Ratiometric Luminescent Nanoprobes Based on Ruthenium and Terbium-Containing Metallopolymers for Intracellular Oxygen Sensing
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Ru- and Tb-Metallopolymer Based NPs (Ru-Tb NPs)
2.3. Oxygen Calibration of Ru-Tb NPs
2.4. Intracellular Localization of Ru-Tb NPs by Confocal Laser Scanning Microscopy
2.5. Characterizations
3. Results and Discussion
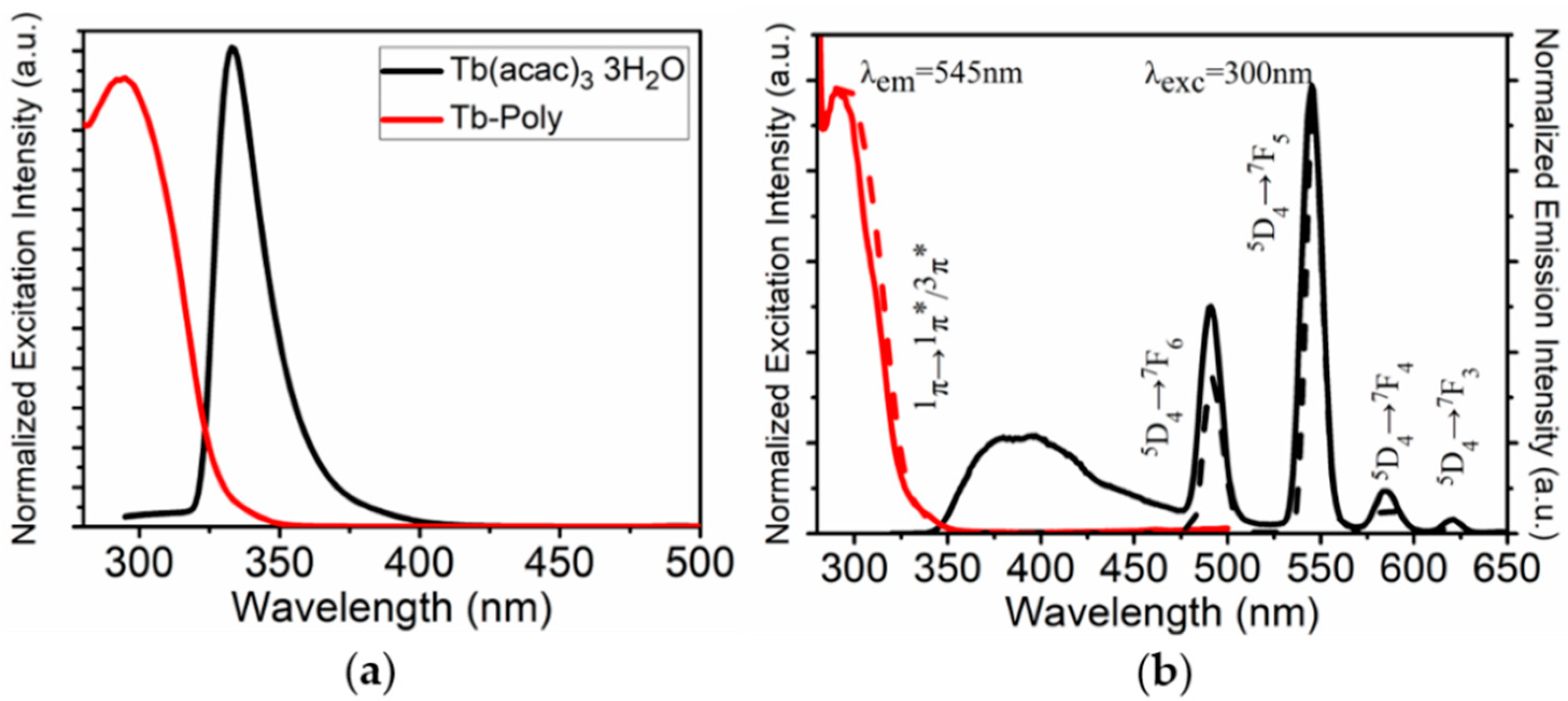
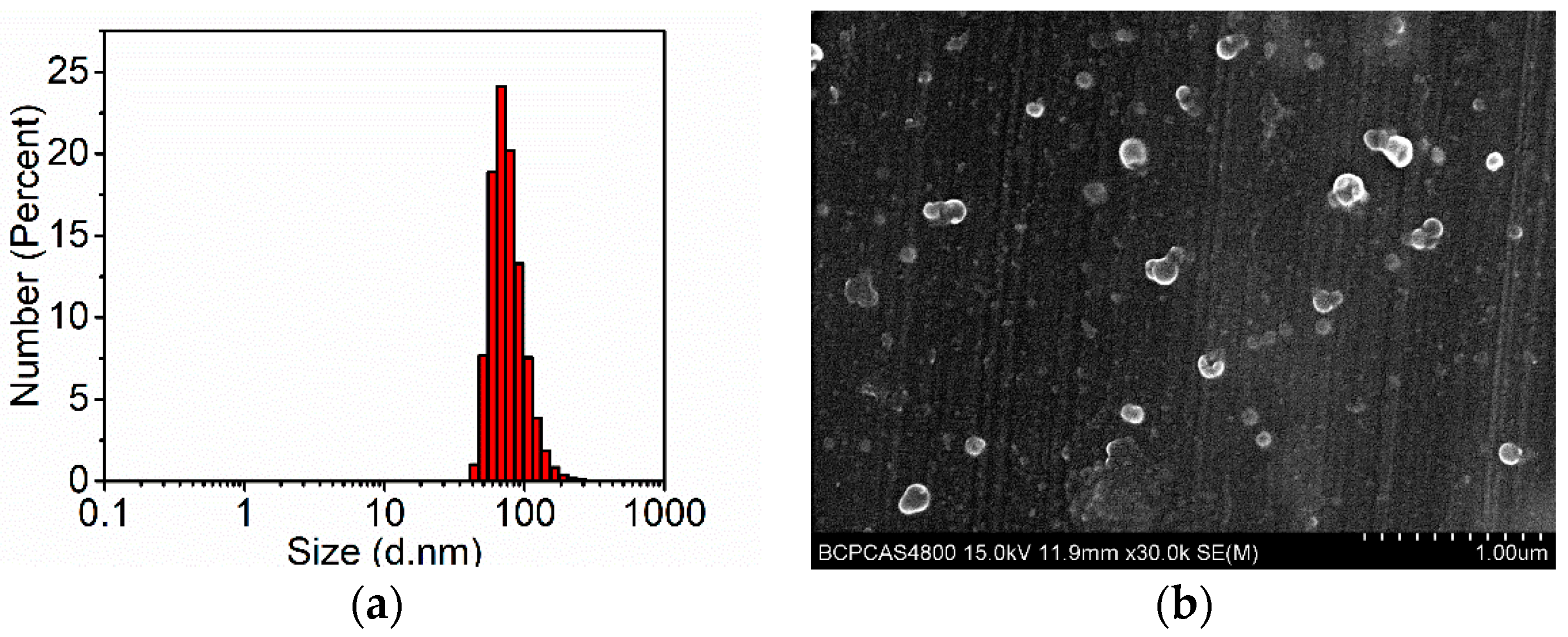
3.1. Preparation, Characterization and Properties of Ru-Tb NPs
3.2. Oxygen Sensitivity and Calibration of the Ratiometric Ru-Tb Nanoprobes
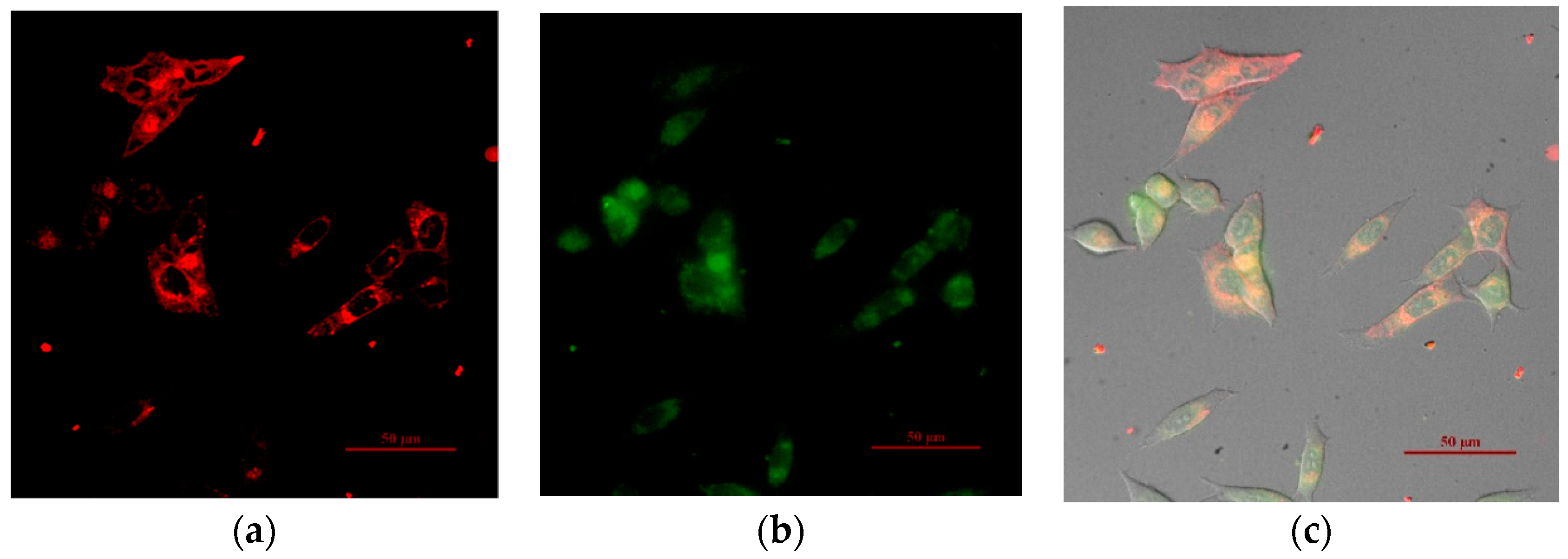
3.3. Intracellular Imaging of the Ratiometric Ru-Tb Nanoprobes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Acker, T.; Acker, H. Cellular oxygen sensing need in CNS function: Physiological and pathological implications. J. Exp. Biol. 2004, 207, 3171–3188. [Google Scholar] [CrossRef] [PubMed]
- Decker, H.; Van Holde, K.E. Oxygen and The Evolution of Life; Springer: Heidelberg, Germany, 2011; p. 172. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Optical methods for sensing and imaging oxygen: Materials, spectroscopies and applications. Chem. Soc. Rev. 2014, 43, 3666–3761. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Gupta, P.; Chen, Y.; Wang, E.; Ji, L.; Chao, H.; Chen, Z.S. The development of anticancer ruthenium (ii) complexes: From single molecule compounds to nanomaterials. Chem. Soc. Rev. 2017, 46, 5771–5804. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.N.; Guo, Z.; Zhu, W.; Yoon, J.; Tian, H. Recent progress on polymer-based fluorescent and colorimetric chemosensors. Chem. Soc. Rev. 2011, 40, 79–93. [Google Scholar] [CrossRef] [PubMed]
- Winter, A.; Schubert, U.S. Synthesis and characterization of metallo-supramolecular polymers. Chem. Soc. Rev. 2016, 45, 5311–5357. [Google Scholar] [CrossRef] [PubMed]
- Dmitriev, R.I.; Papkovsky, D.B. Optical probes and techniques for O2 measurement in live cells and tissue. Cell. Mol. Life Sci. 2012, 69, 2025–2039. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Shumway, B.R.; Meldrum, D.R. A new cross-linkable oxygen sensor covalently bonded into poly(2-hydroxyethyl methacrylate)-co-polyacrylamide thin film for dissolved oxygen sensing. Chem. Mater. 2010, 22, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Wolfbeis, O.S. Luminescent sensing and imaging of oxygen: Fierce competition to the Clark electrode. BioEssays 2015, 37, 921–928. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhao, W.X.; You, F.T.; Geng, Z.X.; Peng, H.S. Highly stable and luminescent oxygen nanosensor based on ruthenium-containing metallopolymer for real-time imaging of intracellular oxygenation. ACS Sens. 2019. [Google Scholar] [CrossRef] [PubMed]
