Preparation of Ag Doped Keratin/PA6 Nanofiber Membrane with Enhanced Air Filtration and Antimicrobial Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Keratin Extraction and Blend Membranes Preparation
2.2.1. Preparation of the Ag-Keratin/PA6 Composite Solution
2.2.2. Preparation of Ag-Keratin/PA6 Composite Membrane
2.3. Characteristic
2.3.1. Determination of Molecular Weight of Keratin
2.3.2. Characterization of the Fibers
2.3.3. Filtration Performance Testing
2.3.4. Pore Size Test
2.3.5. Water–Vapour Transmission (WVT) of the Composite Films
2.3.6. TGA Analysis
2.3.7. Differential Scanning Calorimetry (DSC) Analysis
2.3.8. Antibacterial Properties of AgNP-Doped Composite Films
2.3.9. Bacterial Filtration Efficiency (BFE) Analysis
3. Results and Discussion
3.1. Determination of the Molecular Weight of Keratin
3.2. Structural Characterization of the Nanofibers
3.3. Filtration Performance of the Nanofiber Film
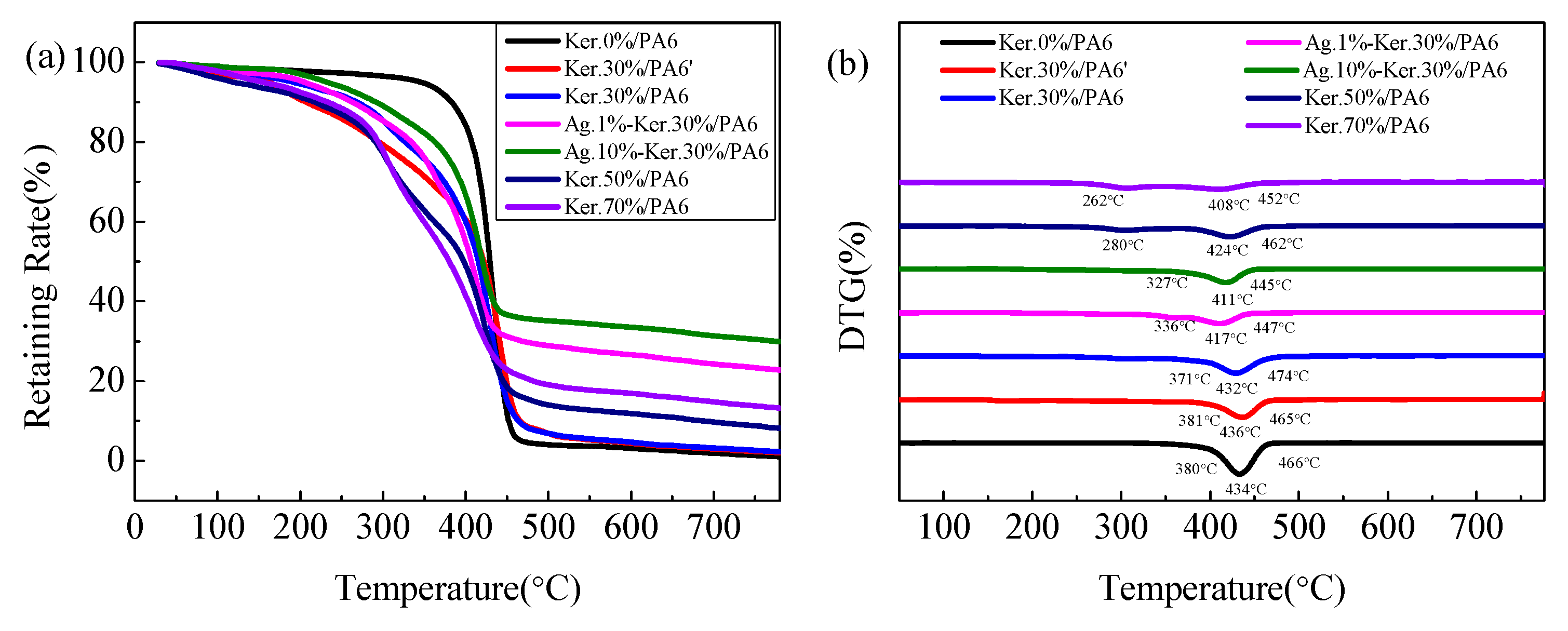
3.4. TG Analysis
3.5. DSC Analysis
3.6. Antibacterial AgNP-Doped Composite Nanofibers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aluigi, A.; Tonetti, C.; Vineis, C. Adsorption of copper (II) ions by keratin/PA6 blend nanofibres. Eur. Polym. J. 2011, 47, 1756–1764. [Google Scholar] [CrossRef]
- Ohgo, K.; Zhao, C.; Kobayashi, M.; Asakura, T. Preparation of non-woven nanofibers of Bombyx mori silk, Samia cynthia ricini silk and recombinant hybrid silk with electrospinning method. Polymer 2003, 44, 841–846. [Google Scholar] [CrossRef]
- Matthews, J.A.; Wnek, G.E.; Simpson, D.G.; Bowlin, G.L. Electrospinning of collagen nanofibers. Biomacromolecules 2002, 3, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Aluigi, A.; Varesano, A.; Vineis, C. Electrospinning of immiscible system: The wool keratin /polyamode-6 case study. Mater. Des. 2017, 127, 144–153. [Google Scholar] [CrossRef]
- Huang, Z.M.; Zhang, Y.Z.; Ramakrishma, S.; Lim, C.T. Electrospinning and mechanical characterization of gelatin nanofiber. Polymer 2004, 45, 5361–5368. [Google Scholar] [CrossRef]
- Esparza, Y.; Ullah, A.; Boluk, Y.; Wu, J. Preparation and characterization of thermally crosslinked poly (vinyl alcohol)/feather keratin nanofiber scaffolds. Mater. Des. 2017, 133, 1–9. [Google Scholar] [CrossRef]
- Hideki, M.; Masayuki, H. Transparent biocompatible wool keratin film prepared by mechanical compression of porous keratin hydrogel. Mater. Sci. Eng. R-Rep. 2018, 91, 19–25. [Google Scholar]
- Buchanan, J.H. A cystine-rich protein fraction from oxidized alpha-keratin. Biochem. J. 1977, 167, 489–491. [Google Scholar] [CrossRef]
- Aluigi, A.; Vineis, C.; Varesano, A.; Mazzuchetti, G.; Ferrero, F.; Tonin, C. Structure and properties of keratin/PEO blend nanofibers. Eur. Polym. J. 2008, 44, 2465–2475. [Google Scholar]
