Mechanistic Insight into the Ring-Opening Polymerization of ε-Caprolactone and L-Lactide Using Ketiminate-Ligated Aluminum Catalysts
Abstract
:1. Introduction
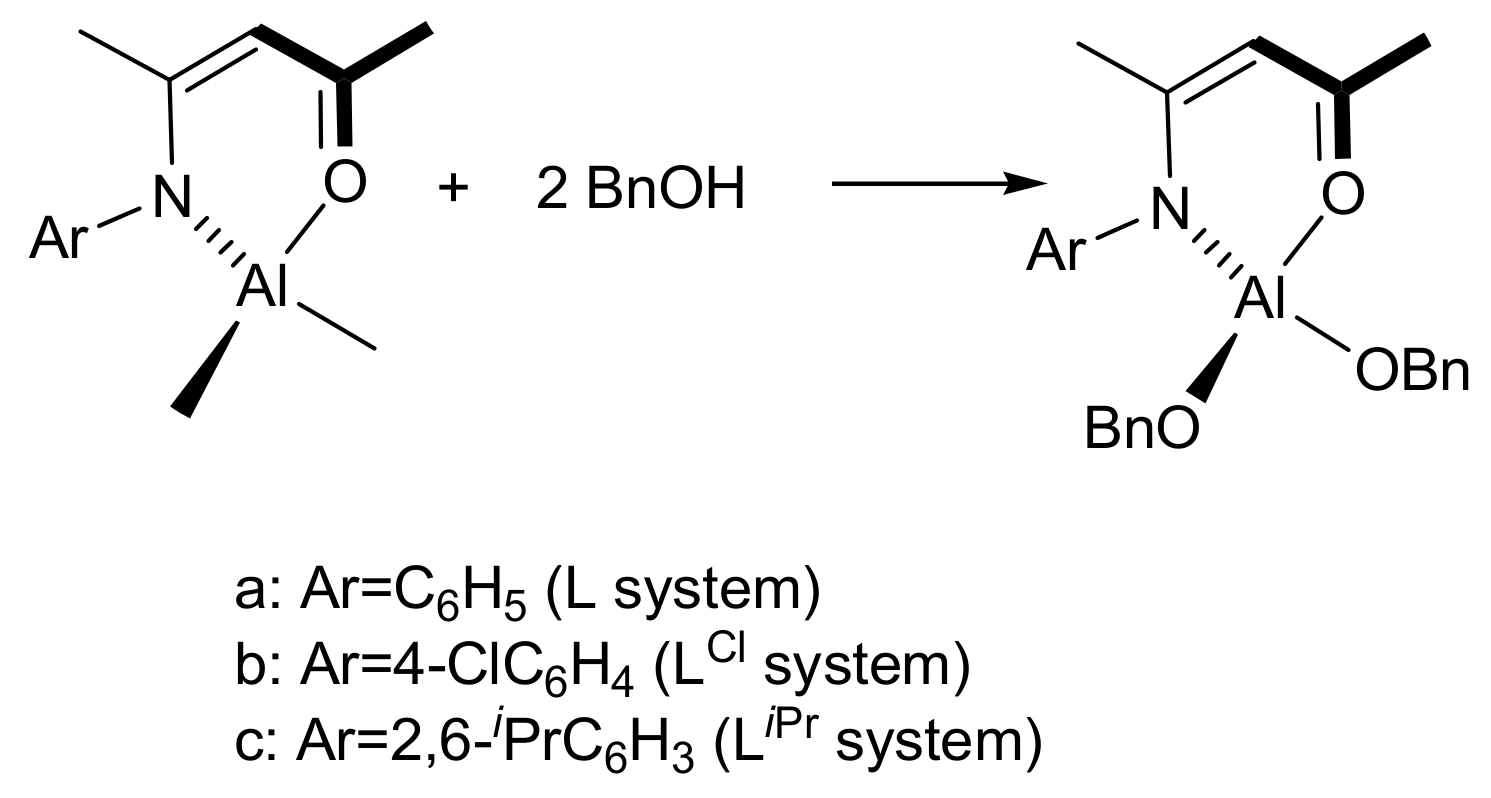
2. Materials and Methods
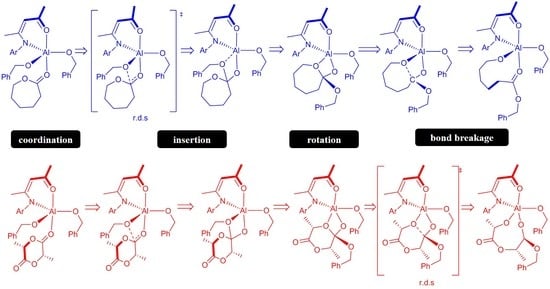
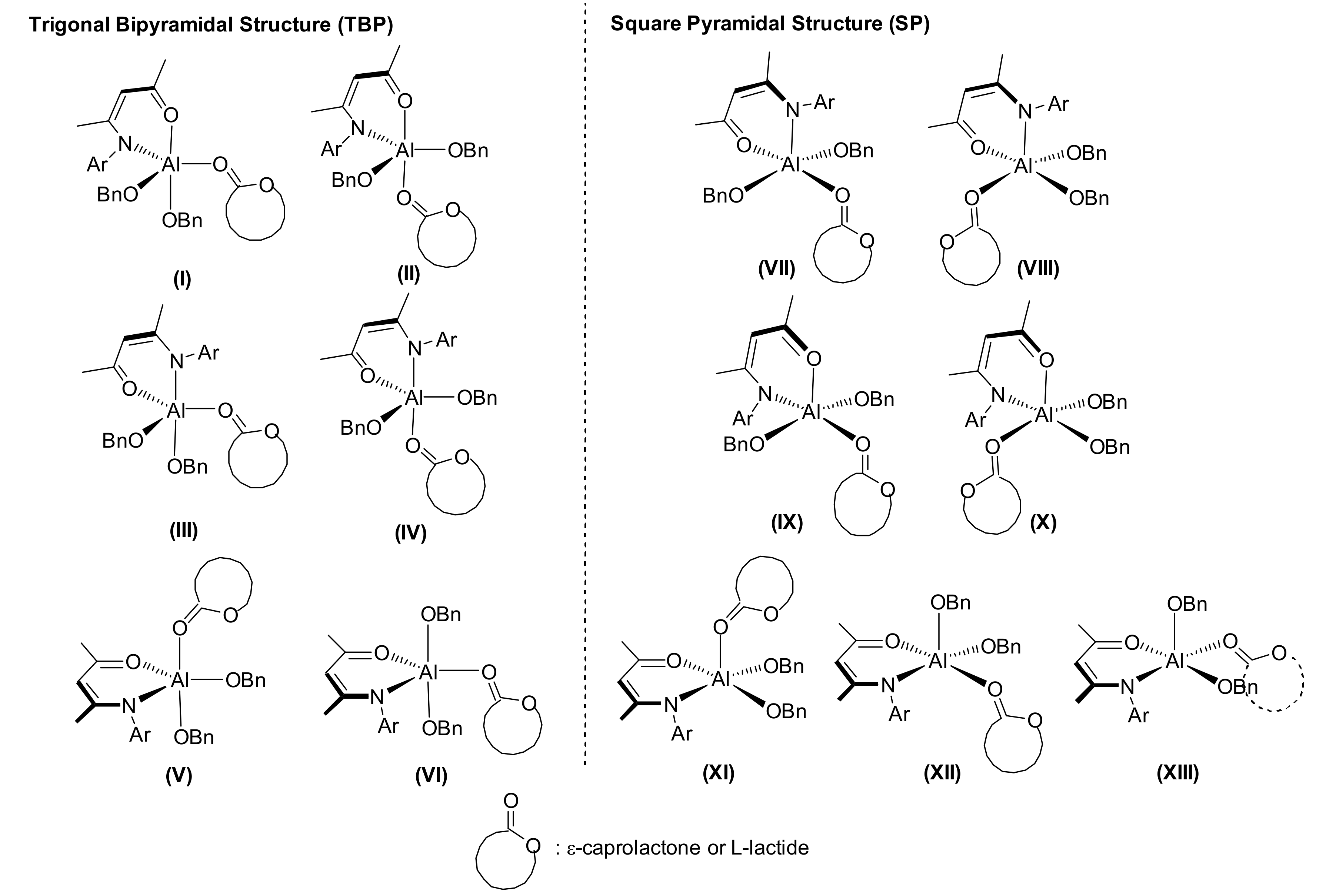
3. Results and Discussion
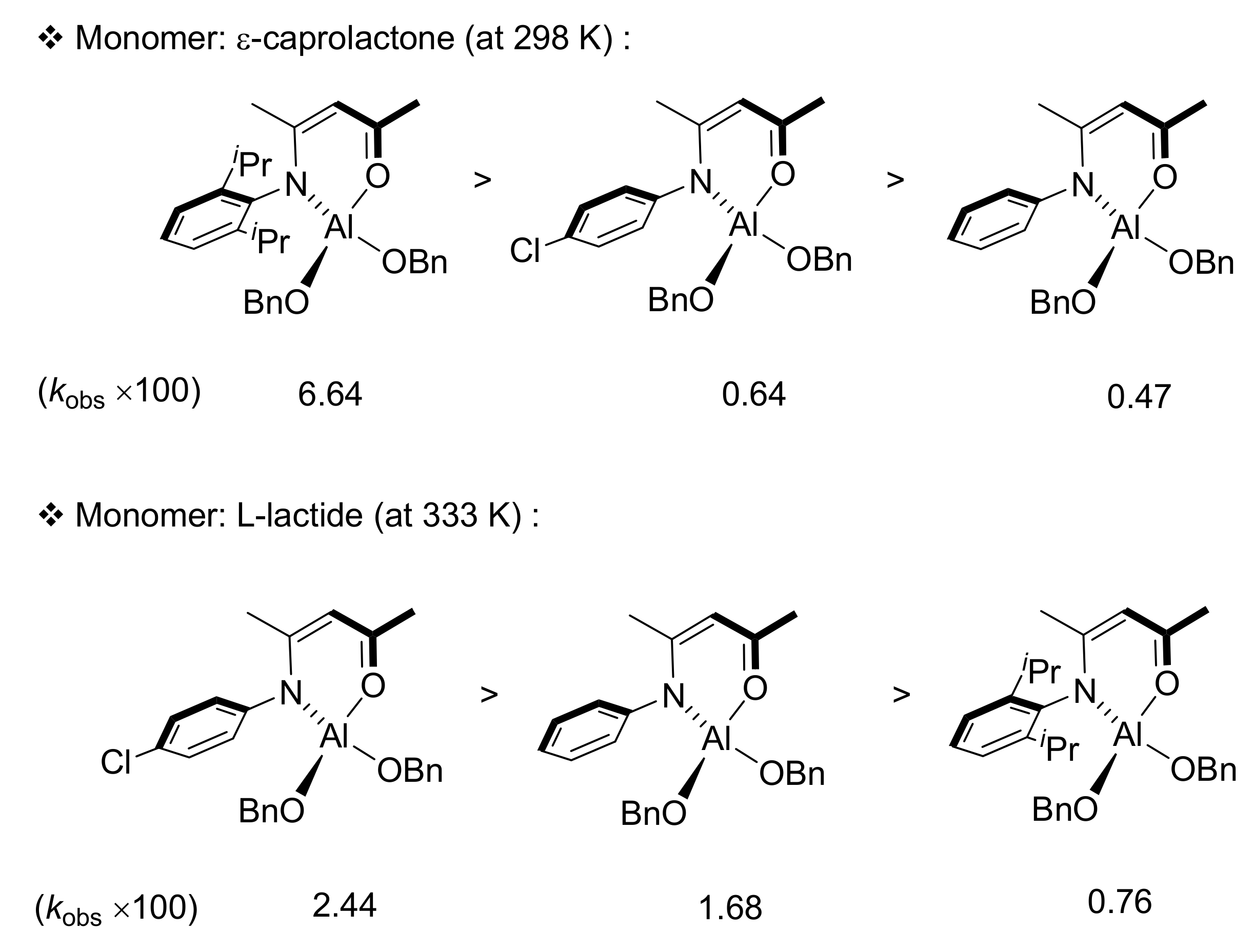
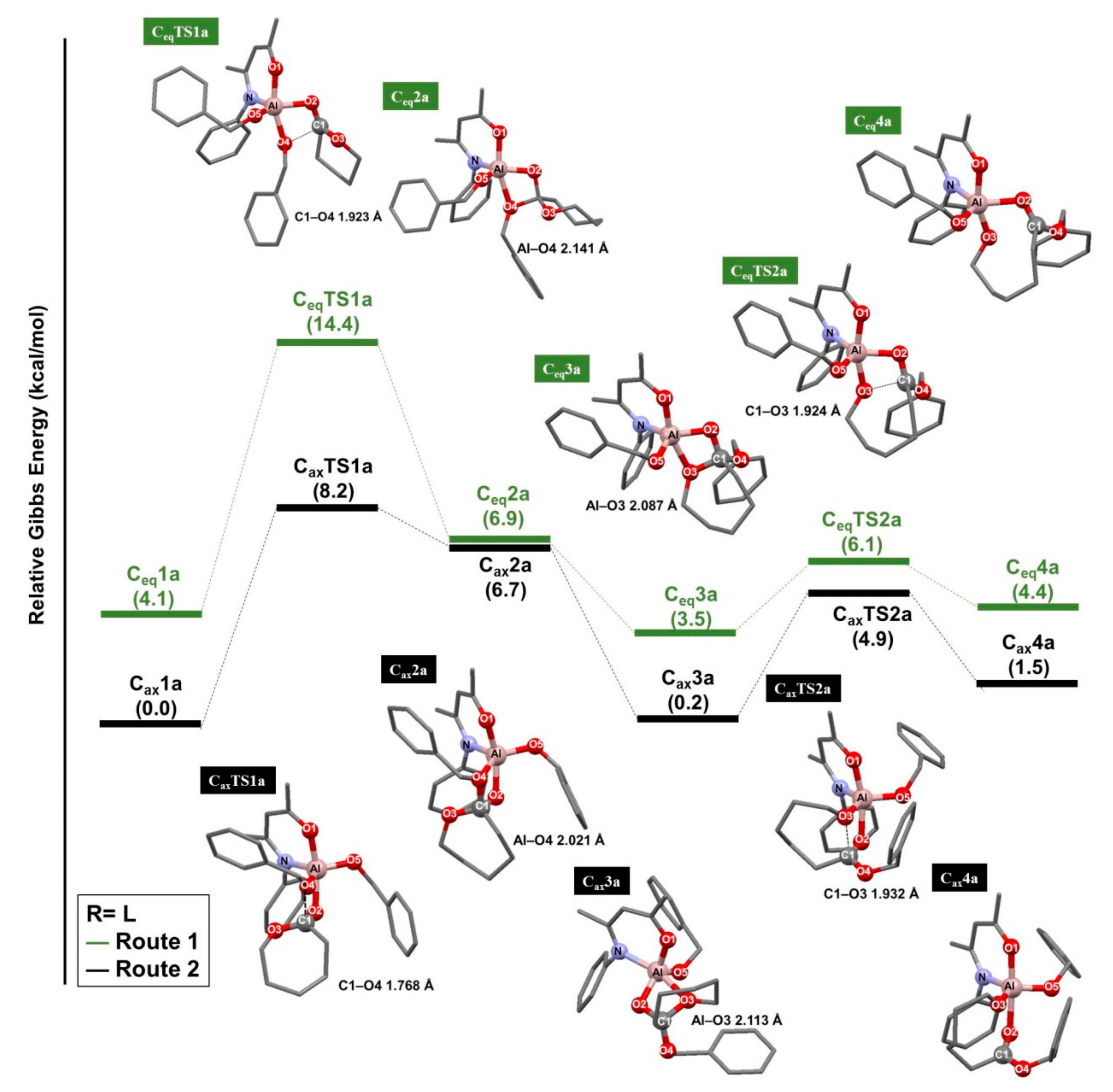
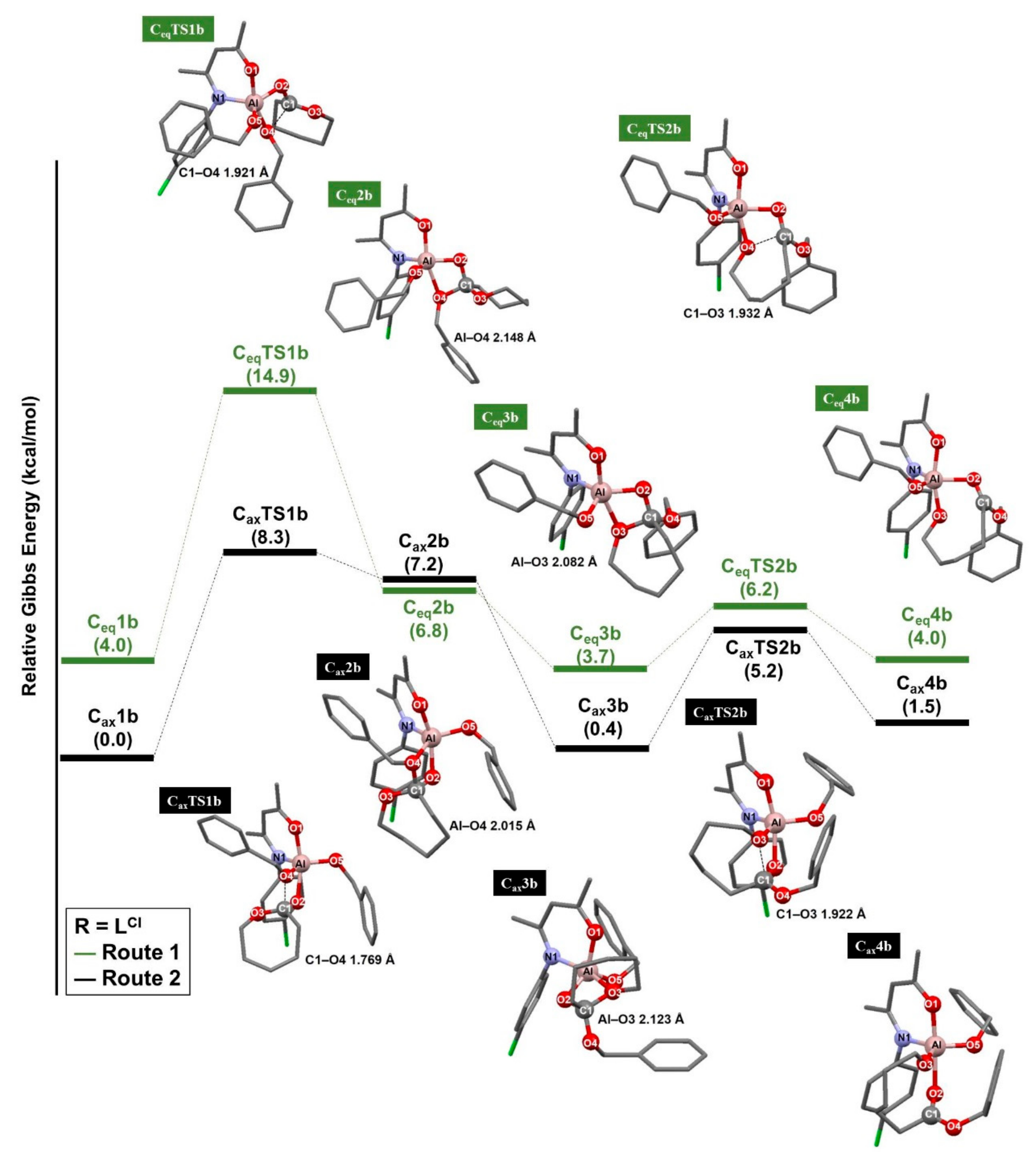
3.1. Ring-Opening Polymerization of ε-Caprolactone
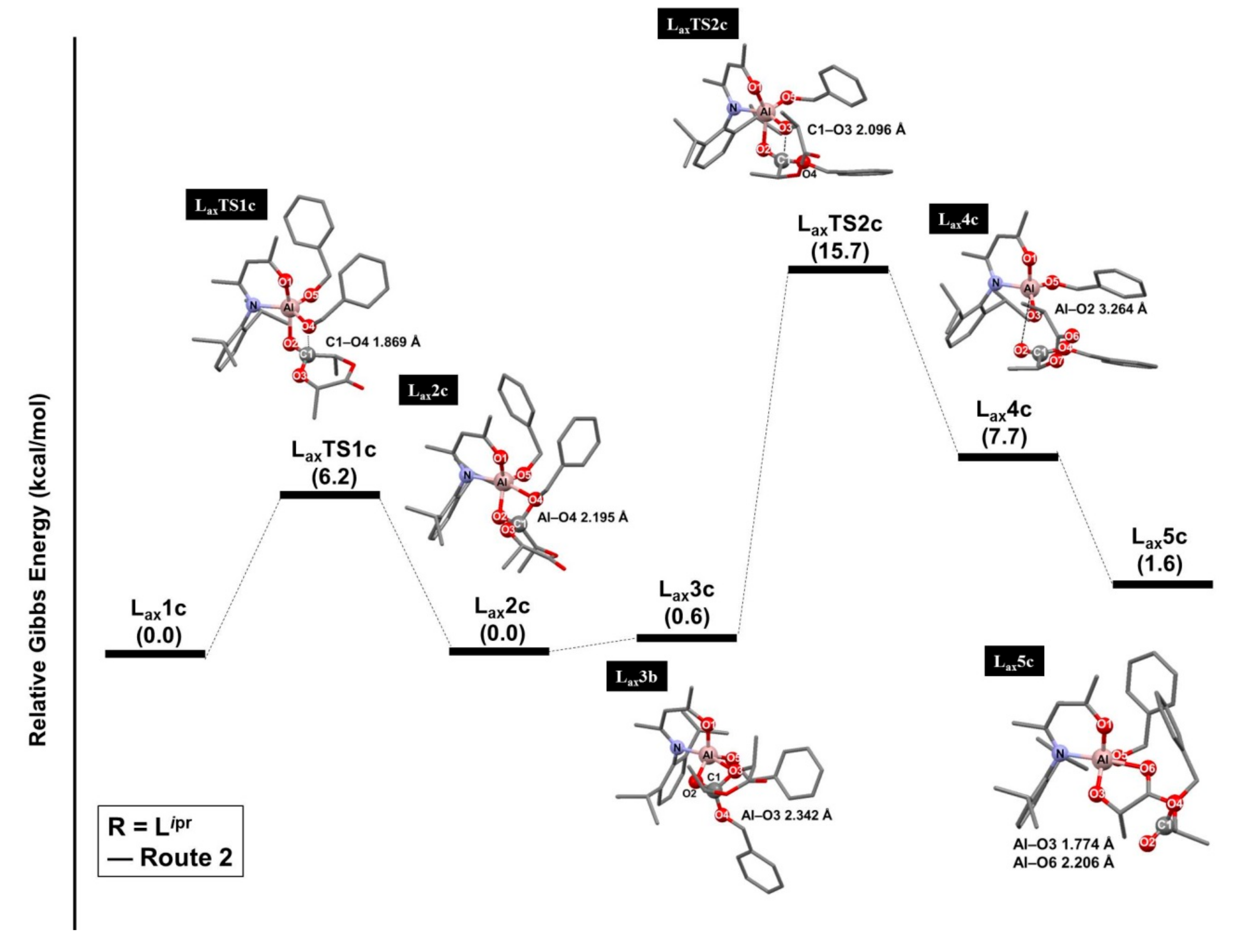
3.2. Ring-Opening Polymerization of L-Lactide
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Chiellini, E.; Solaro, R. Biodegradable Polymeric Materials. Adv. Mater. 1996, 8, 305–313. [Google Scholar] [CrossRef]
- Drumright, R.E.; Gruber, P.R.; Henton, D.E. Polylactic Acid Technology. Adv. Mater. 2000, 12, 1841–1846. [Google Scholar] [CrossRef]
- Albertsson, A.-C.; Varma, I.K. Recent Developments in Ring Opening Polymerization of Lactones for Biomedical Applications. Biomacromolecules 2003, 4, 1466–1486. [Google Scholar] [CrossRef] [PubMed]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef] [PubMed]
- Faÿ, F.; Renard, E.; Langlois, V.; Linossier, I.; Vallée-Rehel, K. Development of Poly(ε-caprolactone-co-l-lactide) and Poly(ε-caprolactone-co-δ-valerolactone) as New Degradable Binder Used for Antifouling Paint. Eur. Polym. J. 2007, 43, 4800–4813. [Google Scholar] [CrossRef]
- Slomkowski, S.; Penczek, S.; Duda, A. Polylactides–an Overview. Polym. Adv. Technol. 2014, 25, 436–447. [Google Scholar] [CrossRef]
- Nomura, N.; Ishii, R.; Akakura, M.; Aoi, K. Stereoselective Ring-Opening Polymerization of Racemic Lactide Using Aluminum-Achiral Ligand Complexes: Exploration of a Chain-End Control Mechanism. J. Am. Chem. Soc. 2002, 124, 5938–5939. [Google Scholar] [CrossRef]
- Zhong, Z.; Dijkstra, P.J.; Feijen, J. [(salen)Al]-Mediated, Controlled and Stereoselective Ring-Opening Polymerization of Lactide in Solution and without Solvent: Synthesis of Highly Isotactic Polylactide Stereocopolymers from Racemic d,l-Lactide. Angew. Chem. Int. Ed. 2002, 41, 4510–4513. [Google Scholar] [CrossRef]
- Alcazar-Roman, L.M.; O’Keefe, B.J.; Hillmyer, M.A.; Tolman, W.B. Electronic Influence of Ligand Substituents on The Rate of Polymerization of ε-Caprolactone by Single-Site Aluminium Alkoxide Catalysts. Dalton Trans 2003, 3082–3087. [Google Scholar] [CrossRef]
