GM-Improved Antiaging Effect of Acrylonitrile Butadiene Styrene in Different Thermal Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Stabilizer GM
2.3. Sample Preparation
2.4. Analysis
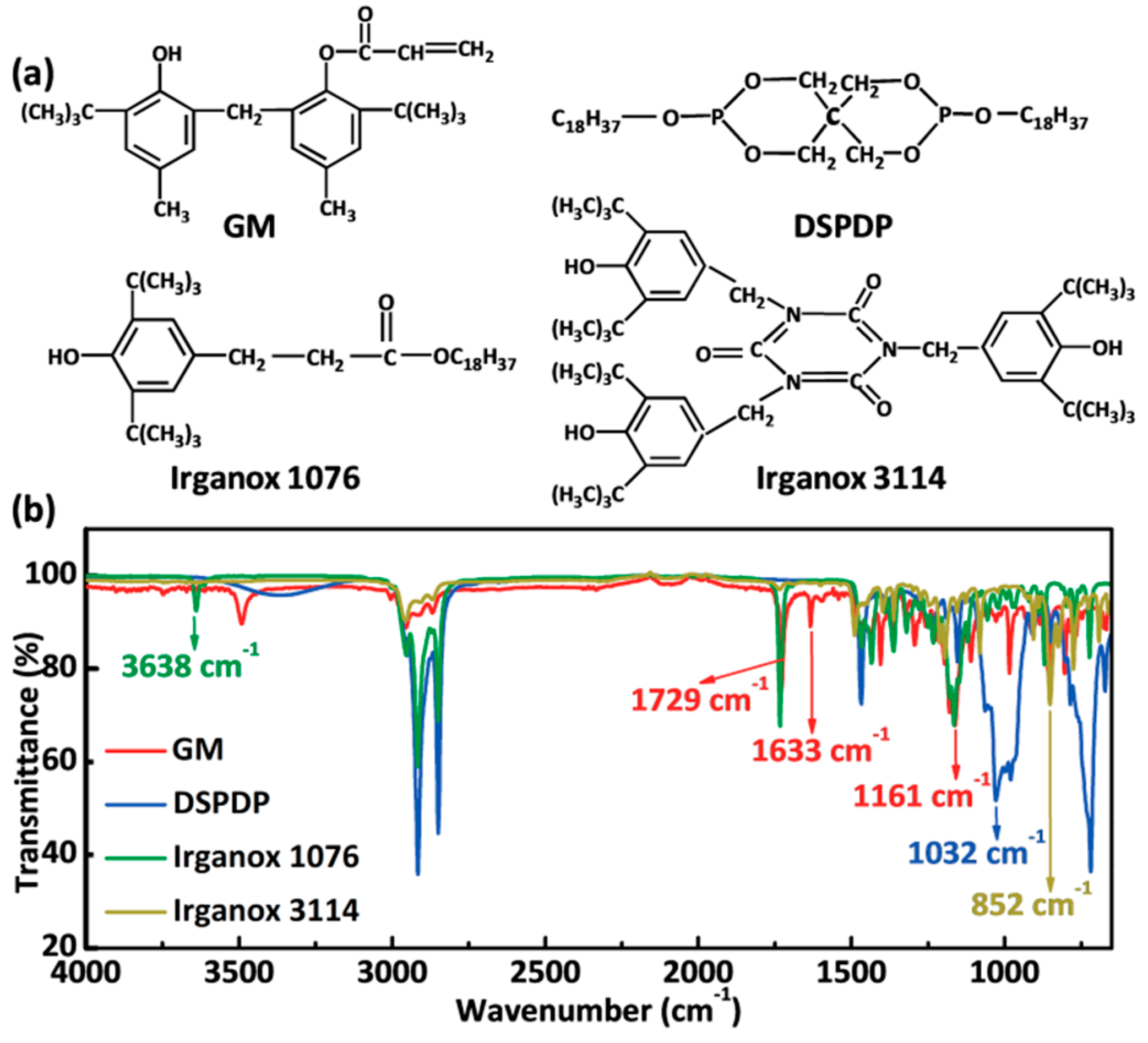
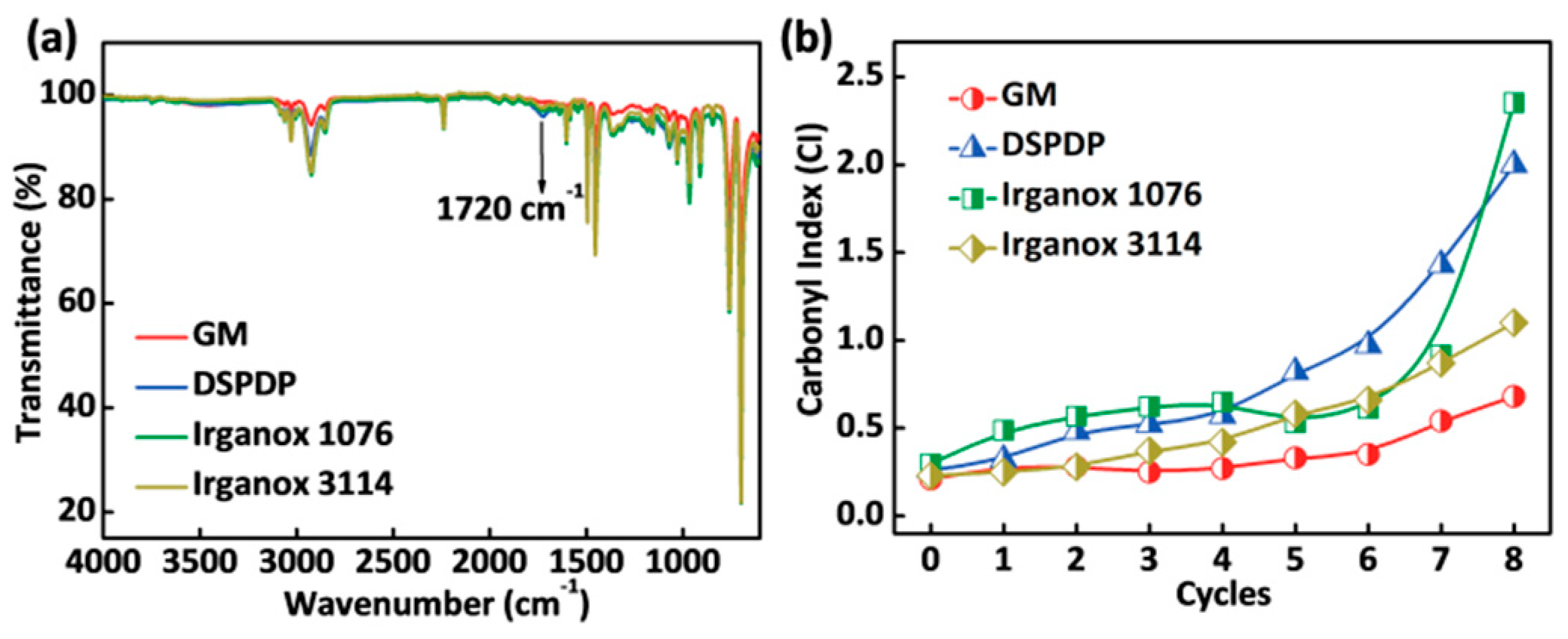
2.4.1. Fourier Transform Infrared Spectroscopy (FTIR)
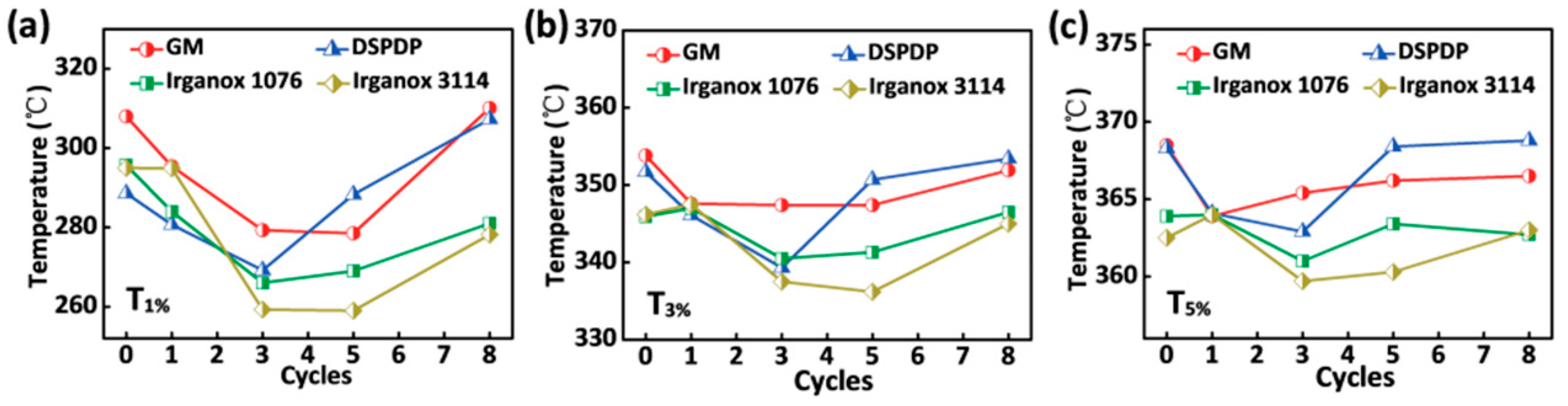
2.4.2. Thermogravimetric Analysis (TGA)
2.4.3. Color Measurements
2.4.4. Mechanical Testing
3. Results
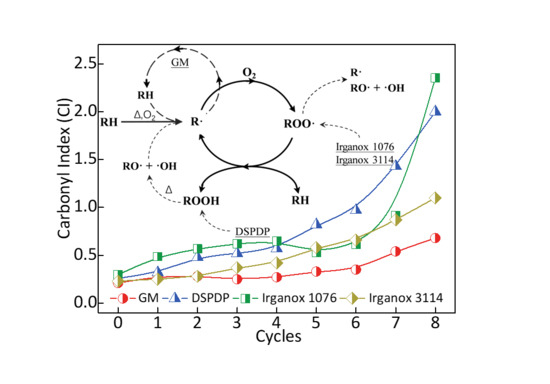
3.1. Additive Selection
3.2. Carbonyl Index
3.3. Thermal Properties
3.4. Optical and Mechanical Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Lin, J.; Li, J.; Wang, J.; Guan, Y.; Wang, G.; Chen, L. Effects of thermoplastic elastomer on the morphology and mechanical properties of glass fiber-reinforced polycarbonate/acrylonitrile-butadiene-styrene. Polym. Eng. Sci. 2019, 59, E144–E151. [Google Scholar] [CrossRef]
- Zhang, A.; Mu, B.; Wang, X.; Wang, A. Microwave hydrothermal assisted preparation of CoAl2O4/kaolin hybrid pigments for reinforcement coloring and mechanical property of acrylonitrile butadiene styrene. Appl. Clay Sci. 2019, 175, 67–75. [Google Scholar] [CrossRef]
- Ahmed, J.K. Studying yhe effect of nano silver on some properties of acrylonitrile butadiene styrene copolymer for medical device. Int. J. Eng. Technol. 2019, 8, 208–213. [Google Scholar]
- Kuram, E. Hybridization effect of talc/glass fiber as a filler in polycarbonate/acrylonitrile-butadiene-styrene composites. Compos. Part B 2019, 173, 106954. [Google Scholar] [CrossRef]
- Rahimi, M.; Esfahanian, M.; Moradi, M. Effect of reprocessing on shrinkage and mechanical properties of ABS and investigating the proper blend of virgin and recycled ABS in injection molding. J. Mater. Process. Technol. 2014, 214, 2359–2365. [Google Scholar] [CrossRef]
