Structure and Physicochemical Properties of Malate Starches from Corn, Potato, and Wrinkled Pea Starches
Abstract
:1. Introduction
2. Materials and methods
2.1. Materials
2.2. Preparation of Malate Starch Samples
2.3. Determination of the Degree of Substitution (DS)
- V0—the volume of HCl solution consumed by the blank, mL;
- V1—the volume of HCl solution consumed by the sample, mL;
- C—the exact concentration of the standard titration solution of hydrochloric acid, mol/L;
- M—the molar content of the 2-hydroxysuccinyl group, g/mol (M(C4H5O4) = 117);
- W—the mass of the sample, g;
- 162—the relative molecular mass of glucosyl;
- 1000—conversion factor.
2.4. Scanning Electron Microscopy (SEM)
2.5. X-Ray Diffraction (XRD)
2.6. Fourier Transform Infrared Spectroscopy (FT–IR)
2.7. Thermogravimetric (TG) Analysis
2.8. In Vitro Starch Digestion
2.9. Statistical Analysis
3. Results and Analysis
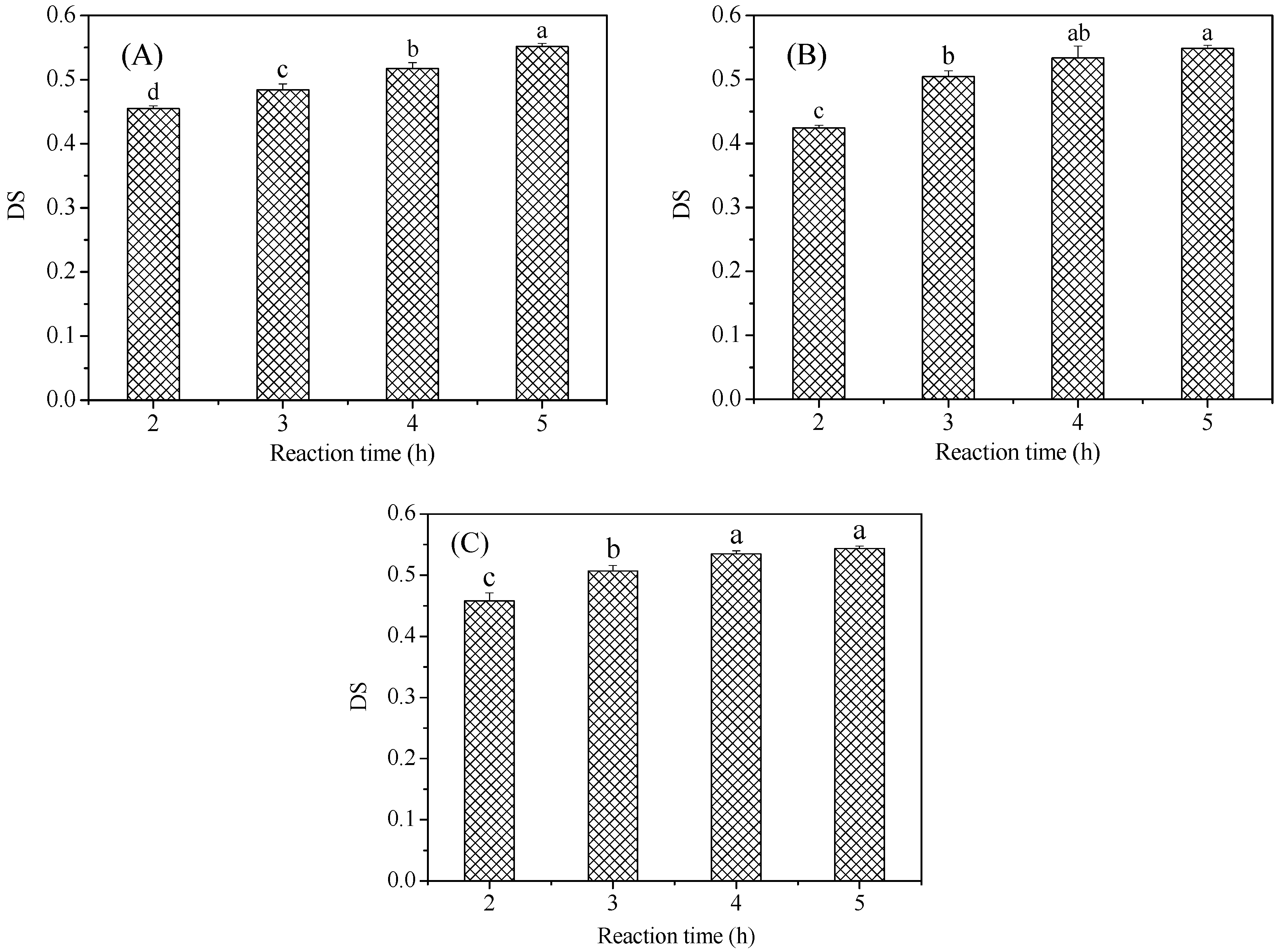
3.1. Degree of Substitution (DS) Analysis of the Malate Starch Samples
3.2. Scanning Electron Microscopy (SEM) of the Malate Starch Samples
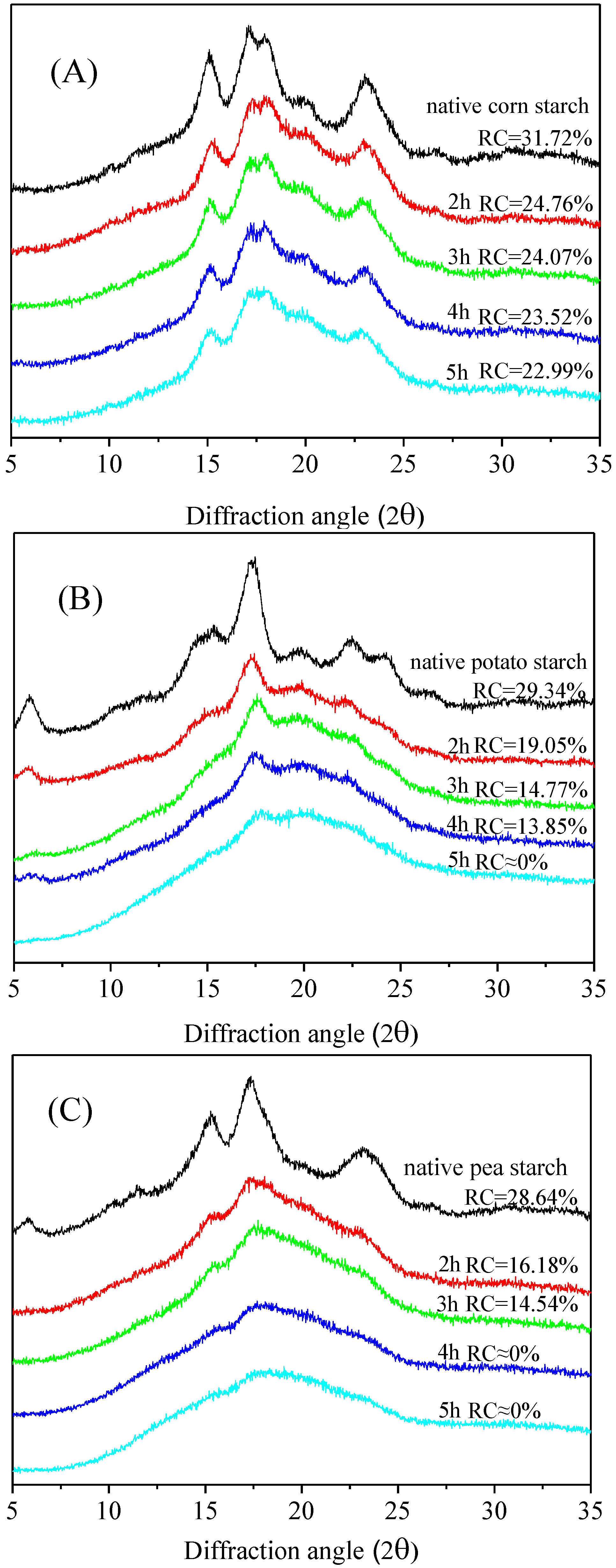
3.3. X-Ray Diffraction (XRD) of the Malate Starch Samples
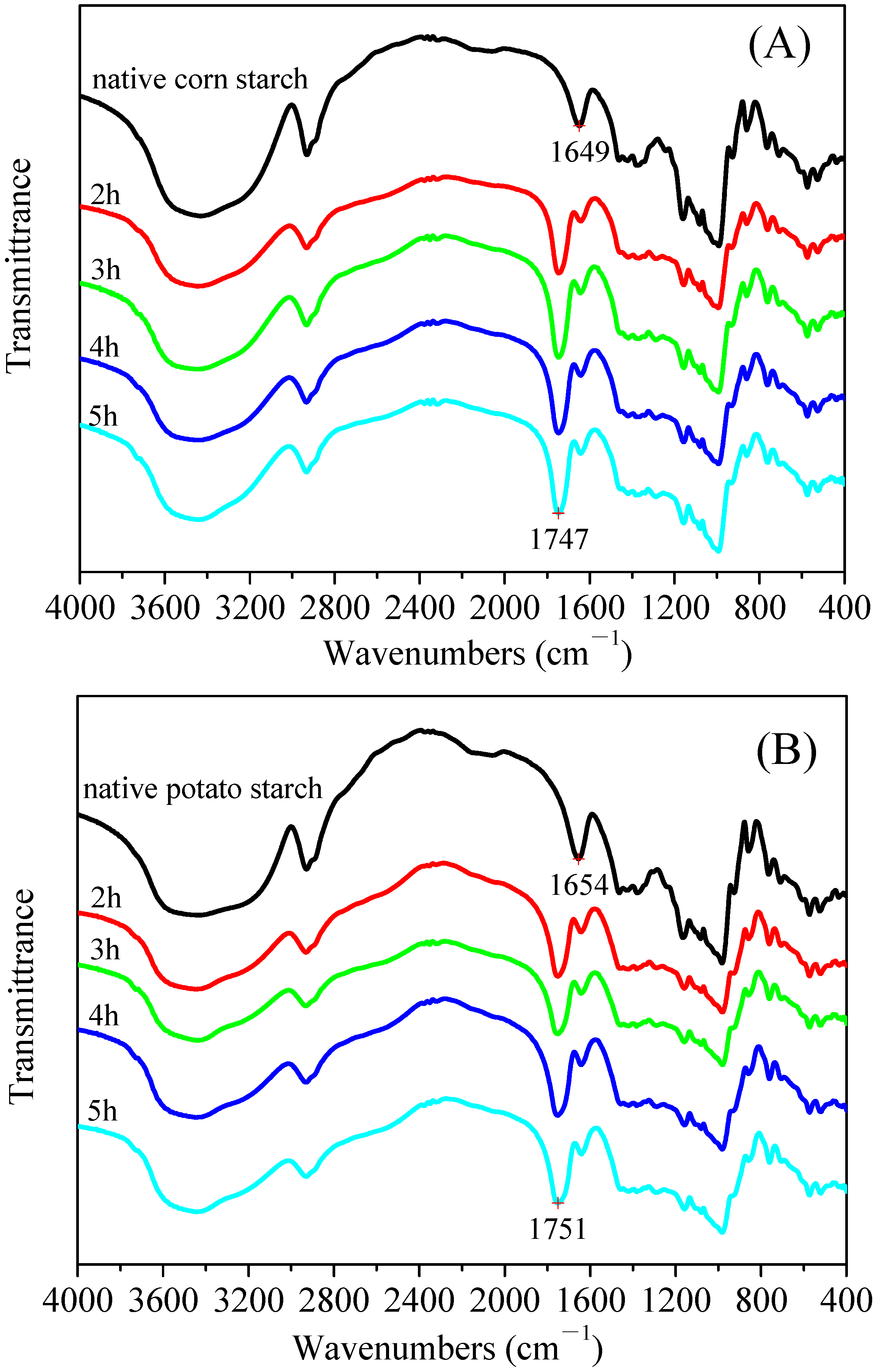
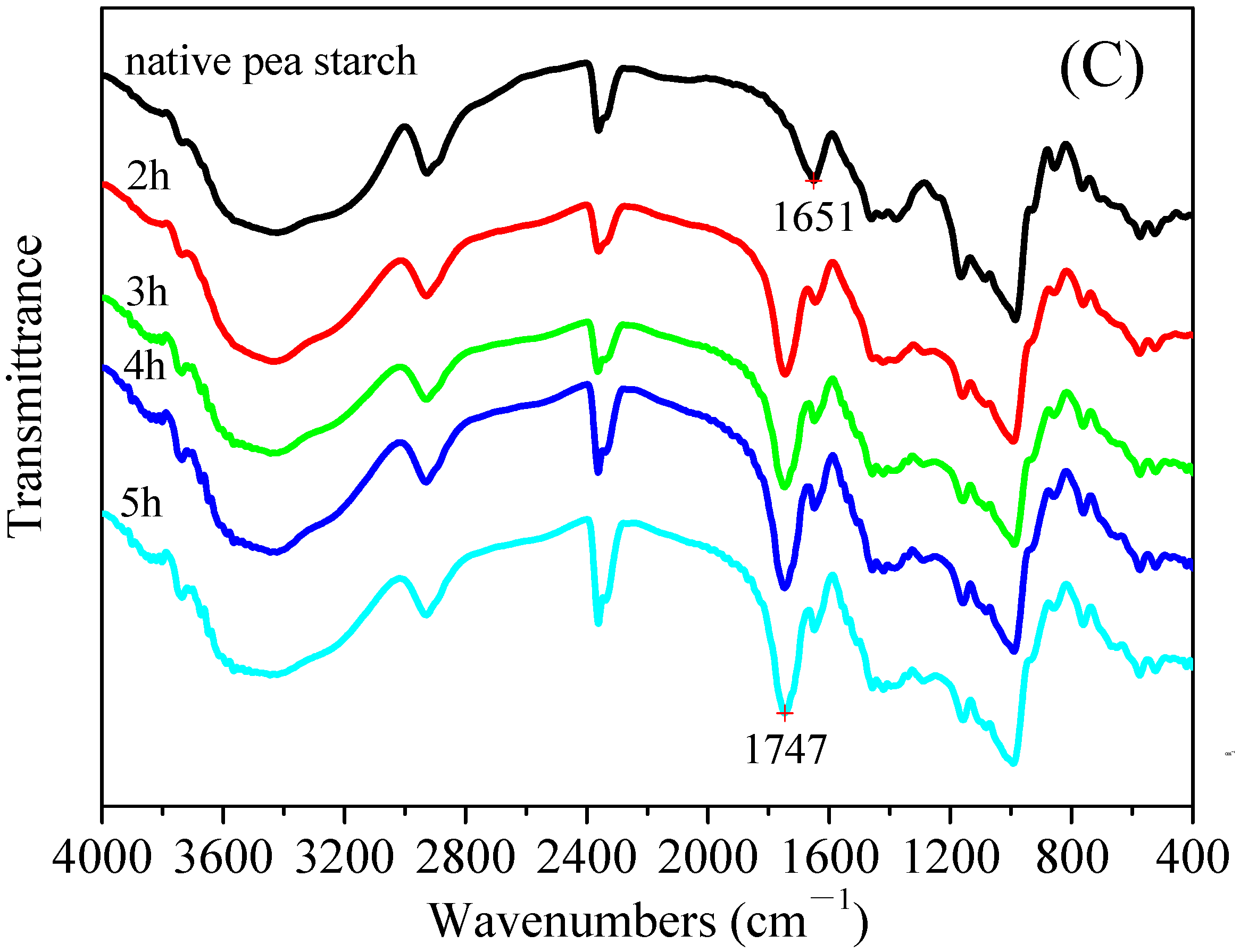
3.4. Fourier Transform Infrared Spectroscopy (FT–IR) of the Malate Starch Samples
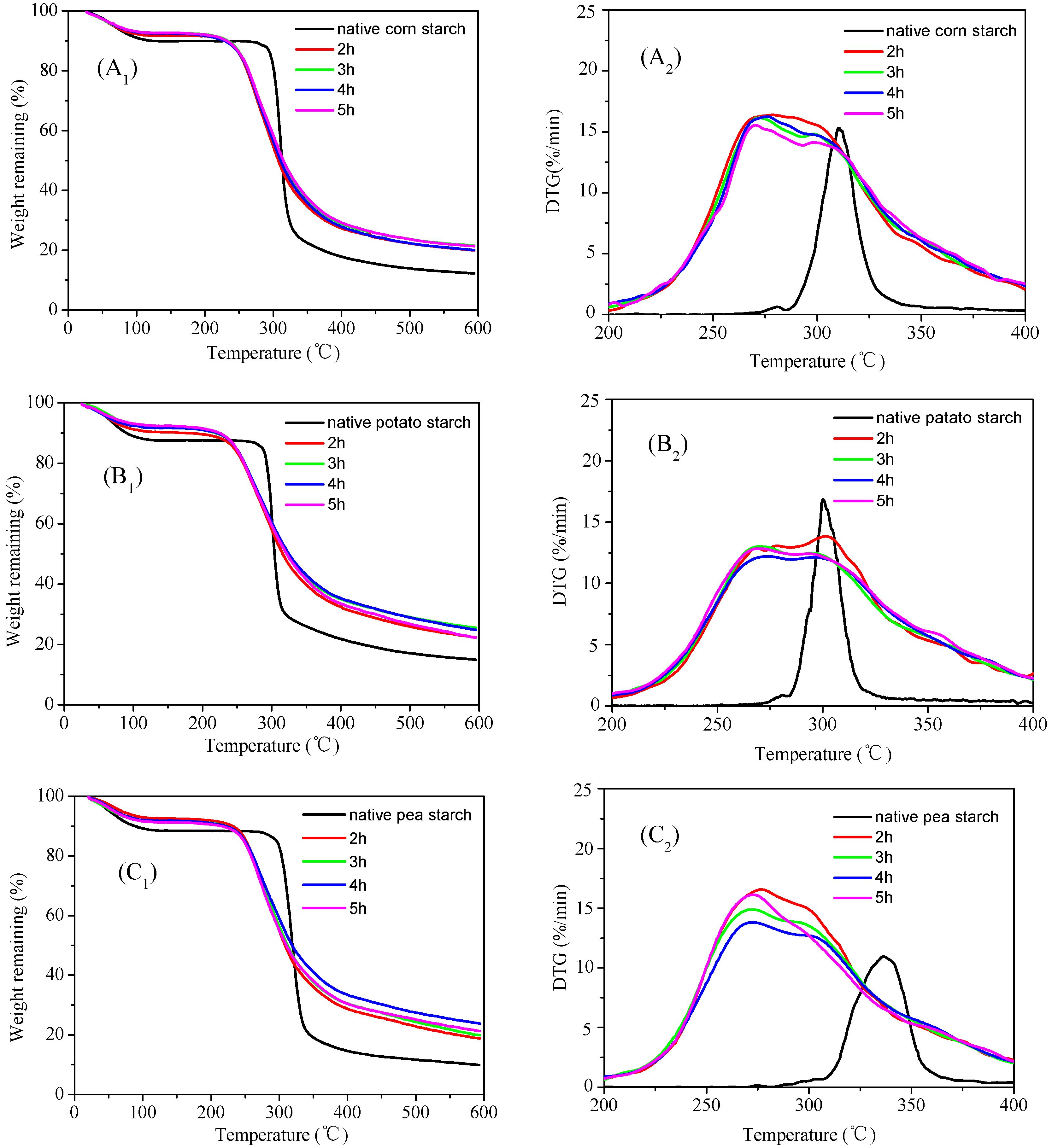