- Hormnirun, P.; Marshall, E.L.; Gibson, V.C.; White, A.J.P.; Williams, D.J. Remarkable Stereocontrol in the Polymerization of Racemic Lactide Using Aluminum Initiators Supported by Tetradentate Aminophenoxide Ligands. J. Am. Chem. Soc. 2004, 126, 2688–2689. [Google Scholar] [CrossRef]
- Ishii, R.; Nomura, N.; Kondo, T. Stereoselective Bulk Polymerization of Racemic Lactide for Stereoblock Poly(racemic lactide) Using an Achiral Aluminum Complex. Polym. J. 2004, 36, 261–264. [Google Scholar] [CrossRef] [Green Version]
- Hormnirun, P.; Marshall, E.L.; Gibson, V.C.; Pugh, R.I.; White, A.J.P. Study of Ligand Substituent Effects on the Rate and Stereoselectivity of Lactide Polymerization Using Aluminum Salen-Type Initiators. Proc. Natl. Acad. Sci. USA 2006, 103, 15343–15348. [Google Scholar] [CrossRef] [PubMed]
- Nomura, N.; Ishii, R.; Yamamoto, Y.; Kondo, T. Stereoselective Ring-Opening Polymerization of a Racemic Lactide by Using Achiral Salen and Homosalen-Aluminum Complexes. Chem. Eur. J. 2007, 13, 4433–4451. [Google Scholar] [CrossRef] [PubMed]
- Chisholm, M.H.; Gallucci, J.C.; Quisenberry, K.T.; Zhou, Z. Complexities in the Ring-Opening Polymerization of Lactide by Chiral Salen Aluminum Initiators. Inorg. Chem. 2008, 47, 2613–2624. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.; Zhang, W.; Nomura, K.; Sun, W.-H. Synthesis and Characterization of Organoaluminum Compounds Containing Quinolin-8-amine Derivatives and Their Catalytic Behaviour for Ring-Opening Polymerization of ε-Caprolactone. Dalton Trans. 2009, 41, 9000–9009. [Google Scholar] [CrossRef]
- Darensbourg, D.J.; Karroonnirun, O. Stereoselective Ring-Opening Polymerization of rac-Lactides Catalyzed by Chiral and Achiral Aluminum Half-Salen Complexes. Organometallics 2010, 29, 5627–5634. [Google Scholar] [CrossRef]
- Sun, W.-H.; Shen, M.; Zhang, W.; Huang, W.; Liu, S.; Redshaw, C. Methylaluminium 8-quinolinolates: Synthesis, characterization and use in ring-opening polymerization (ROP) of ε-caprolactone. Dalton Trans. 2011, 40, 2645–2653. [Google Scholar] [CrossRef]
- Whitelaw, E.L.; Loraine, G.; Mahon, M.F.; Jones, M.D. Salalen Aluminium Complexes and their Exploitation for the Ring Opening Polymerisation of rac-Lactide. Dalton Trans. 2011, 40, 11469–11473. [Google Scholar] [CrossRef]
- Bakewell, C.; Platel, R.H.; Cary, S.K.; Hubbard, S.M.; Roaf, J.M.; Levine, A.C.; White, A.J.P.; Long, N.J.; Haaf, M.; Williams, C.K. Bis(8-quinolinolato)aluminum ethyl Complexes: Iso-Selective Initiators for rac-Lactide Polymerization. Organometallics 2012, 31, 4729–4736. [Google Scholar] [CrossRef]