- Scaffaro, R.; Botta, L.; Benedetto, G.D. Physical properties of virgin-recycled ABS blends: Effect of post-consumer content and of reprocessing cycles. Eur. Polym. J. 2012, 48, 637–648. [Google Scholar] [CrossRef]
- Kim, S.H.; Choi, K.; Choi, H.R.; Kim, T.; Suhr, J.; Kim, K.J.; Choi, H.J.; Nam, J.D. Non-einstein viscosity phenomenon of acrylonitrile-butadiene-styrene composites containing lignin-polycaprolactone particulates highly dispersed by high-shear stress. ACS Omega 2019, 4, 10036–10043. [Google Scholar] [CrossRef]
- Peterson, A.M. Review of acrylonitrile butadiene styrene in fused filament fabrication: A plastics engineering-focused perspective. Addit. Manuf. 2019, 27, 363–371. [Google Scholar] [CrossRef]
- Lu, Y.; Yang, M.; Liu, J. Morphology and properties of thin-walled microcellular polycarbonate/acrylonitrile–butadiene–styrene blend foam. J. Cell. Plast. 2019, 55, 421–432. [Google Scholar] [CrossRef]
- Huang, B.; Meng, S.; He, H.; Jia, Y.; Xu, Y.; Huang, H. Study of processing parameters in fused deposition modeling based on mechanical properties of acrylonitrile-butadiene-styrene filament. Polym. Eng. Sci. 2019, 59, 120–128. [Google Scholar] [CrossRef] [Green Version]
- Rasero, M.Á.P. Metodologia Para el Calculo Dela Viscosidad Empleando Como Caso Practico el Reprocesado de ABS. 3c Tecnología 2012, 1, 33–47. [Google Scholar]
- Tiganis, B.E. Thermal degradation of acrylonitrile–butadiene–styrene (ABS) blends. Polym. Degrad. Stab. 2002, 76, 425–434. [Google Scholar] [CrossRef]
- Belavadi Venkataramaiah, S.; Shridhar, T.N.; Mannikar, A.V.; Mohan Krishna, S.A. Comprehensive investigation of acrylonitrile-butadiene-styrene (ABS) polymer for weathering with the combination of different blends of UV stabilizers, HALS and antioxidant. SAE Tech. Paper Ser. 2019, 26, 0619. [Google Scholar]
