Novel Inulin Derivatives Modified with Schiff Bases: Synthesis, Characterization, and Antifungal Activity
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Analytical Methods
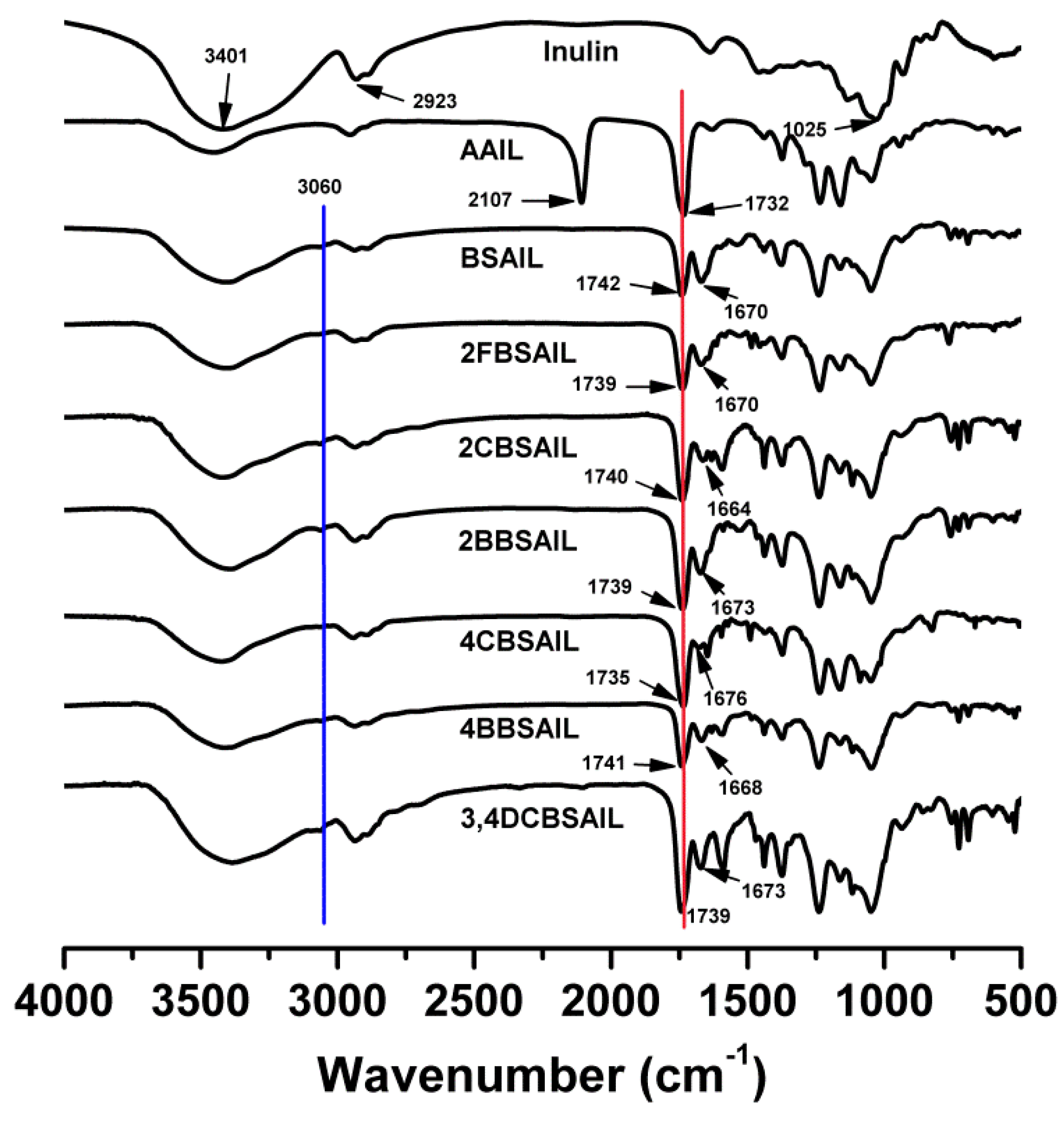
2.2.1. Fourier Transform Infrared (FTIR) Spectroscopy
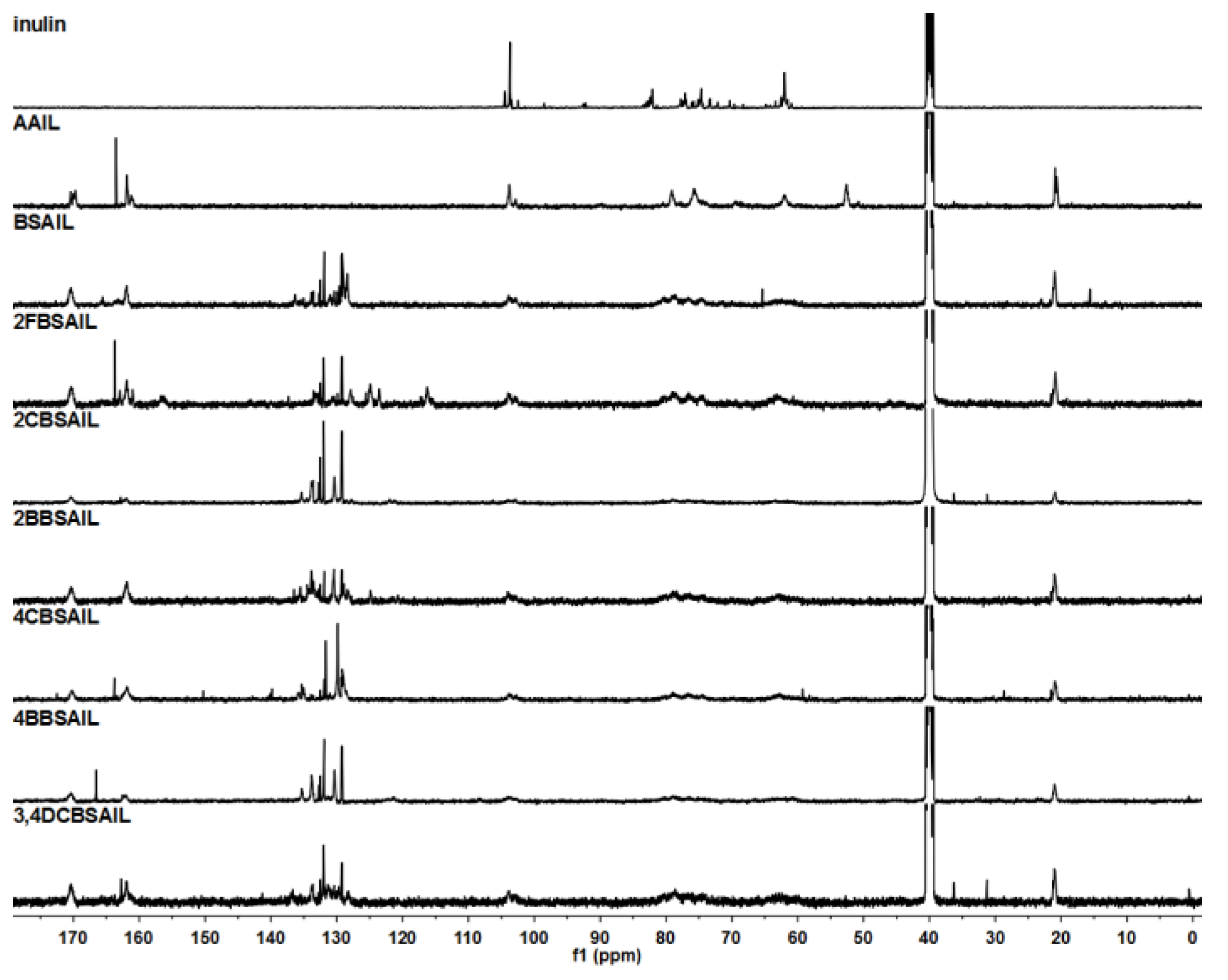
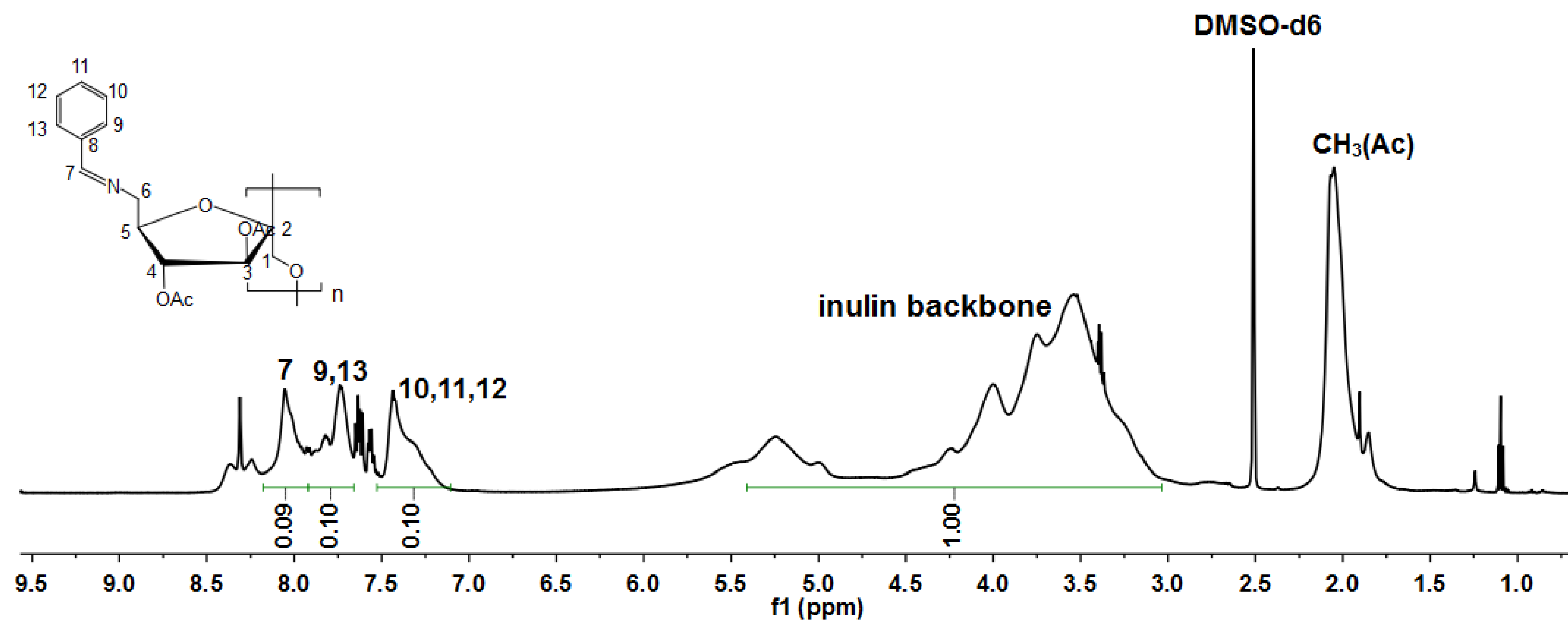
2.2.2. Nuclear Magnetic Resonance (NMR) Spectroscopy
2.3. Synthesis
2.3.1. Synthesis of 6-bromo-6-deoxy-3,4-di-O-acetyl Inulin
2.3.2. Synthesis of AAIL
2.3.3. Synthesis of Inulin Derivatives
2.4. Antifungal Assay
2.5. Statistical Analysis
3. Results and Discussion
3.1. Structure of the Inulin Derivatives
3.2. Degree of Substitution (DS)
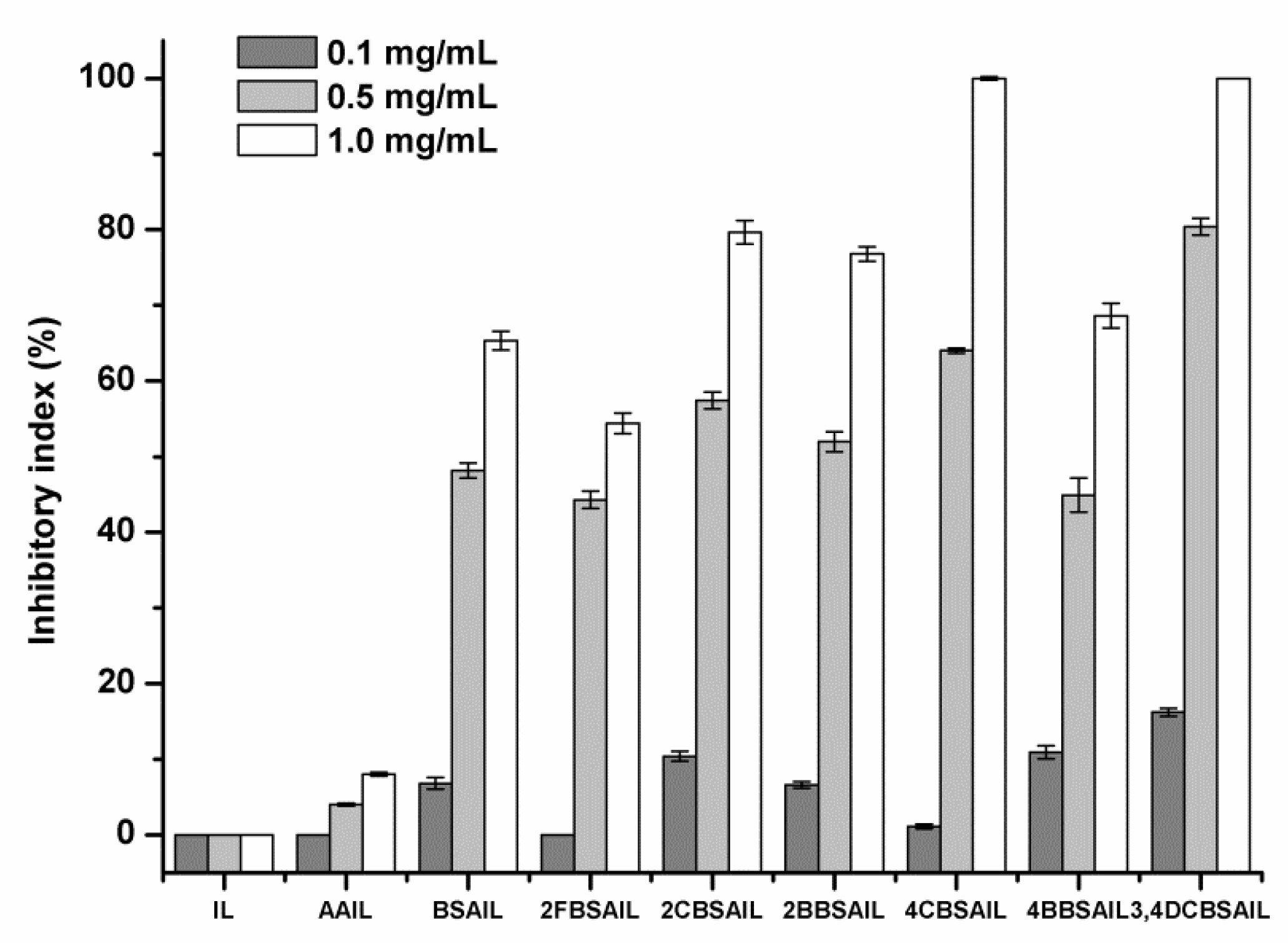
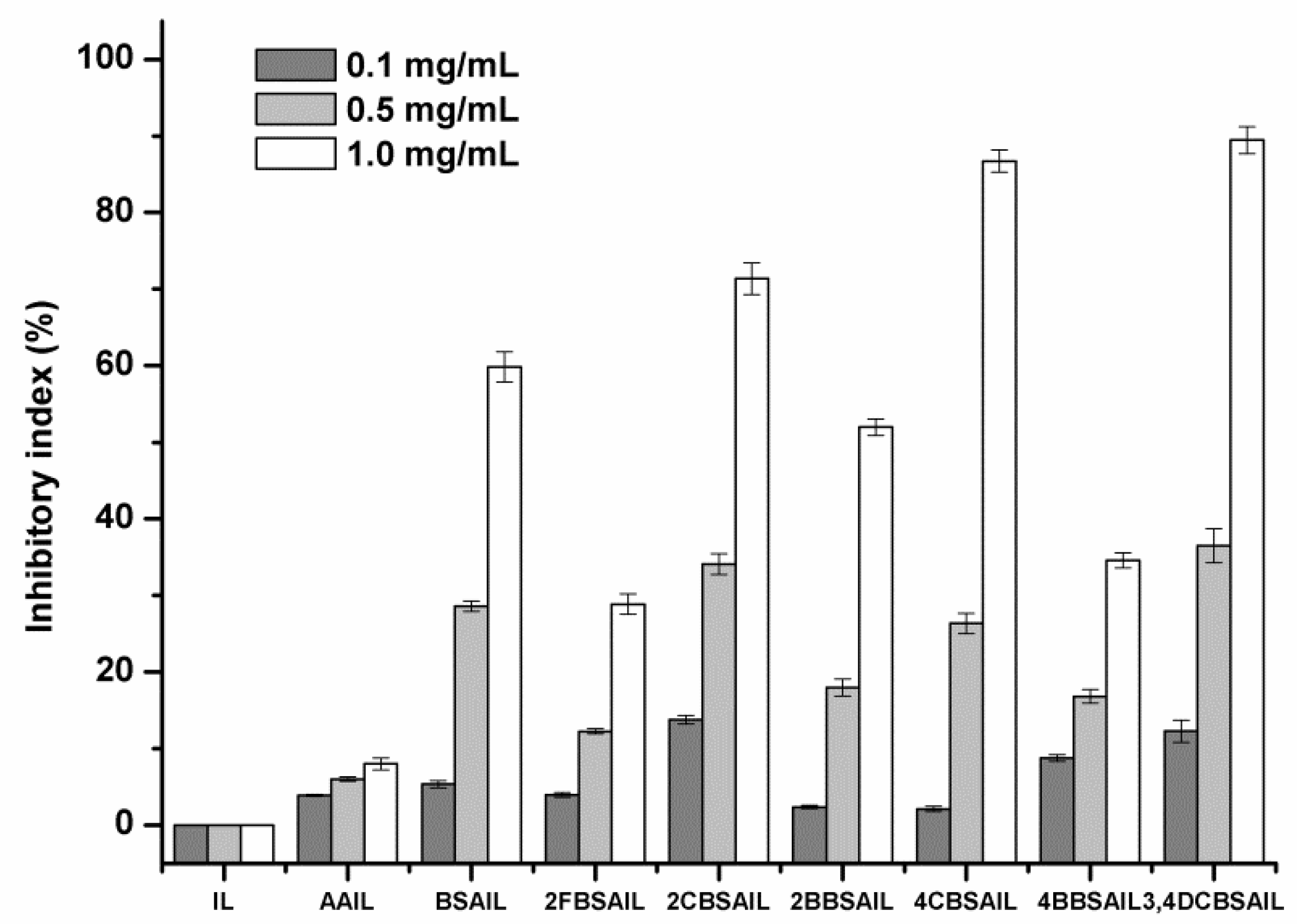
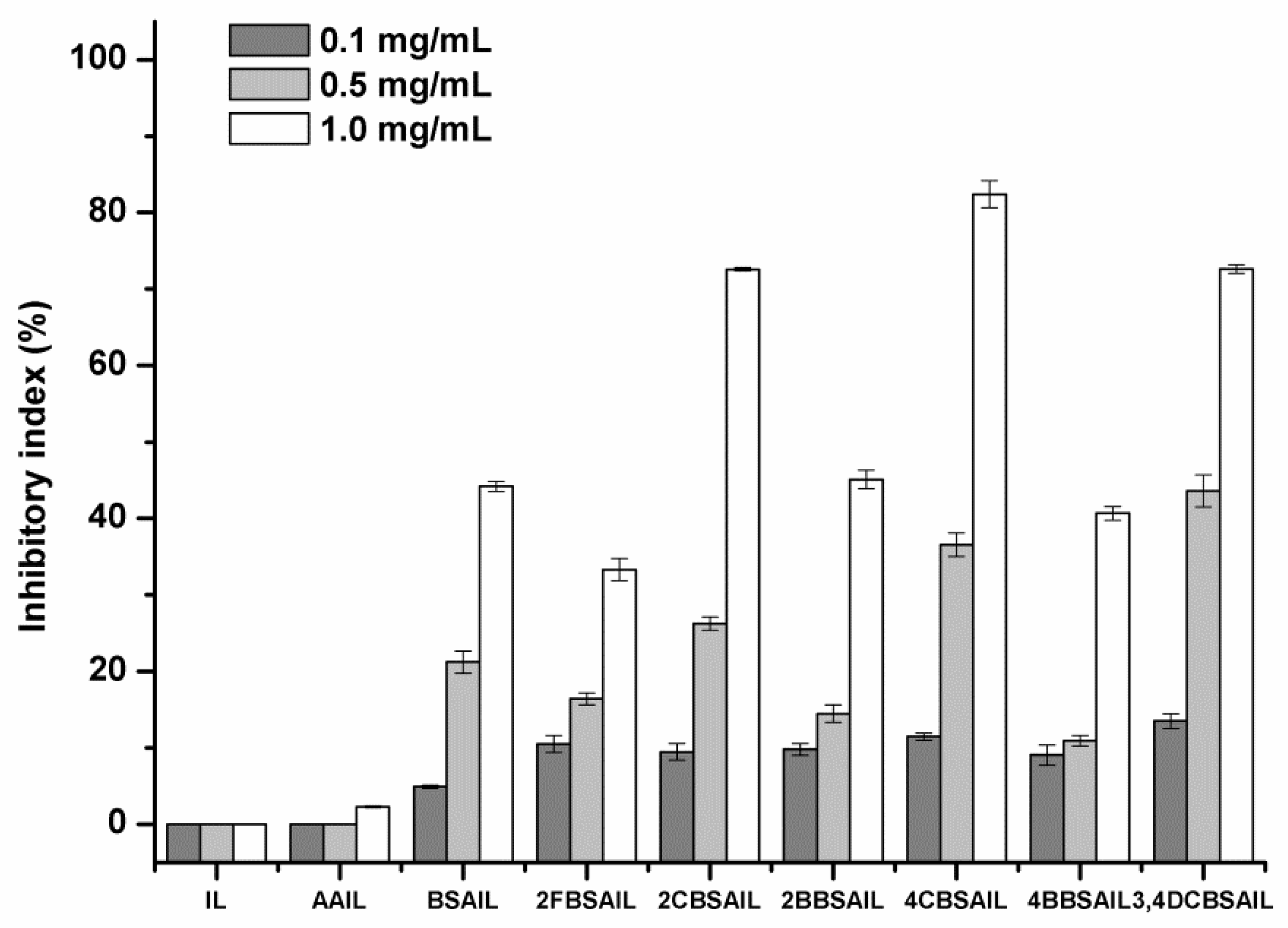
3.3. Solubility and Antifungal Activity
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Shoaib, M.; Shehzad, A.; Omar, M.; Rakha, A.; Raza, H.; Sharif, H.R.; Shakeel, A.; Ansari, A.; Niazi, S. Inulin: Properties, health benefits and food applications. Carbohydr. Polym. 2016, 147, 444–454. [Google Scholar] [CrossRef] [PubMed]
- Rubel, I.A.; Iraporda, C.; Novosad, R.; Cabrera, F.A.; Genovese, D.B.; Manrique, G.D. Inulin rich carbohydrates extraction from Jerusalem artichoke (Helianthus tuberosus L.) tubers and application of different drying methods. Food Res. Int. 2018, 103, 226–233. [Google Scholar]
