Validation of Nanoparticle Response to the Sound Pressure Effect during the Drug-Delivery Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sound Pressure Computational Model of Nanoparticle Drug
2.2. Experimental Validation of the Proposed Nanotechnology-Based Drug-Delivery System
3. Results and Discussion
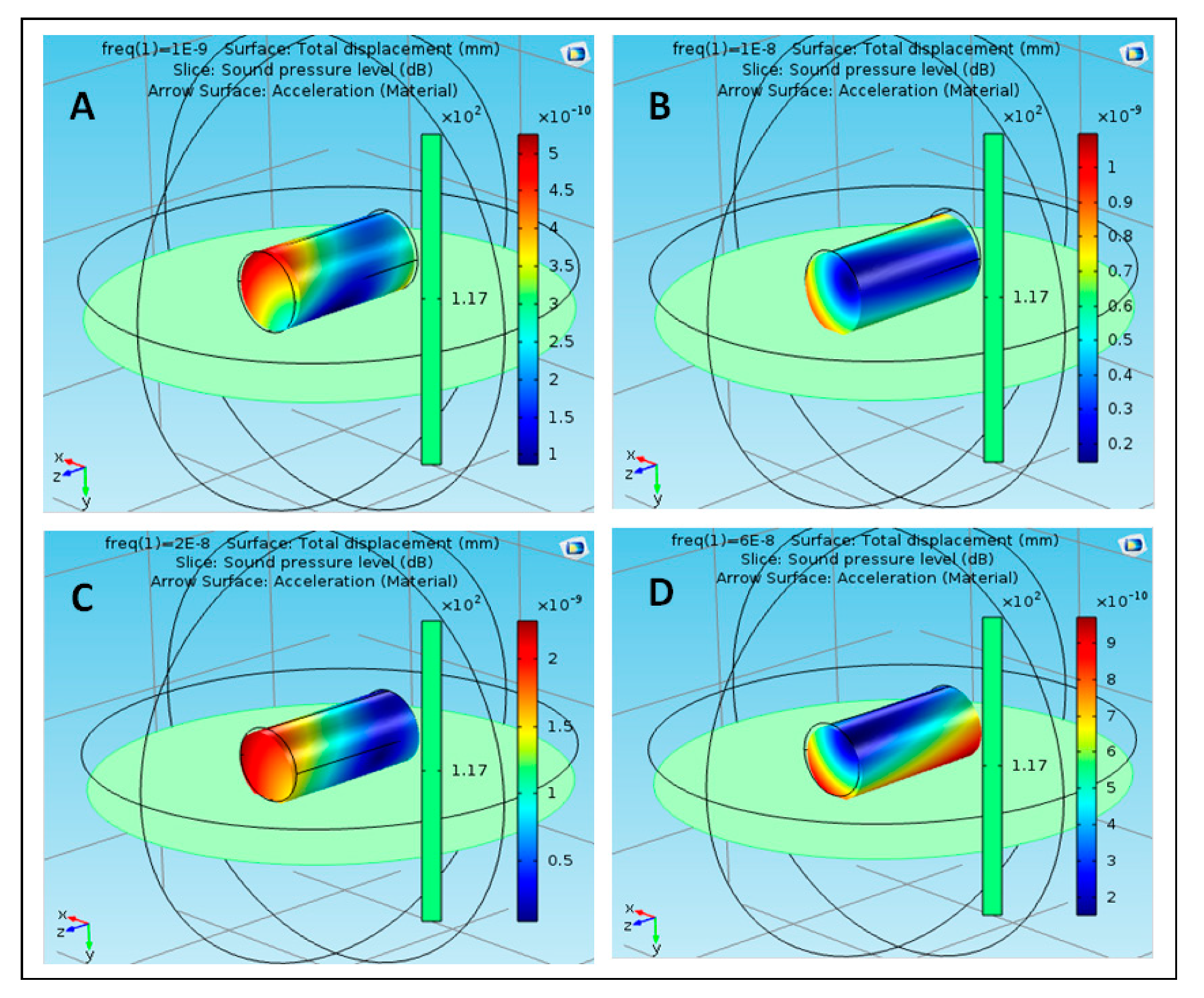
3.1. Validation of the Novel Sound Pressure Computational Model
3.2. Experimental Validation of the Proposed Nanotechnology-Based Drug-Delivery System
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Roet, M.; Hescham, S.; Jahanshahi, A.; Rutten, B.P.; Temel, Y. Progress in neuromodulation of the brain: A role for magnetic nanoparticles? Prog. Neurobiol. 2019, 177, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Sazan, H.; Piperno, S.; Layani, M.; Magdassi, S.; Shpaisman, H. Directed assembly of nanoparticles into continuous microstructures by standing surface acoustic waves. J. Colloid Interface Sci. 2019, 536, 701–709. [Google Scholar] [CrossRef] [PubMed]
- Weiss, L.B.; Marenda, M.; Micheletti, C.; Likos, C.N. Hydrodynamics and Filtering of Knotted Ring Polymers in Nanochannels. Macromolecules 2019, 52, 4111–4119. [Google Scholar] [CrossRef]
- Chahine, G.L.; Gnanaskandan, A.; Mansouri, A.M.; Hsiao, C. Interaction of a cavitation bubble with a polymeric coating–scaling fluid and material dynamics. Int. J. Multiph. Flow 2019, 112, 155–169. [Google Scholar] [CrossRef]
- Lu, L.; Tang, X.; Hu, S.; Pan, Y. Acoustic Field-Assisted Particle Patterning for Smart Polymer Composite Fabrication in Stereolithography. 3D Print. Addit. Manuf. 2018, 5, 151–159. [Google Scholar] [CrossRef]
- Giuriato, U.; Krstulovic, G. Interaction between active particles and quantum vortices leading to Kelvin wave generation. Sci. Rep. 2018, 9, 4839. [Google Scholar] [CrossRef]
- Jalal, J. Interaction of Spherical Particles Owing to Steady Streaming Induced by Ultrasound. Ph.D. Thesis, Swinburne University of Technology, Melbourne, Australia, 2018. [Google Scholar]
- Alziadeh, M. Flow-Sound Interaction Mechanism of a Single Spirally Finned Cylinder in Cross-Flow; University of Ontario Institute of Technology: Oshawa, ON, Canada, 2017. [Google Scholar]
- Mati-Baouche, N.; Baynast, H.D.; Michaud, P.; Dupont, T.; Leclaire, P. Sound absorption properties of a sunflower composite made from crushed stem particles and from chitosan bio-binder. Appl. Acoust. 2016, 111, 179–187. [Google Scholar] [CrossRef] [Green Version]