- Barone, J.R.; Schmidt, W.F.; Liebner, C.F.E. Thermally processed keratin films. J. Appl. Polym. Sci. 2005, 97, 1644–1651. [Google Scholar] [CrossRef]
- Dominici, F.; Peng, R.D.; Bell, M.L.; Pham, L.; McDermott, A.; Zeger, S.L.; Samet, J.M. Fine particulate air pollution and hospital admission for cardiovascular and respiratory diseases. JAMA 2006, 295, 1127–1134. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Kovochich, M.; Brant, J.; Hotze, M.; Sempf, J.; Oberley, T.; Sioutas, C.; Yeh, J.I.; Wiesner, M.R.; Nel, A.E. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006, 6, 1794–1807. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.S.; Otani, Y. Removal of nanoparticles from gas streams by fibrous filters: A review. Ind. Eng. Chem. Res. 2013, 52, 5–17. [Google Scholar] [CrossRef]
- Li, P.; Wang, C.Y.; Zhang, Y.Y.; Wei, F. Air filtration in the free molecular flow regime: A review of high-efficiency particulate air filters based on carbon nanotubes. Small 2014, 10, 4543–4561. [Google Scholar] [CrossRef] [PubMed]
- Kouhi, M.; Jayarama, R.V.; Fathi, M.; Shamanian, M.; Valipouri, A.; Ramakrishna, S. Poly (3-hydroxybutyrate-co-3-hydroxyvalerate)/fibrinogen/bredigite nanofibrous membranes and their integration with osteoblasts for guided bone regeneration. J. Biomed. Mater. Res. 2019, 107, 1154–1165. [Google Scholar] [CrossRef]
- Zoccola, M.; Aluigi, A.; Vineis, C.; Tonin, C.; Ferrero, F.; Piacentino, M.G. Study on cast membranes and electrospun nanofibres made from keratin/fibroin blends. Biomacromolecules 2008, 9, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Baek, D.H.; Ki, C.S.; Um, I.C.; Park, Y.H. Metal ion adsorbability of electrspun wool keratose/silk fibroin blend nanofibres mats. Fibers Polym. 2007, 8, 1–7. [Google Scholar] [CrossRef]
- Aluigi, A.; Corbellini, A.; Rombaldoni, F.; Mazzuchetti, G. Wool-derived keratin nanofiber membranes for dynamic adsorption of heavy-metal ions from aqueous solutions. Text. Res. J. 2013, 83, 1574–1586. [Google Scholar] [CrossRef]
- Gibson, P.; Schreuder-Gibson, H.; Rivin, D. Transport properties of porous membranes based on electrospun nanofibers. Colloids Surf. A-Physicochem. Eng. Asp. 2001, 187, 469–481. [Google Scholar] [CrossRef]
- Uppal, R.; Ramaswamy, G.; Arnold, C.; Goodband, R.; Wang, Y. Hyaluronic acid nanofiber wound dressing-production, characterization, and in vivo behavior. J. Biomed. Mater. Res. B Appl. Biomater. 2011, 97, 20–29. [Google Scholar] [CrossRef]
- Min, B.M.; Lee, G.; Kim, S.H.; Nam, Y.S.; Lee, T.S.; Park, W.H. Electrospinning of silk fibroin nanofibers and its effect on the adhesion and spreading of normal human keratinocytes and fibroblasts in vitro. Biomaterials 2004, 25, 1289–1297. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.J.; Fridrikh, S.V.; Rutledge, G.C.; Kaplan, D.L. Electrospinning Bombyx mori silk with poly(ethylene oxide). Biomacromolecules 2002, 3, 1233–1239. [Google Scholar] [CrossRef] [PubMed]