- Izawa, K.; Hasegawa, T. Tosylated and azidated inulins as key substrates for further chemical modifications to access inulin-based advanced materials: An inulin-based glycocluster. Bioorg. Med. Chem. Lett. 2012, 22, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Laguna, L.; Primo-Martín, C.; Varela, P.; Salvador, A.; Sanz, T. HPMC and inulin as fat replacers in biscuits: Sensory and instrumental evaluation. LWT-Food Sci. Technol. 2014, 56, 494–501. [Google Scholar] [CrossRef]
- Karimi, R.; Azizi, M.H.; Ghasemlou, M.; Vaziri, M. Application of inulin in cheese as prebiotic, fat replacer and texturizer: A review. Carbohydr. Polym. 2015, 119, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Mensink, M.A.; Frijlink, H.W.; Maarschalk, K.V.D.V.; Hinrichs, W.L.J. Inulin, a flexible oligosaccharide. II: Review of its pharmaceutical applications. Carbohydr. Polym. 2015, 134, 418–428. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Jangid, A.K.; Pooja, D.; Kulhari, H. Inulin: A novel and stretchy polysaccharide tool for biomedical and nutritional applications. Int. J. Biol. Macromol. 2019, 132, 852–863. [Google Scholar] [CrossRef]
- Sardo, C.; Farra, R.; Licciardi, M.; Dapas, B.; Scialabba, C.; Giammona, G.; Grassi, M.; Grassi, G.; Cavallaro, G. Development of a simple, biocompatible and cost-effective Inulin-Diethylenetriamine based siRNA delivery system. Eur. J. Pharm.Sci. 2015, 75, 60–71. [Google Scholar] [CrossRef]
- López-Molina, D.; Chazarra, S.; How, C.W.; Pruidze, N.; Navarro-Perán, E.; García-Cánovas, F.; García-Ruiz, P.A.; Rojas-Melgarejo, F.; Rodríguez-López, J.N. Cinnamate of inulin as a vehicle for delivery of colonic drugs. Int. J. Pharm. 2015, 479, 96–102. [Google Scholar] [CrossRef]