- Malakooti, S.; Churu, H.G.; Lee, A.; Xu, T.; Luo, H.; Xiang, N.; Sotiriou-Leventis, C.; Leventis, N.; Lu, H. Sound Insulation Properties in Low-Density, Mechanically Strong and Ductile NanoporousPolyurea Aerogels. J. Non Cryst. Solids 2017, 476, 36–45. [Google Scholar] [CrossRef]
- Kellnberger, S.; Rosenthal, A.; Myklatun, A.; Westmeyer, G.G.; Sergiadis, G.D.; Ntziachristos, V. Magnetoacoustic Sensing of Magnetic Nanoparticles. Phys. Rev. Lett. 2016, 116, 108103. [Google Scholar] [CrossRef]
- Chen, M.L.; Cai, F.; Wang, C.; Wang, Z.; Meng, L.; Li, F.; Zhang, P.; Liu, X.; Zheng, H. Observation of Metal Nanoparticles for Acoustic Manipulation. Adv. Sci. 2017, 4, 1600447. [Google Scholar] [CrossRef]
- Baeza, A.; Vallet-Regí, M. Nanomotors for Nucleic Acid, Proteins, Pollutants and Cells Detection. Int. J. Mol. Sci. 2018, 19, 1579. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Dong, R.; Thamphiwatana, S.; Li, J.; Gao, W.; Zhang, L.; Wang, J. Artificial Micromotors in the Mouse’s Stomach: A Step toward in Vivo Use of Synthetic Motors. ACS Nano 2015, 9, 117–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, L.; Wagner, E.W. Polymeric Carriers for Nucleic Acid Delivery: Current Designs and Future Directions. Biomacromolecules 2019, 20, 3613–3626. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Kagan, D.R.; Hu, C.J.; Campuzano, S.; Lobo-Castañón, M.J.; Lim, N.; Kang, D.Y.; Zimmerman, M.; Zhang, L.; Wang, J. Micromachine-enabled capture and isolation of cancer cells in complex media. Angew. Chem. Int. Ed. 2011, 50, 4161–4164. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Shen, H.; Zhao, K.; Wang, Z.; Peng, H.; Liu, W. Micro/nano machines driven by ultrasound powersources. Chem. Asian J. 2019, 14, 2406–2416. [Google Scholar] [CrossRef] [PubMed]
- Chopra, D.S. Nanocomposites in Drug Delivery and Imaging Applications; IGI Global: Hershey, PA, USA, 2018. [Google Scholar]
- Kumari, P.V.; Rao, Y.S.; Akhila, S. Role of Nanocomposites in Drug Delivery. GSC Biol. Pharm. Sci. 2019, 8, 94–103. [Google Scholar]
- Pitsa, D.; Danikas, M.G. Interfaces Features in Polymer Nanocomposites: A Review of Proposed Models. Nano 2011, 6, 497–508. [Google Scholar] [CrossRef]
- Dell’Orco, D.; Lundqvist, M.; Oslakovic, C.; Cedervall, T.; Linse, S. Modeling the Time Evolution of the Nanoparticle-Protein Corona in a Body Fluid. PLoS ONE 2010, 5, e10949. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, A.D.; Wang, T.Y.; Yuan, L.; Duryea, A.P.; Xu, Z.M.; Cain, C.A. A tissue phantom for visualization and measurement of ultrasound-induced cavitation damage. Ultrasound Med. Boil. 2010, 36, 2132–2143. [Google Scholar] [CrossRef] [Green Version]