3.5. Thermogravimetric (TG) Analysis of the Malate Starch Samples
3.6. Digestibility Properties of the Malate Starch Samples
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Lopez-Rubio, A.; Clarke, J.M.; Scherer, B.; Topping, D.L.; Gilbert, E.P. Structural modifications of granular starch upon acylation with short-chain fatty acids. Food Hydrocoll. 2009, 23, 1940–1946. [Google Scholar] [CrossRef]
- Buléon, A.; Colonna, P.; Planchot, V.; Ball, S. Starch granules: Structure and biosynthesis. Int. J. Biol. Macromol. 1998, 23, 85–112. [Google Scholar] [CrossRef]
- Xie, X.; Liu, Q. Development and physicochemical characterization of new resistant citrate starch from different corn starches. Starch 2004, 56, 364–370. [Google Scholar] [CrossRef]
- Hung, P.V.; Vien, N.L.; Lan Phi, N.T. Resistant starch improvement of rice starches under a combination of acid and heat-moisture treatments. Food Chem. 2016, 191, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Luo, Z.; Shi, Y. Preparation of acetylated waxy, normal, and high-amylose maize starches with intermediate degrees of substitution in aqueous solution and their properties. J. Agric. Food Chem. 2012, 60, 9468–9475. [Google Scholar] [CrossRef] [PubMed]
- Phillips, D.L.; Liu, H.; Pan, D.; Harold, C. General application of Raman Spectroscopy for the determination of level of acetylation in modified starches. Cereal Chem. 2007, 76, 439–443. [Google Scholar] [CrossRef]