- Chen, H.-L.; Dutta, S.; Huang, P.-Y.; Lin, C.-C. Preparation and Characterization of Aluminum Alkoxides Coordinated on salen-Type Ligands: Highly Stereoselective Ring-Opening Polymerization of rac-Lactide. Organometallics 2012, 31, 2016–2025. [Google Scholar] [CrossRef]
- Ikpo, N.; Barbon, S.M.; Drover, M.W.; Dawe, L.N.; Kerton, F.M. Aluminum Methyl and Chloro Complexes Bearing Monoanionic Aminephenolate Ligands: Synthesis, Characterization, and Use in Polymerizations. Organometallics 2012, 31, 8145–8158. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Alonso-Moreno, C.; Márquez-Segovia, I.; Otero, A.; Lara-Sánchez, A.; Fernández-Baeza, J.; Rodríguez, A.M.; Sánchez-Barba, L.F.; García-Martínez, J.C. Synthesis, Structural Characterization and Catalytic Evaluation of the Ring-Opening Polymerization of Discrete Five-Coordinate Alkyl Aluminium Complexes. Dalton Trans. 2013, 42, 9325–9337. [Google Scholar] [CrossRef] [PubMed]
- Gao, B.; Duan, R.; Pang, X.; Li, X.; Qu, Z.; Tang, Z.; Zhuang, X.; Chen, X. Stereoselective Ring-Opening Polymerization of rac-Lactides Catalyzed by Aluminum Hemi-Salen Complexes. Organometallics 2013, 32, 5435–5444. [Google Scholar] [CrossRef]
- Hancock, S.L.; Mahon, M.F.; Jones, M.D. Aluminium Salalen Complexes Based on 1,2-Diaminocyclohexane and their Exploitation for the Polymerisation of rac-Lactide. Dalton Trans. 2013, 42, 9279–9285. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, H. Aluminum Complexes with Bidentate Amido Ligands: Synthesis, Structure and Performance on Ligand-Initiated Ring-Opening Polymerization of rac-Lactide. Dalton Trans. 2014, 43, 9098–9110. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, Y.; Wang, L.; Redshaw, C.; Sun, W.-H. Dialkylaluminium 2-Imidazolylphenolates: Synthesis, Characterization and Ring-Opening Polymerization Behavior Towards Lactides. J. Organomet. Chem. 2014, 750, 65–73. [Google Scholar] [CrossRef]
- Tabthong, S.; Nanok, T.; Sumrit, P.; Kongsaeree, P.; Prabpai, S.; Chuawong, P.; Hormnirun, P. Bis(pyrrolidene) Schiff Base Aluminum Complexes as Isoselective-Biased Initiators for the Controlled Ring-Opening Polymerization of rac-Lactide: Experimental and Theoretical Studies. Macromolecules 2015, 48, 6846–6861. [Google Scholar] [CrossRef]
- Fuoco, T.; Pappalardo, D. Aluminum Alkyl Complexes Bearing Salicylaldiminato Ligands: Versatile Initiators in the Ring-Opening Polymerization of Cyclic Esters. Catalysts 2017, 7, 64. [Google Scholar] [CrossRef]
