Phase Behavior and Thermo-Mechanical Properties of IF-WS2 Reinforced PP–PET Blend-Based Nanocomposites
Abstract
:1. Introduction
2. Experiment Section
2.1. Materials
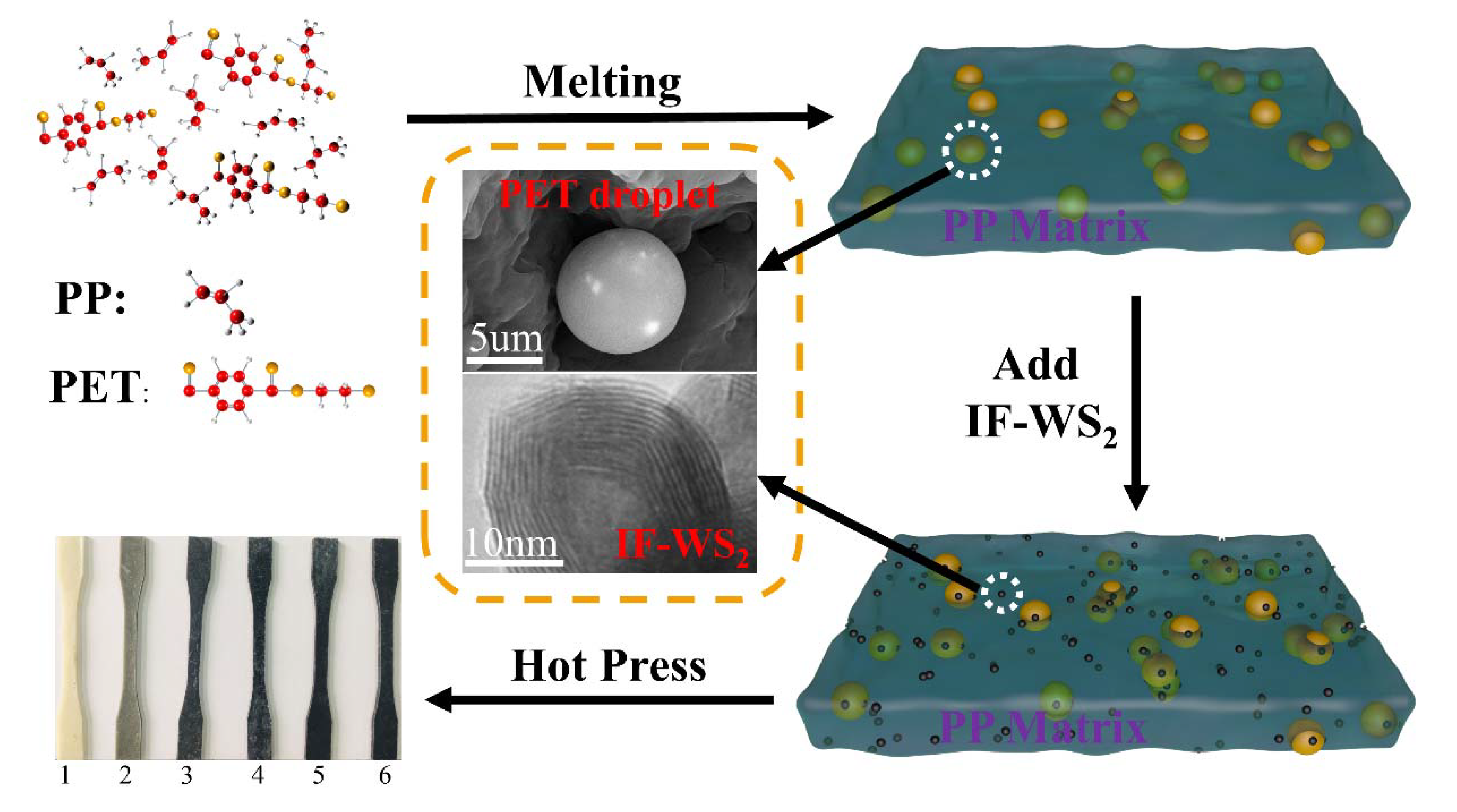
2.2. Preparation of IF-WS2/PP/PET Nanomaterials
2.3. Characterization of IF-WS2/PP/PET Nanocomposites
3. Results and Discussion
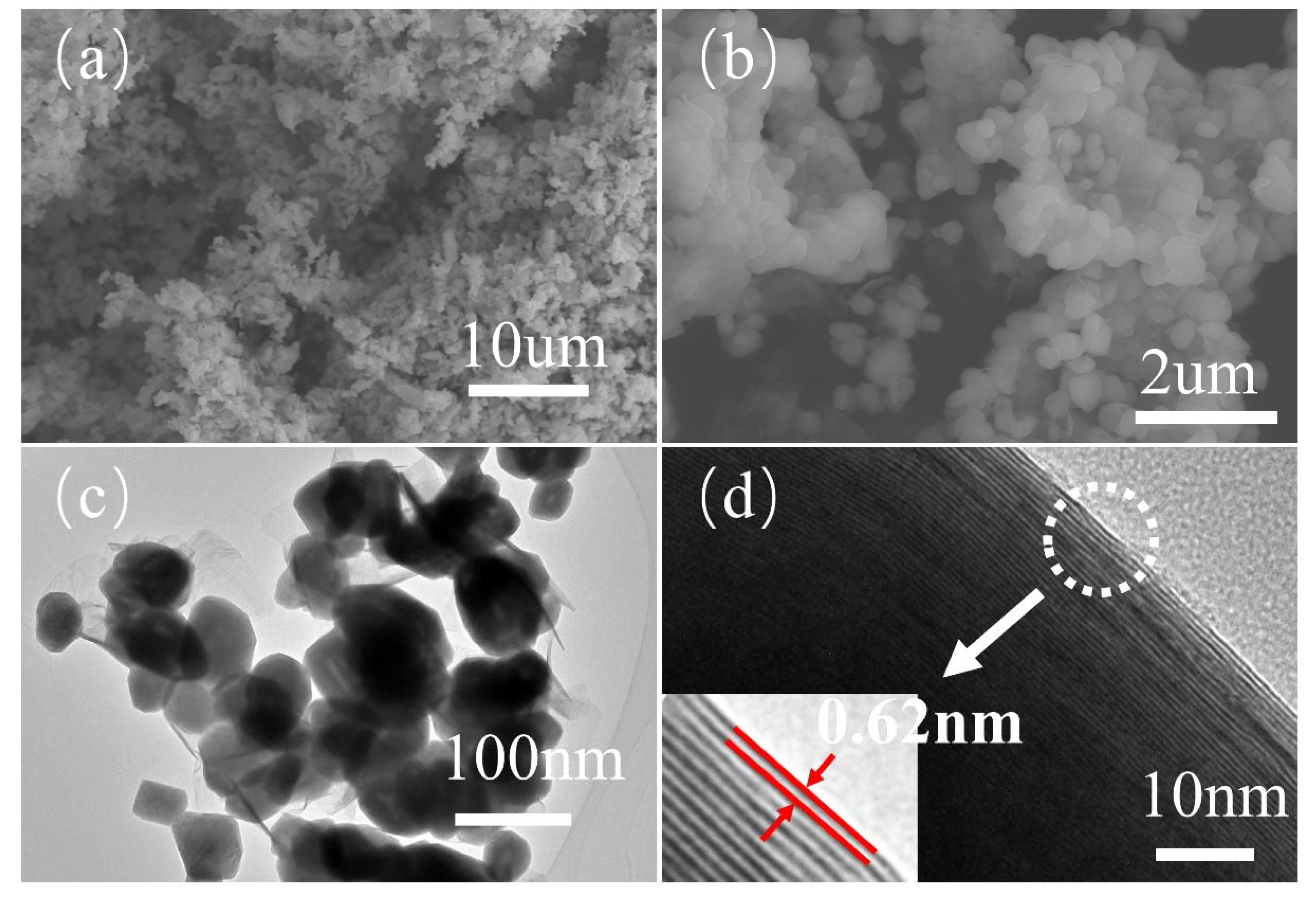
3.1. Structure and Morphology Analysis of IF-WS2 Nanoparticles
3.2. SEM Analysis
3.3. TEM Analysis
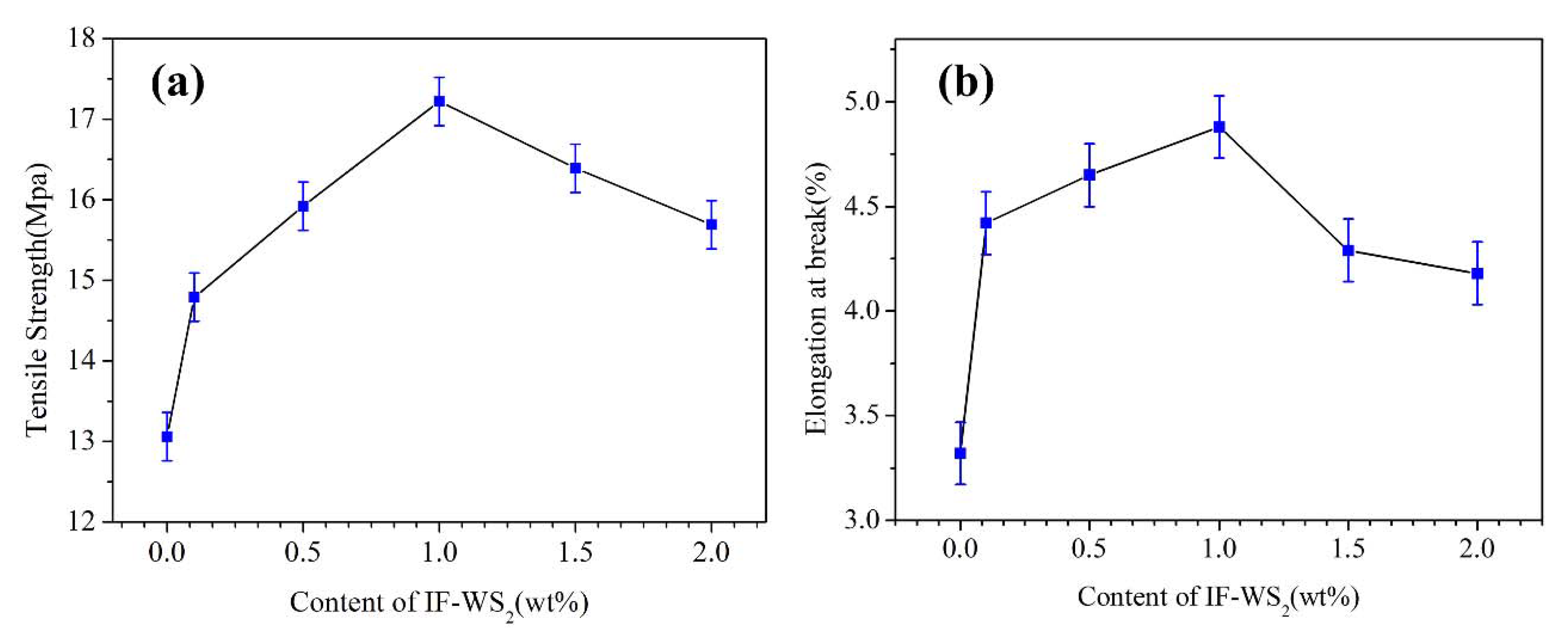
3.4. Tensile Properties
3.5. XRD Analysis
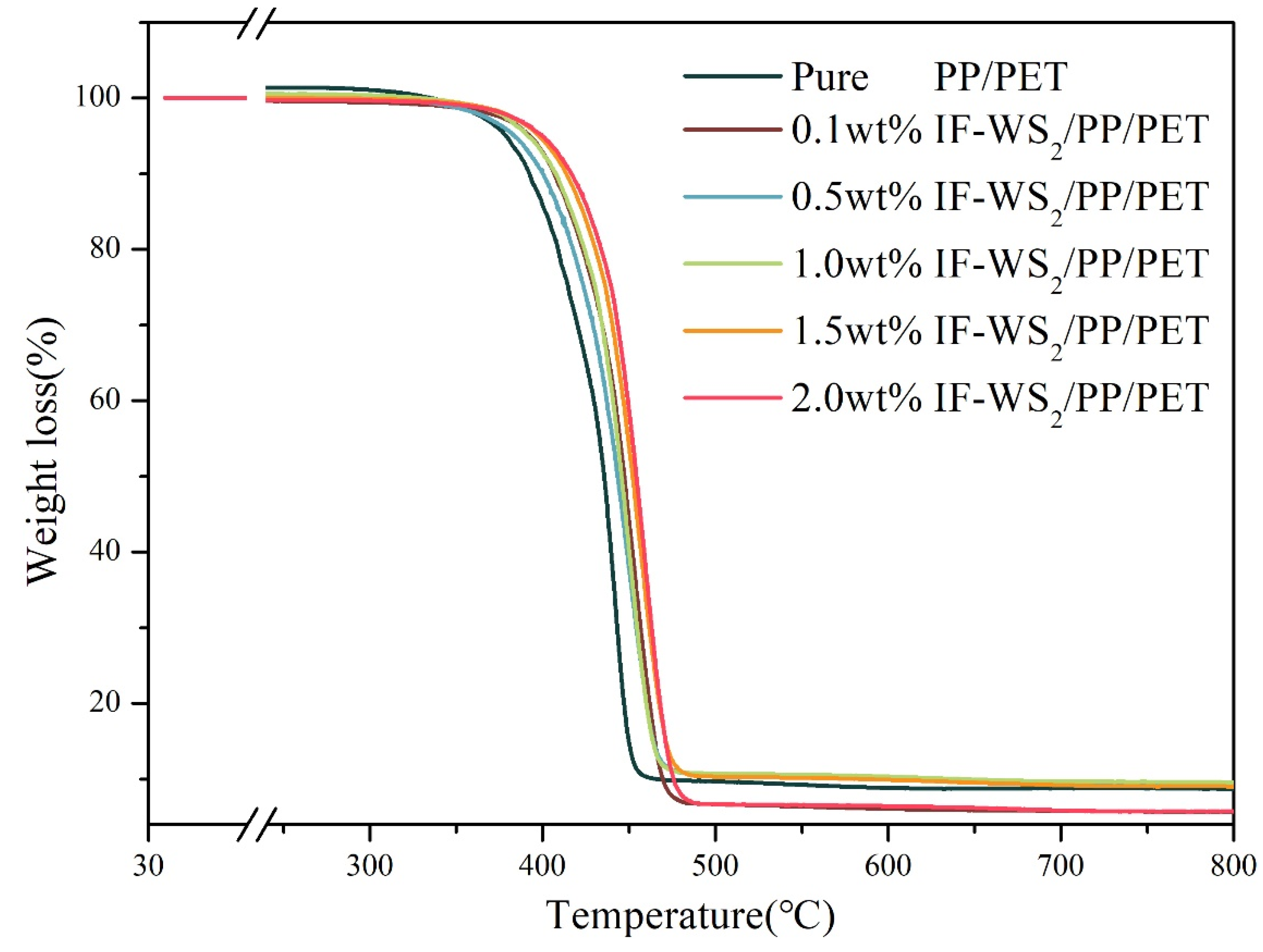
3.6. TGA Analysis
3.7. DSC Analysis
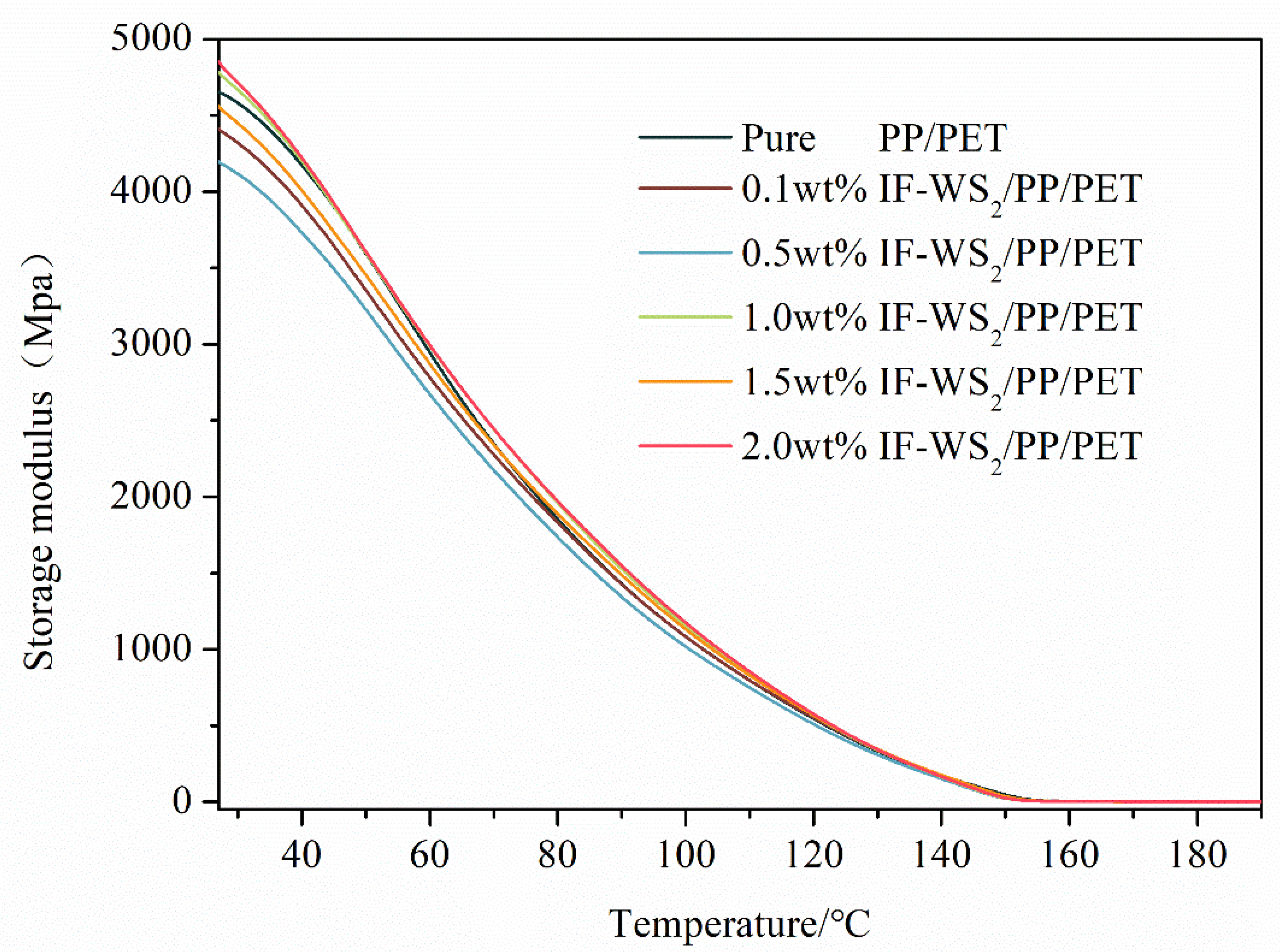
3.8. Storage Modulus Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Gelebart, A.A.H.; Mulder, D.J.; Varga, M.; Konya, A.; Vantomme, G.; Meijer, E.B.; Selinger, R.L.B.; Broer, D.J. Making waves in a photoactive polymer film. Nat. Cell Biol. 2017, 546, 632–636. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Chen, L.; Gadinski, M.R.; Zhang, S.; Zhang, G.; Li, H.U.; Iagodkine, E.; Haque, A.; Chen, L.-Q.; Jackson, T.N.; et al. Correction: Corrigendum: Flexible high-temperature dielectric materials from polymer nanocomposites. Nature 2016, 536, 112. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xing, W.; Feng, X.; Song, L.; Hu, Y. MoS2/polymer nanocomposites: Preparation, properties, and applications. Polym. Rev. 2017, 57, 440–466. [Google Scholar] [CrossRef]