- Shivhare, K.; Garg, C.; Priyam, A.; Gupta, A.; Sharma, A.K.; Kumar, P. Enzyme sensitive smart inulin-dehydropeptide conjugate self-assembles into nanostructures useful for targeted delivery of ornidazole. Int. J. Biol. Macromol. 2018, 106, 775–783. [Google Scholar] [CrossRef]
- Chi, Z.-M.; Zhang, T.; Cao, T.-S.; Liu, X.-Y.; Cui, W.; Zhao, C.-H. Biotechnological potential of inulin for bioprocesses. Bioresour. Technol. 2011, 102, 4295–4303. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Singh, T.; Larroche, C. Biotechnological applications of inulin-rich feedstocks. Bioresour. Technol. 2019, 273, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.S.; Singh, R.P.; Kennedy, J.F. Recent insights in enzymatic synthesis of fructooligosaccharides from inulin. Int. J. Biol. Macromol. 2016, 85, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Meyer, D.; Stasse-Wolthuis, M. The bifidogenic effect of inulin and oligofructose and its consequences for gut health. Eur. J. Clin. Nutr. 2009, 63, 1277–1289. [Google Scholar] [CrossRef] [PubMed]
- Gualtieri, K.D.A.; Guembarovski, R.L.; Oda, J.M.M.; Fiori-Lopes, L.; Carneiro, N.K.; Castro, V.D.D.; Neto, J.S.; Watanabe, M.A.E. Inulin: therapeutic potential, prebiotic properties and immunological aspects. Food Agric. Immunol. 2013, 24, 21–31. [Google Scholar] [CrossRef]
- Rahul, R.; Jha, U.; Sen, G.; Mishra, S. Carboxymethyl inulin: A novel flocculant for wastewater treatment. Int. J. Biol. Macromol. 2014, 63, 1–7. [Google Scholar] [CrossRef]