- Nakonkhet, C.; Nanok, T.; Wattanathana, W.; Chuawong, P.; Hormnirun, P. Aluminium Complexes Containing Salicylbenzothiazole Ligands and their Application in the Ring-Opening Polymerisation of rac-Lactide and ε-Caprolactone. Dalton Trans. 2017, 46, 11013–11030. [Google Scholar] [CrossRef]
- Huang, H.-C.; Wang, B.; Chen, X.-L.; Pan, L.; Li, Y.-S. Ring-Opening Polymerization of (Macro)lactones by Highly Active Mononuclear Salen-Aluminum Complexes Bearing Cyclic β-Ketoiminato Ligand. J. Polym. Sci. Polym. Chem. 2019, 57, 973–981. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Berl, M.; Scharnagl, N. Poly(lactones). 9. Polymerization Mechanism of Metal Alkoxide Initiated Polymerizations of Lactide and Various Lactones. Macromolecules 1988, 21, 286–293. [Google Scholar] [CrossRef]
- Dubois, P.; Barakat, I.; Jerome, R.; Teyssie, P. Macromolecular Engineering of Polyactones and Polyactides. 12. Study of the Depolymerization Reactions of Poly(.epsilon-caprolactone) with Functional Aluminum Alkoxide end Groups. Macromolecules 1993, 26, 4407–4412. [Google Scholar] [CrossRef]
- Yu, R.-C.; Hung, C.-H.; Huang, J.-H.; Lee, H.-Y.; Chen, J.-T. Four- and Five-Coordinate Aluminum Ketiminate Complexes: Synthesis, Characterization, and Ring-Opening Polymerization. Inorg. Chem. 2002, 41, 6450–6455. [Google Scholar] [CrossRef] [PubMed]
- Tabthong, S.; Nanok, T.; Kongsaeree, P.; Prabpai, S.; Hormnirun, P. Monomethylaluminum and Dimethylaluminum Pyrrolylaldiminates for the Ring-Opening Polymerization of rac-Lactide: Effects of Ligand Structure and Coordination Geometry. Dalton Trans. 2014, 43, 1348–1359. [Google Scholar] [CrossRef] [PubMed]
- Sumrit, P.; Chuawong, P.; Nanok, T.; Duangthongyou, T.; Hormnirun, P. Aluminum Complexes Containing Salicylbenzoxazole Ligands and their Application in the Ring-Opening Polymerization of rac-Lactide and ε-Caprolactone. Dalton Trans. 2016, 45, 9250–9266. [Google Scholar] [CrossRef]
- Lee, C.-L.; Lin, Y.-F.; Jiang, M.-T.; Lu, W.-Y.; Vandavasi, J.K.; Wang, L.-F.; Lai, Y.-C.; Chiang, M.Y.; Chen, H.-Y. Improvement in Aluminum Complexes Bearing Schiff Bases in Ring-Opening Polymerization of ε-Caprolactone: A Five-Membered-Ring System. Organometallics 2017, 36, 1936–1945. [Google Scholar] [CrossRef]
- Nomura, N.; Akita, A.; Ishii, R.; Mizuno, M. Random Copolymerization of ε-Caprolactone with Lactide Using a Homosalen−Al Complex. J. Am. Chem. Soc. 2010, 132, 1750–1751. [Google Scholar] [CrossRef] [PubMed]