- Shi, M.; Gao, Q.; Liu, Y. Changes in the structure and digestibility of wrinkled pea starch with malic acid treatment. Polymers 2018, 10, 1359. [Google Scholar] [CrossRef]
- Klaushofer, H.; Berghofer, E.; Steyrer, W. Starch citrates-production and technical application properties (maize, potato). Starch 1978, 30, 47–51. [Google Scholar] [CrossRef]
- Kweon, D.K.; Choi, J.K.; Kim, E.K.; Lim, S.T. Adsorption of divalent metal ions by succinylated and oxidized corn starches. Carbohydr. Polym. 2001, 46, 171–177. [Google Scholar] [CrossRef]
- Chen, L.; Ren, F.; Yu, X.; Zhang, Z.; Xu, D.; Tong, Q. Pasting investigation, SEM observation and the possible interaction study on rice starch-pullulan combination. Int. J. Biol. Macromol. 2015, 73, 45–48. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, H.; Shi, M. Effect of ethanol-water solution on the crystallization of short chain amylose from potato starch. Starch 2016, 68, 683–690. [Google Scholar] [CrossRef]
- Shi, M.; Liang, X.; Yan, Y.; Pan, H.; Liu, Y. Influence of ethanol-water solvent and ultra-high pressure on the stability of amylose-n-octanol complex. Food Hydrocoll. 2018, 74, 315–323. [Google Scholar] [CrossRef]
- Tian, Y.; Li, Y.; Xu, X.; Jin, Z. Starch retrogradation studied by thermogravimetric analysis (TGA). Carbohydr. Polym. 2011, 84, 1165–1168. [Google Scholar] [CrossRef]