- Maddah, H.A. Polypropylene as a promising plastic: A review. Am. J. Polym. Sci. 2016, 6, 1–11. [Google Scholar]
- Sulyman, M.; Haponiuk, J.; Formela, K. Utilization of Recycled Polyethylene Terephthalate (PET) in Engineering Materials: A Review. Int. J. Environ. Sci. Dev. 2016, 7, 100–108. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, A.; Moeini, R.; Yeganeh, J.K. Highly conductive PP/PET polymer blends with high electromagnetic interference shielding performances in the presence of thermally reduced graphene nanosheets prepared through melt compounding. Polym. Compos. 2018, 40, E1461–E1469. [Google Scholar] [CrossRef]
- Liu, Y.; Zhao, Z.; Tang, D.; Kong, M.; Yang, Q.; Huang, Y.; Liao, X.; Niu, Y. Effect of nanoparticles on the morphology and properties of PET/PP in situ microfibrillar reinforced composites. Polym. Compos. 2015, 38, 2718–2726. [Google Scholar] [CrossRef]
- Wang, K.; Xie, J.; Li, T.; Wu, X.; Huang, W.; Tian, Q.; Tu, C.; Yan, W. Surface modification of sepiolite: Effects on thermomechanical properties of PP/PA6 blends. J. Polym. Res. 2020, 27, 25. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. A novel detection approach for serotonin by graphene quantum dots/two-dimensional (2D) hexagonal boron nitride nanosheets with molecularly imprinted polymer. Appl. Surf. Sci. 2018, 458, 648–655. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, X.; Yang, M.; Yang, S.; Wu, H.; Guo, S.; Wang, Y. Ordered multilayer film of (graphene oxide/polymer and boron nitride/polymer) nanocomposites: An ideal EMI shielding material with excellent electrical insulation and high thermal conductivity. Compos. Sci. Technol. 2016, 136, 104–110. [Google Scholar] [CrossRef]
- Krupka, J.; Shakhil, P.; Arun, N.; Ratheesh, R.; Jantunen, H.; Kim, H.; Sebastian, M.T. Low loss polypropylene-silicon composites for millimetre wave applications. Mater. Res. Bull. 2018, 104, 143–148. [Google Scholar] [CrossRef]
- Sebastian, M.T.; Krupka, J.; Arun, S.; Kim, C.; Kim, H. Polypropylene-high resistivity silicon composite for high frequency applications. Mater. Lett. 2018, 232, 92–94. [Google Scholar] [CrossRef]
- Krause, B.; Rzeczkowski, P.; Pötschke, P. Thermal Conductivity and Electrical Resistivity of Melt-Mixed Polypropylene Composites Containing Mixtures of Carbon-Based Fillers. Polymers 2019, 11, 1073. [Google Scholar] [CrossRef] [Green Version]
- Joshi, J.G.; Pattnaik, S.S. Metamaterial-Based Wearable Microstrip Patch Antennas. In Sliding Mode in Intellectual Control and Communication; IGI Global: Hershey, PA, USA, 2014; pp. 518–556. [Google Scholar]
- Matxinandiarena, E.; Múgica, A.; Zubitur, M.; Yus, C.; Sebastian, V.; Irusta, S.; Loaeza, A.D.; Pérez, O.S.; Maspoch, M.L.; Puig, C.; et al. The Effect of Titanium Dioxide Surface Modification on the Dispersion, Morphology, and Mechanical Properties of Recycled PP/PET/TiO2 PBNANOs. Polymers 2019, 11, 1692. [Google Scholar] [CrossRef] [Green Version]