- Koller, J.; Bergman, R.G. Highly Efficient Aluminum-Catalyzed Ring-Opening Polymerization of Cyclic Carbonates, Lactones, and Lactides, Including a Unique Crystallographic Snapshot of an Intermediate. Organometallics 2011, 30, 3217–3224. [Google Scholar] [CrossRef]
- Lamberti, M.; D’Auria, I.; Mazzeo, M.; Milione, S.; Bertolasi, V.; Pappalardo, D. Phenoxy-Thioether Aluminum Complexes as ε-Caprolactone and Lactide Polymerization Catalysts. Organometallics 2012, 31, 5551–5560. [Google Scholar] [CrossRef]
- Hild, F.; Neehaul, N.; Bier, F.; Wirsum, M.; Gourlaouen, C.; Dagorne, S. Synthesis and Structural Characterization of Various N,O,N-Chelated Aluminum and Gallium Complexes for the Efficient ROP of Cyclic Esters and Carbonates: How Do Aluminum and Gallium Derivatives Compare? Organometallics 2013, 32, 587–598. [Google Scholar] [CrossRef]
- McKeown, P.; Davidson, M.G.; Kociok-Köhn, G.; Jones, M.D. Aluminium Salalens vs. Salans: “Initiator Design” for the Isoselective Polymerisation of rac-Lactide. Chem. Commun. 2016, 52, 10431–10434. [Google Scholar] [CrossRef] [PubMed]
- Stasiw, D.E.; Mandal, M.; Neisen, B.D.; Mitchell, L.A.; Cramer, C.J.; Tolman, W.B. Why So Slow? Mechanistic Insights from Studies of a Poor Catalyst for Polymerization of ε-Caprolactone. Inorg. Chem. 2017, 56, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Beament, J.; Mahon, M.F.; Buchard, A.; Jones, M.D. Aluminum Complexes of Monopyrrolidine Ligands for the Controlled Ring-Opening Polymerization of Lactide. Organometallics 2018, 37, 1719–1724. [Google Scholar] [CrossRef]
- Macaranas, J.A.; Luke, A.M.; Mandal, M.; Neisen, B.D.; Marell, D.J.; Cramer, C.J.; Tolman, W.B. Sterically Induced Ligand Framework Distortion Effects on Catalytic Cyclic Ester Polymerizations. Inorg. Chem. 2018, 57, 3451–3457. [Google Scholar] [CrossRef]
- Ropson, N.; Dubois, P.; Jerome, R.; Teyssie, P. Macromolecular Engineering of Polylactones and Polylactides. 20. Effect of Monomer, Solvent, and Initiator on the Ring-Opening Polymerization as Initiated with Aluminum Alkoxides. Macromolecules 1995, 28, 7589–7598. [Google Scholar] [CrossRef]
- Lewiński, J.; Horeglad, P.; Wójcik, K.; Justyniak, I. Chelation Effect in Polymerization of Cyclic Esters by Metal Alkoxides: Structure Characterization of the Intermediate Formed by Primary Insertion of Lactide into the Al−OR Bond of an Organometallic Initiator. Organometallics 2005, 24, 4588–4593. [Google Scholar] [CrossRef]