- Englyst, H.N.; Kingman, S.M.; Cummings, J.H. Classification and measurement of nutritionally important starch fractions. Eur. J. Clin. Nutr. 1992, 46, S33–S50. [Google Scholar] [PubMed]
- Du, S.K.; Jiang, H.; Ai, Y.; Jane, J.L. Physicochemical properties and digestibility of common bean (Phaseolus vulgaris L.) starches. Carbohydr. Polym. 2014, 108, 200–205. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.Y.; Lee, K.Y.; Lee, H.G. Effect of different pH conditions on the in vitro digestibility and physicochemical properties of citric acid-treated potato starch. Int. J. Biol. Macromol. 2017, 107, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Lee, Y.K.; Chang, Y.H. Structure and digestibility properties of resistant rice starch cross-linked with citric acid. Int. J. Food Prop. 2017, 20, 2166–2177. [Google Scholar]
- Zuo, Y.; Gu, J.; Long, Y.; Qiao, Z.; Tan, H.; Zhang, Y. Synthesis and characterization of maleic anhydride esterified corn starch by the dry method. Int. J. Biol. Macromol. 2013, 62, 241–247. [Google Scholar] [CrossRef]
- Majzoobi, M.; Beparva, P.; Farahnaky, A.; Badii, F. Effects of malic acid and citric acid on the functional properties of native and cross-linked wheat starches. Starch 2014, 66, 5–6. [Google Scholar] [CrossRef]
- Andrade, M.M.P.; Oliveira, C.S.D.; Colman, T.A.D.; Costa, F.J.O.G.D.; Schnitzler, E. Effects of heat-moisture treatment on organic cassava starch. J. Therm. Anal. Calorim. 2014, 115, 2115–2122. [Google Scholar] [CrossRef]