- Gao, J.; Yuan, W.; Kong, L.; Xiang, R.; Zhong, S. Efficient ethanol production from inulin by two-stage aerate strategy. Biomass Bioenergy 2015, 80, 10–16. [Google Scholar] [CrossRef]
- Lim, S.-H.; Ryu, J.-M.; Lee, H.; Jeon, J.H.; Sok, D.-E.; Choi, E.-S. Ethanol fermentation from Jerusalem artichoke powder using Saccharomyces cerevisiae KCCM50549 without pretreatment for inulin hydrolysis. Bioresour. Technol. 2011, 102, 2109–2111. [Google Scholar] [CrossRef]
- Zhang, L.; Li, Y.; Wang, C.; Li, G.; Zhao, Y.; Yang, Y. Synthesis of methylprednisolone loaded ibuprofen modified inulin based nanoparticles and their application for drug delivery. Mater. Sci. Eng: C 2014, 42, 111–115. [Google Scholar] [CrossRef]
- Palumbo, F.S.; Fiorica, C.; Di Stefano, M.; Pitarresi, G.; Gulino, A.; Agnello, S.; Giammona, G. In situ forming hydrogels of hyaluronic acid and inulin derivatives for cartilage regeneration. Carbohydr. Polym. 2015, 122, 408–416. [Google Scholar] [CrossRef]
- Stevens, C.V.; Meriggi, A.; Booten, K. Chemical Modification of Inulin, a Valuable Renewable Resource, and Its Industrial Applications. Biomacromolecules 2001, 2, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.M.S.; Krausová, G.; Carneiro, J.W.P.; Gonçalves, J.E.; Gonçalves, R.A.C.; de Oliveira, A.J.B. A new natural source for obtainment of inulin and fructo-oligosaccharides from industrial waste of Stevia rebaudiana Bertoni. Food Chem. 2017, 225, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Krivorotova, T.; Staneviciene, R.; Luksa, J.; Serviene, E.; Sereikaite, J. Preparation and characterization of nisin-loaded pectin-inulin particles as antimicrobials. LWT-Food Sci. Technol. 2016, 72, 518–524. [Google Scholar] [CrossRef]