- Parenté, V.; Brédas, J.-L.; Dubois, P.; Ropson, N.; Jérôme, R. Theoretical Investigation of the Molecular Structure of Aluminium Triisopropoxide and its Complexes in Ring-Opening Polymerization. Macromol. Theory Simul. 1996, 5, 525–546. [Google Scholar] [CrossRef]
- Chen, Y.-H.; Chen, Y.-J.; Tseng, H.-C.; Lian, C.-J.; Tsai, H.-Y.; Lai, Y.-C.; Hsu, S.C.N.; Chiang, M.Y.; Chen, H.-Y. Comparing l-Lactide and ε-Caprolactone Polymerization by Using Aluminum Complexes Bearing Ketiminate Ligands: Steric, Electronic, and Chelating Effects. RSC Adv. 2015, 5, 100272–100280. [Google Scholar] [CrossRef]
- Tseng, H.-C.; Chiang, M.Y.; Lu, W.-Y.; Chen, Y.-J.; Lian, C.-J.; Chen, Y.-H.; Tsai, H.-Y.; Lai, Y.-C.; Chen, H.-Y. A Closer Look at ε-Caprolactone Polymerization Catalyzed by Alkyl Aluminum Complexes: The Effect of Induction Period on Overall Catalytic Activity. Dalton Trans. 2015, 44, 11763–11773. [Google Scholar] [CrossRef]
- Chang, M.-C.; Lu, W.-Y.; Chang, H.-Y.; Lai, Y.-C.; Chiang, M.Y.; Chen, H.-Y.; Chen, H.-Y. Comparative Study of Aluminum Complexes Bearing N,O- and N,S-Schiff Base in Ring-Opening Polymerization of ε-Caprolactone and l-Lactide. Inorg. Chem. 2015, 54, 11292–11298. [Google Scholar] [CrossRef]
- Chang, C.-C.; Chang, Y.-C.; Lu, W.-Y.; Lai, Y.-C.; Wu, K.-H.; Lin, Y.-F.; Chen, H.-Y. Novel Aluminum Complexes Bearing 2-(Aminomethylene)malonate Ligands with High Efficiency and Controllability in Ring-Opening Polymerization of ε-Caprolactone. Eur. Polym. J. 2019, 115, 399–408. [Google Scholar] [CrossRef]
- Mandal, M.; Luke, A.M.; Dereli, B.; Elwell, C.E.; Reineke, T.M.; Tolman, W.B.; Cramer, C.J. Computational Prediction and Experimental Verification of ε-Caprolactone Ring-Opening Polymerization Activity by an Aluminum Complex of an Indolide/Schiff-Base Ligand. ACS Catal. 2019, 9, 885–889. [Google Scholar] [CrossRef]
- Von Schenck, H.; Ryner, M.; Albertsson, A.-C.; Svensson, M. Ring-Opening Polymerization of Lactones and Lactides with Sn(IV) and Al(III) Initiators. Macromolecules 2002, 35, 1556–1562. [Google Scholar] [CrossRef]
- Marshall, E.L.; Gibson, V.C.; Rzepa, H.S. A Computational Analysis of the Ring-Opening Polymerization of rac-Lactide Initiated by Single-Site β-Diketiminate Metal Complexes: Defining the Mechanistic Pathway and the Origin of Stereocontrol. J. Am. Chem. Soc. 2005, 127, 6048–6051. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.O.; DePorre, Y.; Vazquez-Lima, H.; Johnson, M.A.; Marell, D.J.; Cramer, C.J.; Tolman, W.B. Understanding the Mechanism of Polymerization of ε-Caprolactone Catalyzed by Aluminum Salen Complexes. Inorg. Chem. 2013, 52, 13692–13701. [Google Scholar] [CrossRef] [PubMed]
