Zhang, J.; Zhang, F. Carbon dots modified mesoporous organosilica as an adsorbent for the removal of 2,4-dichlorophenol and heavy metal ions. J. Mater. Chem. A 2015, 3, 13357–13364. [Google Scholar] [CrossRef]
- Zhang, X.; Li, Z.; Liu, K.; Jiang, L. Bioinspired multifunctional foam with self-cleaning and oil/water separation. Adv. Funct. Mater. 2013, 23, 2881–2886. [Google Scholar] [CrossRef]
- Bi, H.; Yin, Z.; Cao, X.; Xie, X.; Tan, C.; Huang, X.; Chen, B.; Chen, F.; Yang, Q.; Bu, X.; et al. Carbon fiber aerogel made from raw cotton: a novel, efficient and recyclable sorbent for oils and organic solvents. Adv. Mater. 2013, 25, 5916–5921. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Zeng, Z.; Lin, Z.; Gan, Q.; Xiang, R.; Zhu, Y.; Cao, A.; Tang, Z. Magnetic and highly recyclable macroporous carbon nanotubes for spilled oil sorption and separation. ACS Appl. Mater. Interfaces 2013, 5, 5845–5850. [Google Scholar] [CrossRef] [PubMed]
- Gui, X.; Wei, J.; Wang, K.; Cao, A.; Zhu, H.; Jia, Y.; Shu, Q.; Wu, D. Carbon nanotube sponges. Adv. Mater. 2010, 22, 617–621. [Google Scholar] [CrossRef] [PubMed]
- Radetic, M.; Ilic, V.; Radojevic, D.; Miladinovic, R.; Jocic, D.; Jovancic, P. Efficiency of recycled wool-based nonwoven material for the removal of oils from water. Chemosphere 2008, 70, 525–530. [Google Scholar] [CrossRef]
- Sajab, M.S.; Chia, C.H.; Zakaria, S.; Jani, S.M.; Ayob, M.K.; Chee, K.L.; Khiew, P.S.; Chiu, W.S. Citric acid modified kenaf core fibres for removal of methylene blue from aqueous solution. Bioresour. Technol. 2011, 102, 7237–7243. [Google Scholar] [CrossRef] [PubMed]
- Deng, H.; Lu, J.; Li, G.; Zhang, G.; Wang, X. Adsorption of methylene blue on adsorbent materials produced from cotton stalk. Chem. Eng. J. 2011, 172, 326–334. [Google Scholar] [CrossRef]
- Dural, M.U.; Cavas, L.; Papageorgiou, S.K.; Katsaros, F.K. Methylene blue adsorption on activated carbon prepared from Posidonia oceanica (L.) dead leaves: Kinetics and equilibrium studies. Chem. Eng. J. 2011, 168, 77–85. [Google Scholar] [CrossRef]
- Bhatnagar, A.; Sillanpää, M.; Witek-Krowiak, A. Agricultural waste peels as versatile biomass for water purification—A review. Chem. Eng. J. 2015, 270, 244–271. [Google Scholar] [CrossRef]
- Yu, J.-X.; Chi, R.-A.; Guo, J.; Zhang, Y.-F.; Xu, Z.-G.; Xiao, C.-Q. Desorption and photodegradation of methylene blue from modified sugarcane bagasse surface by acid TiO2 hydrosol. Appl. Surf. Sci. 2012, 258, 4085–4090. [Google Scholar] [CrossRef]
- Adam, F.; Appaturi, J.N.; Khanam, Z.; Thankappan, R.; Nawi, M.A.M. Utilization of tin and titanium incorporated rice husk silica nanocomposite as photocatalyst and adsorbent for the removal of methylene blue in aqueous medium. Appl. Surf. Sci. 2013, 264, 718–726. [Google Scholar] [CrossRef]
- Auta, M.; Hameed, B.H. Chitosan–clay composite as highly effective and low-cost adsorbent for batch and fixed-bed adsorption of methylene blue. Chem. Eng. J. 2014, 237, 352–361. [Google Scholar] [CrossRef]
- Yan, L.; Chang, P.R.; Zheng, P.; Ma, X. Characterization of magnetic guar gum-grafted carbon nanotubes and the adsorption of the dyes. Carbohydr. Polym. 2012, 87, 1919–1924. [Google Scholar] [CrossRef]
- Hassan, A.F.; Abdel-Mohsen, A.M.; Fouda, M.M.G. Comparative study of calcium alginate, activated carbon, and their composite beads on methylene blue adsorption. Carbohydr. Polym. 2014, 102, 192–198. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, W.; Wang, A. Efficient adsorption of methylene blue on an alginate-based nanocomposite hydrogel enhanced by organo-illite/smectite clay. Chem. Eng. J. 2013, 228, 132–139. [Google Scholar] [CrossRef]