- Tripodo, G.; Perteghella, S.; Grisoli, P.; Trapani, A.; Torre, M.L.; Mandracchia, D. Drug delivery of rifampicin by natural micelles based on inulin: Physicochemical properties, antibacterial activity and human macrophages uptake. Eur. J. Pharm. Biopharm. 2019, 136, 250–258. [Google Scholar] [CrossRef]
- Chen, Y.; Tan, W.; Li, Q.; Dong, F.; Gu, G.; Guo, Z. Synthesis of inulin derivatives with quaternary phosphonium salts and their antifungal activity. Int. J. Biol. Macromol. 2018, 113, 1273–1278. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.-W.; Zhang, H.; Yang, Q.; Wang, H.; Zhang, G. Preparation, characterization and antibacterial activity of octenyl succinic anhydride modified inulin. Int. J. Biol. Macromol. 2015, 78, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, G.G.; Mahmoud, W.H.; Diab, M.A.; El-Sonbati, A.Z.; Abbas, S.Y. Synthesis, characterization, theoretical study and biological activity of Schiff base nanomaterial analogues. J. Mol. Struct. 2019, 1181, 645–659. [Google Scholar] [CrossRef]
- Xu, R.; Aotegen, B.; Zhong, Z. Synthesis, characterization and biological activity of C6-Schiff bases derivatives of chitosan. Int. J. Biol. Macromol. 2017, 105, 1563–1571. [Google Scholar] [CrossRef]