- Vieira, I.d.S.; Whitelaw, E.L.; Jones, M.D.; Herres-Pawlis, S. Synergistic Empirical and Theoretical Study on the Stereoselective Mechanism for the Aluminum Salalen Complex Mediated Polymerization of rac-Lactide. Chem. Eur. J. 2013, 19, 4712–4716. [Google Scholar] [CrossRef]
- Roymuhury, S.K.; Chakraborty, D.; Ramkumar, V. Aluminium Complexes Bearing N,O-Aminophenol Ligands as Efficient Catalysts for the Ring Opening Polymerization of Lactide. Eur. Polym. J. 2015, 70, 203–214. [Google Scholar] [CrossRef]
- Jitonnom, J.; Molloy, R.; Punyodom, W.; Meelua, W. Theoretical Studies on Aluminum Trialkoxide-Initiated Lactone Ring-Opening Polymerizations: Roles of Alkoxide Substituent and Monomer Ring Structure. Computa. Theor. Chem. 2016, 1097, 25–32. [Google Scholar] [CrossRef]
- Chandanabodhi, D.; Nanok, T. A DFT Study of the Ring-Opening Polymerization Mechanism of l-Lactide and ε-Caprolactone Using aluminium Salen-Type Initiators: Towards an Understanding of their Reactivities in Homo- and Copolymerization. Mol. Catal. 2017, 436, 145–156. [Google Scholar] [CrossRef]
- Robert, C.; Schmid, T.E.; Richard, V.; Haquette, P.; Raman, S.K.; Rager, M.-N.; Gauvin, R.M.; Morin, Y.; Trivelli, X.; Guérineau, V.; et al. Mechanistic Aspects of the Polymerization of Lactide Using a Highly Efficient Aluminum(III) Catalytic System. J. Am. Chem. Soc. 2017, 139, 6217–6225. [Google Scholar] [CrossRef]
- Wei, J.; Riffel, M.N.; Diaconescu, P.L. Redox Control of Aluminum Ring-Opening Polymerization: A Combined Experimental and DFT Investigation. Macromolecules 2017, 50, 1847–1861. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Xi, Y.; Li, L.; Li, H.; Sun, W.-H.; Lei, M. A DFT Study on Ring-Opening Polymerization of ε-Caprolactone Initiated by Mg and Al Complexes. Inorg. Chim. Acta 2018, 477, 34–39. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Petersson, G.A.; Nakatsuji, H.; et al. Gaussian 16 Rev. A.03. Wallingford CT. 2016. Available online: https://gaussian.com/citation (accessed on 14 September 2019).
- Ditchfield, R.; Hehre, W.J.; Pople, J.A. Self-Consistent Molecular-Orbital Methods. IX. An Extended Gaussian-Type Basis for Molecular-Orbital Studies of Organic Molecules. J. Chem. Phys. 1971, 54, 724–728. [Google Scholar] [CrossRef]