- Mo, X.; Sun, B.; Wu, T.; Li, D. Electrospun Nanofibers for Tissue Engineering. In Electrospinning: Nanofabrication and Applications; William Andrew Publishing: Burlington, MA, USA, 2019; pp. 719–734. [Google Scholar]
- Aluigi, A.; Rombaldoni, F.; Tonetti, C.; Jannoke, L. Study of Methylene Blue adsorption on keratin nanofibrous membranes. J. Hazard. Mater. 2014, 268, 156–165. [Google Scholar] [CrossRef] [PubMed]
- Cipitria, A.; Skelton, A.; Dargaville, T.; Dalton, P.; Hutmacher, D. Design, fabrication and characterization of PCL electrospun scaffolds-a review. J. Mater. Chem. 2011, 21, 9419–9453. [Google Scholar] [CrossRef]
- Edwards, A.; Jarvis, D.; Hopkins, T.; Pixley, S.; Bhattarai, N. Poly(ε-caprolactone)/keratin-based composite nanofibers for biomedical applications. J. Biomed. Mater. Res. B. Appl. Biomater. 2015, 103, 21–30. [Google Scholar] [CrossRef]
- Xiu, Z.M.; Zhang, Q.B.; Puppala, H.L.; Colvin, V.L.; Alvarez, P.J.J. Negligible particle-specific antibacterial activity of silver nanoparticles. Nano Lett. 2012, 12, 4271–4275. [Google Scholar] [CrossRef] [PubMed]
- Quadros, M.E.; Marr, L.C. Silver nanoparticles and total aerosols emitted by nanotechnology-related consumer spray products. Environ. Sci. Technol. 2011, 45, 10713–10719. [Google Scholar] [CrossRef]
- Wang, C.Y.; Wu, S.Y.; Jian, M.Q.; Xie, J.R.; Xu, L.P.; Yang, X.D.; Zheng, Q.S. Silk nanofibers as high efficient and lightweight air filter. Nano Res. 2016, 9, 2590–2597. [Google Scholar] [CrossRef]
- Martin, J.J.; Cardamone, J.M.; Irwin, P.L.; Brown, E.M. Keratin capped silver nanoparticles-Synthesis and characterization of a nanomaterial with desirable handling properties. Colloid Surf. B-Biointerfaces 2011, 88, 354–361. [Google Scholar] [CrossRef]
- Liu, Z.; Du, J.; Jin, X.; Li, P.F.; Jiang, X.F.; Jiang, Y. Polyurethane/Keratin/AgNPs nanofibrous mats as catalyst support for 4-nitroaniline reduction. Mater. Lett. 2019, 237, 9–13. [Google Scholar] [CrossRef]
- Wu, P.P.; Dai, X.Q.; Chen, K.; Li, R.; Xing, Y.J. Fabrication of regenerated wool keratin/polycaprolactone nanofiber membranes for cell culture. Int. J. Biol. Macromol. 2018, 114, 1168–1173. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Panthi, G.; Liu, Y.; Kim, J.; Chae, S.H.; Lee, H.Y. Keratin/poly (vinyl alcohol) blended nanofibers with high optical transmittance. Polymer 2015, 58, 146–152. [Google Scholar] [CrossRef]
- Sanchez Ramirez, D.O.; Carletto, R.A.; Tonetti, C.; Truffa Giachet, F.; Varesano, A.; Vineis, C. Wool keratin film plasticized by citric acid for food packaging. Food Packag. Shelf 2017, 12, 100–106. [Google Scholar] [CrossRef]
- Zhao, L.; Jiang, L.H.; Li, H.Y.; Hu, C.; Sun, J.X.; Li, L.; Meng, F.R.; Dong, Z.H.; Zhou, C.C. Synthesis and characterization of silver-incorporated calcium phosphate antibacterial nanocomposites for mask filtration material. Compos. Part B-Eng. 2018, 153, 387–392. [Google Scholar] [CrossRef]