- Pandiyaraj, K.N.; Selvarajan, V.; Deshmukh, K.; Gao, C. Adhesive properties of polypropylene (PP) and polyethylene terephthalate (PET) film surfaces treated by DC glow discharge plasma. Vacuum 2008, 83, 332–339. [Google Scholar] [CrossRef]
- Calcagno, C.; Mariani, C.; Teixeira, S.R.; Mauler, R.S. The role of the MMT on the morphology and mechanical properties of the PP/PET blends. Compos. Sci. Technol. 2008, 68, 2193–2200. [Google Scholar] [CrossRef]
- Kuzmanović, M.; Delva, L.; Mi, D.; Martins, C.I.; Cardon, L.; Ragaert, K. Development of Crystalline Morphology and Its Relationship with Mechanical Properties of PP/PET Microfibrillar Composites Containing POE and POE-g-MA. Polymers 2018, 10, 291. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Kobayashi, T.; Yang, Z.; Sekine, T.; Chang, H.; Wang, N.; Xia, Y.; Zhu, Y. How the Toughest Inorganic Fullerene Cages Absorb Shockwave Pressures in a Protective Nanocomposite: Experimental Evidence from Two In Situ Investigations. ACS Nano 2017, 11, 8114–8121. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Dominguez, J.M.; Tesa-Serrate, M.A.; Ansón-Casaos, A.; Díez-Pascual, A.M.; Gómez-Fatou, M.A.; Martínez, M.T. Wrapping of SWCNTs in Polyethylenoxide-Based Amphiphilic Diblock Copolymers: An Approach to Purification, Debundling, and Integration into the Epoxy Matrix. J. Phys. Chem. C 2012, 116, 7399–7408. [Google Scholar] [CrossRef]