- Hehre, W.J.; Ditchfield, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Hariharan, P.C.; Pople, J.A. The influence of Polarization Functions on Molecular Orbital Hydrogenation Energies. Theoretica chimica acta 1973, 28, 213–222. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; DeFrees, D.J.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XXIII. A Polarization-Type Basis set for Second-Row Elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-Functional Thermochemistry. III. the Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Stephens, P.J.; Devlin, F.J.; Chabalowski, C.F.; Frisch, M.J. Ab Initio Calculation of Vibrational Absorption and Circular Dichroism Spectra Using Density Functional Force Fields. J. Chem. Phys. 1994, 98, 11623–11627. [Google Scholar] [CrossRef]
- Grimme, S. Semiempirical GGA-Type Density Functional Constructed with a Long-Range Dispersion Correction. J. Comput. Chem. 2006, 27, 1787–1799. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3094. [Google Scholar] [CrossRef] [PubMed]
- Fukui, K. Formulation of the Reaction Coordinate. J. Phys. Chem. 1970, 74, 4161–4163. [Google Scholar] [CrossRef]
- Fukui, K. The Path of Chemical Reactions-the IRC Approach. Acc. Chem. Res. 1981, 14, 363–368. [Google Scholar] [CrossRef]
- Gonzalez, C.; Schlegel, H.B. Reaction Path Following in Aass-Weighted Internal Voordinates. J. Phys. Chem. 1990, 94, 5523–5527. [Google Scholar] [CrossRef]
- Maeda, S.; Harabuchi, Y.; Ono, Y.; Taketsugu, T.; Morokuma, K. Intrinsic Reaction Coordinate: Calculation, Bifurcation, and Automated Search. Int. J. Quantum Chem. 2015, 115, 258–269. [Google Scholar] [CrossRef]
- Chan, M.S.W.; Deng, L.; Ziegler, T. Density Functional Study of Neutral Salicylaldiminato Nickel(II) Complexes as Olefin Polymerization Catalysts. Organometallics 2000, 19, 2741–2750. [Google Scholar] [CrossRef]






© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Y.-F.; Jheng, N.-Y. Mechanistic Insight into the Ring-Opening Polymerization of ε-Caprolactone and L-Lactide Using Ketiminate-Ligated Aluminum Catalysts. Polymers 2019, 11, 1530. https://doi.org/10.3390/polym11091530
Lin Y-F, Jheng N-Y. Mechanistic Insight into the Ring-Opening Polymerization of ε-Caprolactone and L-Lactide Using Ketiminate-Ligated Aluminum Catalysts. Polymers. 2019; 11(9):1530. https://doi.org/10.3390/polym11091530
Chicago/Turabian StyleLin, Ya-Fan, and Nai-Yuan Jheng. 2019. "Mechanistic Insight into the Ring-Opening Polymerization of ε-Caprolactone and L-Lactide Using Ketiminate-Ligated Aluminum Catalysts" Polymers 11, no. 9: 1530. https://doi.org/10.3390/polym11091530