- Fornal, J.; Sadowska, J.; Błaszczak, W.; Jeliński, T.; Stasiak, M.; Molenda, M.; Hajnos, M. Influence of some chemical modifications on the characteristics of potato starch powders. J. Food Eng. 2012, 108, 515–522. [Google Scholar] [CrossRef]
- Kaur, L.; Singh, N.; Singh, J. Factors influencing the properties of hydroxypropylated potato starches. Carbohydr. Polym. 2004, 55, 211–223. [Google Scholar] [CrossRef]
- Hong, J.; Zeng, X.; Buckow, R.; Han, Z. Structural, thermodynamic and digestible properties of maize starches esterified by conventional and dual methods: Differentiation of amylose contents. Food Hydrocoll. 2018, 83, 419–429. [Google Scholar] [CrossRef]
- Ma, X.; Chang, P.; Yu, J.; Stumborg, M. Properties of biodegradable citirc acid-modified granular starch/thermoplastic pea starch composites. Carbohydr. Polym. 2009, 75, 1–8. [Google Scholar] [CrossRef]
- Kawabata, A.; Takase, N.; Miyoshi, E.; Sawayama, S. Microscopic observation and x-ray diffractometry of heat/moisture-treated starch granules. Starch 1994, 46, 463–469. [Google Scholar] [CrossRef]
- Garg, S.; Jana, A.K. Characterization and evaluation of acylated starch with different acyl groups and degrees of substitution. Carbohydr. Polym. 2011, 83, 1623–1630. [Google Scholar] [CrossRef]