- Godoy-Alcántar, C.; Yatsimirsky, A.K.; Lehn, J.-M. Structure-stability correlations for imine formation in aqueous solution. J. Phys. Org. Chem. 2005, 18, 979–985. [Google Scholar] [CrossRef]
- Belowich, M.E.; Stoddart, J.F. Dynamic imine chemistry. Chem. Soc. Rev. 2012, 41, 2003–2024. [Google Scholar] [CrossRef]
- Antony, R.; Arun, T.; Manickam, S.T.D. A review on applications of chitosan-based Schiff bases. Int. J. Biol. Macromol. 2019, 129, 615–633. [Google Scholar] [CrossRef] [PubMed]
- Anush, S.M.; Vishalakshi, B.; Kalluraya, B.; Manju, N. Synthesis of pyrazole-based Schiff bases of Chitosan: Evaluation of antimicrobial activity. Int. J. Biol. Macromol. 2018, 119, 446–452. [Google Scholar] [CrossRef] [PubMed]
- Kenawy, E.-R.; Ali, S.S.; Al-Etewy, M.; Sun, J.; Wu, J.; El-Zawawy, N. Synthesis, characterization and biomedical applications of a novel Schiff base on methyl acrylate-functionalized chitosan bearing p-nitrobenzaldehyde groups. Int. J. Biol. Macromol. 2019, 122, 833–843. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Edgar, K.J. Synthesis of curdlan derivatives regioselectively modified at C-6: O-(N)-Acylated 6-amino-6-deoxycurdlan. Carbohydr. Polym. 2014, 105, 161–168. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Liu, S.; Edgar, K.J. Efficient synthesis of secondary amines by reductive amination of curdlan Staudinger ylides. Carbohydr. Polym. 2017, 171, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Edgar, K.J. Water-soluble aminocurdlan derivatives by chemoselective azide reduction using NaBH4. Carbohydr. Polym. 2015, 122, 84–92. [Google Scholar] [CrossRef]