- Hara, D.; Komatsu, H.; Son, A.; Nishimoto, S.; Tanabe, K. Water-soluble phosphorescent ruthenium complex with a fluorescent coumarin unit for ratiometric sensing of oxygen levels in living cells. Bioconj. Chem. 2015, 26, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chang, H.; Xie, J.; Zhao, B.; Liu, B.; Xu, S.; Pei, W.; Ren, N.; Huang, L.; Huang, W. Recent developments in lanthanide-based luminescent probes. Coord. Chem. Rev. 2014, 273, 201–212. [Google Scholar] [CrossRef]
- Badiane, A.M.; Freslon, S.; Daiguebonne, C.; Suffren, Y.; Bernot, K.; Calvez, G.; Costuas, K.; Camara, M.; Guillou, O. Lanthanide-based coordination polymers with a 4,5-dichlorophthalate ligand exhibiting highly tunable luminescence: Toward luminescent bar codes. Inorg. Chem. 2018, 57, 3399–3410. [Google Scholar] [CrossRef] [PubMed]
- Kotova, O.; Bradberry, S.J.; Savyasachi, A.J.; Gunnlaugsson, T. Recent advances in the development of luminescent lanthanide-based supramolecular polymers and soft materials. Dalton Transact. 2018, 47, 16377–16387. [Google Scholar] [CrossRef] [PubMed]
- Beer, P.D.; Szemes, F.; Passaniti, P.; Maestri, M. Luminescent Ruthenium(II) Bipyridine−Calix[4]arene complexes as receptors for lanthanide cations. Inorg. Chem. 2004, 43, 3965–3975. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, H.; Feng, Y.; Zhang, H.; Calzaferri, G.; Ren, T. Orienting Zeolite L Microcrystals with a functional linker. Angewandte Chem. Int. Ed. 2010, 49, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Parra, D.F.; Mucciolo, A.; Brito, H.F. Green luminescence system containing a Tb3+ -β-diketonate complex doped in the epoxy resin as sensitizer. J. Appl. Polym. Sci. 2004, 94, 865–870. [Google Scholar] [CrossRef]