- Diop, C.I.K.; Li, H.; Xie, B.; Shi, J. Effects of acetic acid/acetic anhydride ratios on the properties of corn starch acetates. Food Chem. 2011, 126, 1662–1669. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, J.; Xu, J.; Mao, D. The impact of heat-moisture treatment on the molecular structure and physicochemical properties of Coix seed starches. Starch 2016, 68, 662–674. [Google Scholar] [CrossRef]
- Altuna, L.; Herrera, M.L.; Foresti, M.L. Synthesis and characterization of octenyl succinic anhydride modified starches for food applications. A review of recent literature. Food Hydrocoll. 2018, 80, 97–110. [Google Scholar] [CrossRef]
- Zhang, L.; Xie, W.; Zhao, X.; Liu, Y.; Gao, W. Study on the morphology, crystalline structure and thermal properties of yellow ginger starch acetates with different degrees of substitution. Thermochim. Acta 2009, 495, 57–62. [Google Scholar] [CrossRef]
- Xu, Y.; Miladinov, V.; Hanna, M. Synthesis and characterization of starch acetates with high substitution. Cereal Chem. 2004, 81, 735–740. [Google Scholar] [CrossRef]
- Li, M.; Xie, Y.; Chen, H.; Zhang, B. Effects of heat-moisture treatment after citric acid esterification on structural properties and digestibility of wheat starch, A- and B-type starch granules. Food Chem. 2019, 272, 523–529. [Google Scholar] [CrossRef] [PubMed]