- Ren, J.; Wang, P.; Dong, F.; Feng, Y.; Peng, D.; Guo, Z. Synthesis and antifungal properties of 6-amino-6-deoxyinulin, a kind of precursors for facile chemical modifications of inulin. Carbohydr. Polym. 2012, 87, 1744–1748. [Google Scholar] [CrossRef]
- Tan, W.; Li, Q.; Dong, F.; Wei, L.; Guo, Z. Synthesis, characterization, and antifungal property of chitosan ammonium salts with halogens. Int. J. Biol. Macromol. 2016, 92, 293–298. [Google Scholar] [CrossRef]
- Li, Q.; Tan, W.; Zhang, C.; Gu, G.; Guo, Z. Synthesis of water soluble chitosan derivatives with halogeno-1,2,3-triazole and their antifungal activity. Int. J. Biol. Macromol. 2016, 91, 623–629. [Google Scholar] [CrossRef]
- Yang, G.; Jin, Q.; Xu, C.; Fan, S.; Wang, C.; Xie, P. Synthesis, characterization and antifungal activity of coumarin-functionalized chitosan derivatives. Int. J. Biol. Macromol. 2018, 106, 179–184. [Google Scholar] [CrossRef]
- Marin, L.; Ailincai, D.; Mares, M.; Paslaru, E.; Cristea, M.; Nica, V.; Simionescu, B.C. Imino-chitosan biopolymeric films. Obtaining, self-assembling, surface and antimicrobial properties. Carbohydr. Polym. 2015, 117, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Lopes, S.M.S.; Krausová, G.; Rada, V.; Gonçalves, J.E.; Gonçalves, R.A.C.; De Oliveira, A.J.B. Isolation and characterization of inulin with a high degree of polymerization from roots of Stevia rebaudiana (Bert.) Bertoni. Carbohydr. Res. 2015, 411, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Apolinário, A.C.; Carvalho, E.M.D.; Lima Damasceno, B.P.G.D.; Silva, P.C.D.D.; Converti, A.; Pessoa, A.; Silva, J.A.D. Extraction, isolation and characterization of inulin from Agave sisalana boles. Ind. Crops Prod. 2017, 108, 355–362. [Google Scholar] [CrossRef]