- Naffakh, M.; Díez-Pascual, A.M.; Marco, C.; Ellis, G. Morphology and thermal properties of novel poly(phenylene sulfide) hybrid nanocomposites based on single-walled carbon nanotubes and inorganic fullerene-like WS2nanoparticles. J. Mater. Chem. 2012, 22, 1418–1425. [Google Scholar] [CrossRef] [Green Version]
- Naffakh, M.; Díez-Pascual, A.M.; Marco, C.; Ellis, G. Novel polypropylene/inorganic fullerene-like WS2 nanocomposites containing a β-nucleating agent: Mechanical, tribological and rheological properties. Mater. Chem. Phys. 2014, 144, 98–106. [Google Scholar] [CrossRef] [Green Version]
- Niste, V.B.; Ratoi, M.; Tanaka, H.; Xu, F.; Zhu, Y.; Sugimura, J. Self-lubricating Al-WS2 composites for efficient and greener tribological parts. Sci. Rep. 2017, 7, 14665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, N.; Yang, Z.; Thummavichai, K.; Xu, F.; Hu, C.; Chen, H.; Xia, Y.; Zhu, Y. Novel graphitic carbon coated IF-WS 2 reinforced poly(ether ether ketone) nanocomposites. RSC Adv. 2017, 7, 35265–35273. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Yang, Z.; Wang, Y.; Thummavichai, K.; Xia, Y.; Ghita, O.; Zhu, Y.Q. Interface and properties of inorganic fullerene tungsten sulphide nanoparticle reinforced poly (ether ether ketone) nanocomposites. Results Phys. 2017, 7, 2417–2424. [Google Scholar] [CrossRef]