- Dontulwar, J.R.; Borikar, D.K.; Gogte, B.B. An esteric polymer synthesis and its characterization using starch, glycerol and maleic anhydride as precursor. Carbohydr. Polym. 2006, 65, 207–210. [Google Scholar] [CrossRef]
- Barud, H.S.; Ribeiro, C.A.; Crespi, M.S.; Martines, M.A.U.; Dexpert-Ghys, J.; Marques, R.F.C.; Messaddeq, Y.; Ribeiro, S.J.L. Thermal characterization of bacterial cellulose-phosphate composite membranes. J. Therm. Anal. Calorim. 2007, 87, 815–818. [Google Scholar] [CrossRef]
- Thiebaud, S.; Aburto, J.; Alric, I.; Borredon, E. Properties of fatty-acid esters of starch and their blends with LDPE. J. Appl. Polym. Sci. 2015, 65, 705–721. [Google Scholar] [CrossRef]
- Cyras, V.P.; Zenklusen, M.C.T.; Vazquez, A. Relationship between structure and properties of modified potato starch biodegradable films. J. Appl. Polym. Sci. 2006, 101, 4313–4319. [Google Scholar] [CrossRef]

| Samples | RDS (%) | SDS (%) | RS (%) |
|---|---|---|---|
| Native corn Starch | 79.27 ± 3.83 a† | 5.50 ± 4.18 c | 15.22 ± 3.53 h |
| Malate corn starch-2h | 33.63 ± 1.10 d | 2.75 ± 1.34 d | 63.61 ± 2.18 f |
| Malate corn starch-3h | 19.52 ± 0.66 f | 2.22 ± 0.28 d | 78.26 ± 0.93 d |
| Malate corn starch-4h | 9.61 ± 0.77 h | 0.71 ± 0.43 d | 89.68 ± 0.43 b |
| Malate corn starch-5h | 7.11 ± 0.61 ij | 0.92 ± 0.67 d | 91.97 ± 0.20 ab |
| Native potato Starch | 42.73 ± 1.36 c | 36.10 ± 3.32 a | 21.18 ± 4.32 g |
| Malate potato starch-2h | 35.74 ± 1.09 d | 1.30 ± 0.19 d | 62.96 ± 0.91 f |