- Ma, T.; Chang, P.R.; Zheng, P.; Zhao, F.; Ma, X. Fabrication of ultra-light graphene-based gels and their adsorption of methylene blue. Chem. Eng. J. 2014, 240, 595–600. [Google Scholar] [CrossRef]
- Liu, L.; Wan, Y.; Xie, Y.; Zhai, R.; Zhang, B.; Liu, J. The removal of dye from aqueous solution using alginate-halloysite nanotube beads. Chem. Eng. J. 2012, 187, 210–216. [Google Scholar] [CrossRef]
- Draget, K.I.; Taylor, C. Chemical, physical and biological properties of alginates and their biomedical implications. Food Hydrocoll. 2011, 25, 251–256. [Google Scholar] [CrossRef]
- Fuks, L.; Filipiuk, D.; Majdan, M. Transition metal complexes with alginate biosorbent. J. Mol. Struct. 2006, 792–793, 104–109. [Google Scholar] [CrossRef]
- Robitzer, M.; David, L.; Rochas, C.; Di Renzo, F.; Quignard, F. Nanostructure of calcium alginate aerogels obtained from multistep solvent exchange route. Langmuir 2008, 24, 12547–12552. [Google Scholar] [CrossRef]
- Grant, G.T.; Morris, E.R.; Rees, D.A.; Smith, P.J.C.; Thom, D. Biological interactions between polysaccharides and divalent cations: The egg-box model. FEBS Lett. 1973, 32, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Obeid, L.; El Kolli, N.; Dali, N.; Talbot, D.; Abramson, S.; Welschbillig, M.; Cabuil, V.; Bée, A. Adsorption of a cationic surfactant by a magsorbent based on magnetic alginate beads. J. Colloid Interfaces Sci. 2014, 432, 182–189. [Google Scholar] [CrossRef] [PubMed]
- Peretz, S.; Cinteza, O. Removal of some nitrophenol contaminants using alginate gel beads. Colloids Surf. A 2008, 319, 165–172. [Google Scholar] [CrossRef]
- Deze, E.G.; Papageorgiou, S.K.; Favvas, E.P.; Katsaros, F.K. Porous alginate aerogel beads for effective and rapid heavy metal sorption from aqueous solutions: Effect of porosity in Cu2+ and Cd2+ ion sorption. Chem. Eng. J. 2012, 209, 537–546. [Google Scholar] [CrossRef]
- Shi, H.; Li, W.; Zhong, L.; Xu, C. Methylene blue adsorption from aqueous solution by magnetic cellulose/graphene oxide composite: Equilibrium, kinetics, and thermodynamics. Ind. Eng. Chem. Res. 2014, 53, 1108–1118. [Google Scholar] [CrossRef]
- Liu, G.; Hu, Z.; Guan, R.; Zhao, Y.; Zhang, H.; Zhang, B. Efficient removal of methylene blue in aqueous solution by freeze-dried calcium alginate beads. Korean J. Chem. Eng. 2016, 33, 3141–3148. [Google Scholar] [CrossRef]
- Gupta, V.K.; Nayak, A.; Agarwal, S. Bioadsorbents for remediation of heavy metals: Current status and their future prospects. Environ. Eng. Res. 2015, 20, 1–18. [Google Scholar] [CrossRef]







| Isotherm Model | Parameter | Value |
|---|---|---|
| Langmuir model | Qm (mg g−1) | 1317 |
| KL × 10−2 (L mg−1) | 1.90 | |
| R2 | 0.9991 | |
| Freundlich model | KF (mg g−1) (L mg−1)1/n | 98.5845 |
| n | 2.4643 | |
| R2 | 0.8515 |
| Isotherm Model | Parameter | Value |
|---|---|---|
| Pseudo-First-Order Model | Qe (mg g−1) | 694 |
| k1 × 10−2 (min−1) | 0.567 | |
| R2 | 0.9890 | |
| Pseudo-Second-Order Model | Qe (mg g−1) | 943 |
| k2 × 10−4 [g (mg·min)−1] | 0.130 | |
| R2 | 0.9988 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, X.; Huang, P.; Li, F.; Wang, X.; Yuan, T.; Sun, R. Compressive Alginate Sponge Derived from Seaweed Biomass Resources for Methylene Blue Removal from Wastewater. Polymers 2019, 11, 961. https://doi.org/10.3390/polym11060961
Shen X, Huang P, Li F, Wang X, Yuan T, Sun R. Compressive Alginate Sponge Derived from Seaweed Biomass Resources for Methylene Blue Removal from Wastewater. Polymers. 2019; 11(6):961. https://doi.org/10.3390/polym11060961
Chicago/Turabian StyleShen, Xiaojun, Panli Huang, Fengfeng Li, Xiluan Wang, Tongqi Yuan, and Runcang Sun. 2019. "Compressive Alginate Sponge Derived from Seaweed Biomass Resources for Methylene Blue Removal from Wastewater" Polymers 11, no. 6: 961. https://doi.org/10.3390/polym11060961