- Saviello, D.; Pouyet, E.; Toniolo, L.; Cotte, M.; Nevin, A. Synchrotron-based FTIR microspectroscopy for the mapping of photo-oxidation and additives in acrylonitrile-butadiene-styrene model samples and historical objects. Anal. Chim. Acta 2014, 843, 59–72. [Google Scholar] [CrossRef]
- Davis, P. The effect of photo-oxidative degradation on fracture in ABS pipe resins. Polym. Degrad. Stab. 2004, 84, 233–242. [Google Scholar] [CrossRef]
- Cristea, E.; Sturza, R.; Auregi, P.; Niculaua, J.M.; Ghendov-Mosanu, A.; Patras, A. Influence of pH and ionic strength on the color parameters and antioxidant properties of an ethanolic red grape marc extract. J. Food Biochem. 2019, 43, e12788. [Google Scholar] [CrossRef]
- Wang, J.; Cai, X.-F. Kinetics study of thermal oxidative degradation of ABS containing flame retardant components. J. Therm. Anal. Calorim. 2011, 107, 725–732. [Google Scholar] [CrossRef]
- Conway, K.M.; Pataky, G.J. Crazing in additively manufactured acrylonitrile butadiene styrene. Eng. Fract. Mech. 2019, 211, 114–124. [Google Scholar] [CrossRef]
- Guessasma, S.; Belhabib, S.; Nouri, H. Microstructure, Thermal and mechanical behavior of 3D printed acrylonitrile styrene acrylate. Macromol. Mater. Eng. 2019, 304, 1800793. [Google Scholar] [CrossRef]
- Blom, H.; Yeh, R.; Wojnarowski, R.; Ling, M. Retracted: Detection of degradation of ABS materials via DSC. Thermochim. Acta 2006, 442, 64–66. [Google Scholar] [CrossRef]
- Salari, D. Study on the recycling of ABS resins: Simulation of reprocessing and thermo-oxidation. Iran. Polym. J. 2008, 17, 599–610. [Google Scholar]
- Fiorio, R.; D’Hooge, D.R.; Ragaert, K.; Cardon, L. A statistical analysis on the effect of antioxidants on the thermal-oxidative stability of commercial mass- and emulsion-polymerized ABS. Polymers 2018, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ong, T.K.; Tshai, K.Y.; Khiew, P.S.; Yap, E.H. Thermal and mechanical properties of chemically treated oil palm fiber filled acrylonitrile butadiene styrene composites. Mater. Werkst. 2019, 50, 240–247. [Google Scholar] [CrossRef]