- Almarhaby, A.M.; Lees, J.E.; Bugby, S.L.; Alqahtani, M.S.; Jambi, L.K.; McKnight, W.R.; Perkins, A.C. Characterisation of a near-infrared (NIR) fluorescence imaging systems intended for hybrid gamma-NIR fluorescence image guided surgery. J. Instrum. 2019, 14, P07007. [Google Scholar] [CrossRef]
- PhilipsWebsite. Available online: https://www.usa.philips.com/healthcare/product/HC795200C/epiq-7ultrasound-system-for-cardiology/specifications (accessed on 6 December 2019).
- Available online: https://www.comsol.com/model/acoustic-structure-interaction-417 (accessed on 4 July 2019).
- Stride, E.; Mulvana, H.; Rademeyer, P.; Carugo, D.; Owen, J.; Browning, R.J.; Tang, M.; Eckersley, R.J. Characterisation of FunctionalisedMicrobubbles for Ultrasound Imaging and Therapy; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Versluis, M. Sonoprinting promotes drug delivery with liposome-loaded microbubbles and ultrasound. J. Acoust. Soc. Am. 2019, 146, 2990. [Google Scholar] [CrossRef]
- Abou-Elkacem, L.; Wang, H.; Chowdhury, S.M.; Kimura, R.H.; Bachawal, S.V.; Gambhir, S.S.; Tian, L.; Willmann, J.K. Thy1-Targeted Microbubbles for Ultrasound Molecular Imaging of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2018, 24, 1574–1585. [Google Scholar] [CrossRef] [PubMed] [Green Version]










© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abbas, M.; Alqahtani, M.; Algahtani, A.; Kessentini, A.; Loukil, H.; Parayangat, M.; Ijyas, T.; Mohammed, A.W. Validation of Nanoparticle Response to the Sound Pressure Effect during the Drug-Delivery Process. Polymers 2020, 12, 186. https://doi.org/10.3390/polym12010186
Abbas M, Alqahtani M, Algahtani A, Kessentini A, Loukil H, Parayangat M, Ijyas T, Mohammed AW. Validation of Nanoparticle Response to the Sound Pressure Effect during the Drug-Delivery Process. Polymers. 2020; 12(1):186. https://doi.org/10.3390/polym12010186
Chicago/Turabian StyleAbbas, Mohamed, Mohammed Alqahtani, Ali Algahtani, Amir Kessentini, Hassen Loukil, Muneer Parayangat, Thafasal Ijyas, and Abdul Wase Mohammed. 2020. "Validation of Nanoparticle Response to the Sound Pressure Effect during the Drug-Delivery Process" Polymers 12, no. 1: 186. https://doi.org/10.3390/polym12010186