- Xu, F.; Yan, C.; Shyng, Y.-T.; Chang, H.; Xia, Y.; Zhu, Y. Ultra-toughened nylon 12 nanocomposites reinforced with IF-WS2. Nanotechnology 2014, 25, 325701. [Google Scholar] [CrossRef] [PubMed]
- Xu, F. Large Scale Manufacturing of WS2 Nanomaterials and Their Application in Polymer Nanocomposites. Ph.D. Thesis, University of Exeter, Penryn, UK, 2013. [Google Scholar]
- Mojtabaei, A.; Otadi, M.; Goodarzi, V.; Khonakdar, H.; Jafari, S.-H.; Reuter, U.; Wagenknecht, U. Influence of fullerene-like tungsten disulfide (IF-WS2) nanoparticles on thermal and dynamic mechanical properties of PP/EVA blends: Correlation with microstructure. Compos. Part B Eng. 2017, 111, 74–82. [Google Scholar] [CrossRef]
- Naffakh, M.; Martín, Z.; Fanegas, N.; Marco, C.; Gómez, M.A.; Jiménez, I. Influence of inorganic fullerene-like WS2 nanoparticles on the thermal behavior of isotactic polypropylene. J. Polym. Sci. Part B 2007, 45, 2309–2321. [Google Scholar] [CrossRef]
- Dodiuk, H.; Kariv, O.; Kenig, S.; Tenne, R. The effect of tungsten disulphide nanoparticles on the properties of polyurethane adhesives. J. Adhes. Sci. Technol. 2013, 28, 38–52. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Gómez, M.A.; Jiménez, I. Unique Nucleation Activity of Inorganic Fullerene-like WS2 Nanoparticles in Polyphenylene Sulfide Nanocomposites: Isokinetic and Isoconversional Study of Dynamic Crystallization Kinetics. J. Phys. Chem. B 2009, 113, 7107–7115. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Ellis, G. Novel Polypropylene/Inorganic Fullerene-like WS2Nanocomposites Containing a β-Nucleating Agent: Dynamic Crystallization and Melting Behavior. J. Phys. Chem. B 2011, 115, 10836–10843. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Gómez, M.A.; Gómez-Herrero, J.; Jiménez, I. Use of Inorganic Fullerene-like WS2to Produce New High-Performance Polyphenylene Sulfide Nanocomposites: Role of the Nanoparticle Concentration. J. Phys. Chem. B 2009, 113, 10104–10111. [Google Scholar] [CrossRef] [PubMed]
- Naffakh, M.; Díez-Pascual, A.M.; Marco, C.; Gómez, M.A.; Jiménez, I. Novel melt-processable poly (ether ether ketone) (PEEK)/inorganic fullerene-like WS2 nanoparticles for critical applications. J. Phys. Chem. B 2010, 114, 11444–11453. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golbang, A.; Harkin-Jones, E.; Wegrzyn, M.; Campbell, G.; Archer, E.; McIlhagger, A. Production and characterization of PEEK/IF-WS2 nanocomposites for additive manufacturing: Simultaneous improvement in processing characteristics and material properties. Addit. Manuf. 2020, 31, 100920. [Google Scholar] [CrossRef]
- Zhang, T.; Ma, T.; Li, W. Preparation of Ultrahigh Molecular Weight Polyethylene/WS 2 Composites for Bulletproof Materials and Study on Their Bulletproof Mechanism. J. Macromol. Sci. Part B 2015, 54, 992–1000. [Google Scholar] [CrossRef]