- Chan, M.Y.; Teh, P.L.; Yeoh, C.K. Effect of blend ratio on the properties of polystyrene/acrylonitrile butadiene styrene/carbon black (PS/ABS/CB) conductive materials. J. Eng. Sci. 2019, 15, 63–75. [Google Scholar] [CrossRef]
- Gao, A.; Zhao, F.; Wang, F.; Zhang, G.; Zhao, S.; Cui, J.; Yan, Y. Highly conductive and light-weight acrylonitrile-butadiene-styrene copolymer/reduced graphene nanocomposites with segregated conductive structure. Compos. Part A 2019, 122, 1–7. [Google Scholar] [CrossRef]
- Allen, N.S.; Barcelona, A.; Edge, M.; Wilkinson, A.; Merchan, C.G.; Quiteria, V.R.S. Aspects of the thermal and photostabilisation of high styrene–butadiene copolymer (SBC). Polym. Degrad. Stab. 2006, 91, 1395–1416. [Google Scholar] [CrossRef]
- Triantou, M.I.; Chatzigiannakis, E.M.; Tarantili, P.A. Evaluation of thermal degradation mechanisms and their effect on the gross calorific value of ABS/PC/organoclay nanocomposites. J. Therm. Anal. Calorim. 2014, 119, 337–347. [Google Scholar] [CrossRef]
- Yachigo, S.; Sasaki, M.; Takahashi, Y.; Kojima, F.; Takada, T.; Okita, T. Studies on polymer stabilisers: Part I—A novel thermal stabiliser for butadiene polymers. Polym. Degrad. Stab. 1988, 22, 63–77. [Google Scholar] [CrossRef]
- Shimada, J.; Kabuki, K. The mechanism of oxidative degradation of ABS resin. Part I. The mechanism of thermooxidative degradation. J. Appl. Polym. Sci. 1968, 12, 655–669. [Google Scholar] [CrossRef]
- Wyzgoski, M.G. Effects of oven aging on ABS, poly (acrylonitrile-butadiene-styrene). Polym. Eng. Sci. 1976, 16, 265–269. [Google Scholar] [CrossRef]