- Zhang, R.J.; Yang, K.Z.; Yu, A.C.; Zhao, X.S. Fluorescence lifetime and energy transfer of rare earth β-diketone complexes in organized molecular film. Thin Solid Films 2000, 363, 275–278. [Google Scholar] [CrossRef]
- Huang, H.; Yang, L.; Zhang, P.; Qiu, K.; Huang, J.; Chen, Y.; Diao, J.; Liu, J.; Ji, L.; Long, J.; et al. Real-time tracking mitochondrial dynamic remodeling with two-photon phosphorescent iridium (III) complexes. Biomaterials 2016, 83, 321–331. [Google Scholar] [CrossRef] [PubMed]





© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.-x.; Zhou, C.; Peng, H.-s. Ratiometric Luminescent Nanoprobes Based on Ruthenium and Terbium-Containing Metallopolymers for Intracellular Oxygen Sensing. Polymers 2019, 11, 1290. https://doi.org/10.3390/polym11081290
Zhao W-x, Zhou C, Peng H-s. Ratiometric Luminescent Nanoprobes Based on Ruthenium and Terbium-Containing Metallopolymers for Intracellular Oxygen Sensing. Polymers. 2019; 11(8):1290. https://doi.org/10.3390/polym11081290
Chicago/Turabian StyleZhao, Wu-xing, Chao Zhou, and Hong-shang Peng. 2019. "Ratiometric Luminescent Nanoprobes Based on Ruthenium and Terbium-Containing Metallopolymers for Intracellular Oxygen Sensing" Polymers 11, no. 8: 1290. https://doi.org/10.3390/polym11081290