- Ferro, M.; Castiglione, F.; Panzeri, W.; Dispenza, R.; Santini, L.; Karlsson, H.J.; Wit, P.P.D.; Mele, A. Non-destructive and direct determination of the degree of substitution of carboxymethyl cellulose by HR-MAS 13C NMR spectroscopy. Carbohydr. Polym. 2017, 169, 16–22. [Google Scholar] [CrossRef] [PubMed]




| Compound | Yield | Degree of Substitution |
|---|---|---|
| Inulin | / | / |
| BSAIL | 64% | 0.34 |
| 2FBSAIL | 68% | 0.53 |
| 2CBSAIL | 75% | 0.94 |
| 2BBSAIL | 82% | 0.71 |
| 4CBSAIL | 79% | 0.69 |
| 4BBSAIL | 71% | 0.50 |
| 3,4DCBSAIL | 88% | 0.68 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Mi, Y.; Sun, X.; Zhang, J.; Li, Q.; Ji, N.; Guo, Z. Novel Inulin Derivatives Modified with Schiff Bases: Synthesis, Characterization, and Antifungal Activity. Polymers 2019, 11, 998. https://doi.org/10.3390/polym11060998
Chen Y, Mi Y, Sun X, Zhang J, Li Q, Ji N, Guo Z. Novel Inulin Derivatives Modified with Schiff Bases: Synthesis, Characterization, and Antifungal Activity. Polymers. 2019; 11(6):998. https://doi.org/10.3390/polym11060998
Chicago/Turabian StyleChen, Yuan, Yingqi Mi, Xueqi Sun, Jingjing Zhang, Qing Li, Naiyun Ji, and Zhanyong Guo. 2019. "Novel Inulin Derivatives Modified with Schiff Bases: Synthesis, Characterization, and Antifungal Activity" Polymers 11, no. 6: 998. https://doi.org/10.3390/polym11060998