- Boldizar, A.; Möller, K. Degradation of ABS during repeated processing and accelerated ageing. Polym. Degrad. Stab. 2003, 81, 359–366. [Google Scholar] [CrossRef]
- Rosik, L.; Kovářová, J.; Pospíšil, J. Lifetime prediction of ABS polymers based on thermoanalytical data. J. Therm. Anal. 1996, 46, 465–470. [Google Scholar] [CrossRef]
- Bai, X.; Isaac, D.H.; Smith, K. Reprocessing acrylonitrile–butadiene–styrene plastics: Structure–property relationships. Polym. Eng. Sci. 2007, 47, 120–130. [Google Scholar] [CrossRef]
- Hassinen, J. Deterioration of polyethylene pipes exposed to chlorinated water. Polym. Degrad. Stab. 2004, 84, 261–267. [Google Scholar] [CrossRef]
- Santos, R.M.; Botelho, G.L.; Machado, A.V. Artificial and natural weathering of ABS. J. Appl. Polym. Sci. 2010, 116, 2005–2014. [Google Scholar] [CrossRef]
- Suzuki, M.; Wilkie, C.A. The thermal degradation of acrylonitrile-butadiene-styrene terpolymei as studied by TGA/FTIR. Polym. Degrad. Stab. 1995, 47, 217–221. [Google Scholar] [CrossRef]
- Griesser, R. Assessment of whiteness and tint of fluorescent substrates with good interinstrument correlation. Color Res. Appl. 1994, 19, 446–460. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Chen, M.; Lan, M.; Li, Z.; Lu, S.; Wu, G. GM-Improved Antiaging Effect of Acrylonitrile Butadiene Styrene in Different Thermal Environments. Polymers 2020, 12, 46. https://doi.org/10.3390/polym12010046
Wang Y, Chen M, Lan M, Li Z, Lu S, Wu G. GM-Improved Antiaging Effect of Acrylonitrile Butadiene Styrene in Different Thermal Environments. Polymers. 2020; 12(1):46. https://doi.org/10.3390/polym12010046
Chicago/Turabian StyleWang, Yuchao, Ming Chen, Miaoyu Lan, Zhi Li, Shulai Lu, and Guangfeng Wu. 2020. "GM-Improved Antiaging Effect of Acrylonitrile Butadiene Styrene in Different Thermal Environments" Polymers 12, no. 1: 46. https://doi.org/10.3390/polym12010046