| Malate potato starch-3h | 16.37 ± 0.38 g | 1.27 ± 0.47 d | 82.35 ± 0.73 c |
| Malate potato starch-4h | 14.68 ± 0.20 g | 1.57 ± 0.52 d | 83.75 ± 0.71 c |
| Malate potato starch-5h | 8.71 ± 0.68 hi | 0.80 ± 0.50 d | 90.49 ± 0.33 b |
| Native pea Starch | 66.36 ± 2.49 b | 14.12 ± 2.25 b | 19.53 ± 4.43 g |
| Malate pea starch-2h | 26.88 ± 0.51 e | 1.56 ± 0.29 d | 71.56 ± 0.80 e |
| Malate pea starch-3h | 6.20 ± 0.32 jk | 0.87 ± 0.09 d | 92.93 ± 0.31 ab |
| Malate pea starch-4h | 5.05 ± 0.27 jk | 0.79 ± 0.47 d | 94.16 ± 0.48 a |
| Malate pea starch-5h | 4.02 ± 0.25 l | 0.74 ± 0.51 d | 95.23 ± 0.27 a |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, M.; Jing, Y.; Yang, L.; Huang, X.; Wang, H.; Yan, Y.; Liu, Y. Structure and Physicochemical Properties of Malate Starches from Corn, Potato, and Wrinkled Pea Starches. Polymers 2019, 11, 1523. https://doi.org/10.3390/polym11091523
Shi M, Jing Y, Yang L, Huang X, Wang H, Yan Y, Liu Y. Structure and Physicochemical Properties of Malate Starches from Corn, Potato, and Wrinkled Pea Starches. Polymers. 2019; 11(9):1523. https://doi.org/10.3390/polym11091523
Chicago/Turabian StyleShi, Miaomiao, Yue Jing, Liuzhi Yang, Xianqing Huang, Hongwei Wang, Yizhe Yan, and Yanqi Liu. 2019. "Structure and Physicochemical Properties of Malate Starches from Corn, Potato, and Wrinkled Pea Starches" Polymers 11, no. 9: 1523. https://doi.org/10.3390/polym11091523