- Shavandi, A.; Carne, A.; Bekhit, A.A.; Ala Bekhit, E.A. An improved method for solubilisation of wool keratin using peracetic acid. J. Environ. Eng. 2017, 5, 1977–1984. [Google Scholar] [CrossRef]
- Bhardwaj, N.; Kundu, S.C. Electrospinning: A fascinating fiber fabrication technique. Biotechnol. Adv. 2010, 28, 325–347. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Yang, X.H. Fabrication and characterization of electrospun wool keratin/poly(-vinyl alcohol) blend nanofibers. Adv. Mater. Sci. Eng. 2014, 2014, 163678. [Google Scholar] [CrossRef]
- He, W.T.; Gao, J.; Liao, S.T.; Wang, X.Y.; Qin, S.H.; Song, P.A. A facile method to improve thermal stability and flame retardancy of polyamide 6. Compos. Commun. 2019, 13, 143–150. [Google Scholar]
- Campoy, I.; Gómez, M.A.; Marco, C.; Campoy, M.; Gómez, A.; Marco, C. Structure and thermal properties of blends of nylon 6 and a liquid crystal copolyester. Polymer 1998, 39, 6279–6288. [Google Scholar] [CrossRef]
- Liu, H.; Huang, J.Y.; Mao, J.J.; Chen, Z.; Chen, G.Q.; Lai, Y.K. Transparent antibacterial nanofiber air filters with highly efficient moisture resistance for sustainable particulate matter capture. iScience 2019, 19, 214–223. [Google Scholar] [CrossRef]





| Samples | Diameter (nm) | Viscosity (Pa·S) | Conductivity (mS/cm) |
|---|---|---|---|
| Ker.0%/PA6 | 293 ± 70 | 2.48 | 3.18 |
| Ker.30%/PA6′ | 229 ± 74 | 1.95 | 4.15 |
| Ker.30%/PA6 | 192 ± 65 | 1.09 | 4.45 |
| Ag.1%-Ker.30%/PA6 | 183 ± 40 | 0.94 | 4.67 |
| Ag.10%-Ker.30%/PA6 | 168 ± 60 | 0.85 | 6.83 |
| Ker.50%/PA6 | 174 ± 49 | 0.78 | 5.26 |
| Ker.70%/PA6 | 155 ± 50 | 0.70 | 6.53 |
| Samples | Bacterial Inhibition Zone (mm) | Bacterial Inhibition Rate (%) | ||
|---|---|---|---|---|
| E. coli | S. aureus | E. coli | S. aureus | |
| Ker.30%/PA6 | 0 | 0 | 0 | 0 |
| Ag.1%-Ker.30%/PA6 | <1 | 0 | 19.95 | 20.65 |
| Ag.10%-Ker.30%/PA6 | >1 | >1 | 99.10 | 99.62 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shen, B.; Zhang, D.; Wei, Y.; Zhao, Z.; Ma, X.; Zhao, X.; Wang, S.; Yang, W. Preparation of Ag Doped Keratin/PA6 Nanofiber Membrane with Enhanced Air Filtration and Antimicrobial Properties. Polymers 2019, 11, 1511. https://doi.org/10.3390/polym11091511
Shen B, Zhang D, Wei Y, Zhao Z, Ma X, Zhao X, Wang S, Yang W. Preparation of Ag Doped Keratin/PA6 Nanofiber Membrane with Enhanced Air Filtration and Antimicrobial Properties. Polymers. 2019; 11(9):1511. https://doi.org/10.3390/polym11091511
Chicago/Turabian StyleShen, Baolei, Dongyu Zhang, Yujuan Wei, Zihua Zhao, Xiaofei Ma, Xiaodan Zhao, Shuo Wang, and Wenxiu Yang. 2019. "Preparation of Ag Doped Keratin/PA6 Nanofiber Membrane with Enhanced Air Filtration and Antimicrobial Properties" Polymers 11, no. 9: 1511. https://doi.org/10.3390/polym11091511