- Flores, A.; Naffakh, M.; Díez-Pascual, A.M.; Ania, F.; Gómez-Fatou, M.A. Evaluating the Reinforcement of Inorganic Fullerene-like Nanoparticles in Thermoplastic Matrices by Depth-Sensing Indentation. J. Phys. Chem. C 2013, 117, 20936–20943. [Google Scholar] [CrossRef]
- Naffakh, M.; Marco, C.; Gómez, M.; Jiménez, I. Time-resolved synchrotron X-ray study of the dynamic crystallization and melting behaviour of nylon 6/inorganic fullerene-like WS2 nanocomposites. Mater. Chem. Phys. 2011, 128, 265–273. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Chrissafis, K.; Bikiaris, D.N. β-Nucleated polypropylene: Processing, properties and nanocomposites. Polym. Rev. 2015, 55, 596–629. [Google Scholar] [CrossRef]
- De Rosa, C.; Scoti, M.; De Ballesteros, O.R.; Di Girolamo, R.; Auriemma, F.; Malafronte, A. Propylene–Butene Copolymers: Tailoring Mechanical Properties from Isotactic Polypropylene to Polybutene. Macromolecules 2020, 53, 4407–4421. [Google Scholar] [CrossRef]
- Girija, B.; Sailaja, R.; Madras, G. Thermal degradation and mechanical properties of PET blends. Polym. Degrad. Stab. 2005, 90, 147–153. [Google Scholar] [CrossRef]
- Kuzmanovic, M.; Delva, L.; Cardon, L.; Ragaert, K. The Effect of Injection Molding Temperature on the Morphology and Mechanical Properties of PP/PET Blends and Microfibrillar Composites. Polymers 2016, 8, 355. [Google Scholar] [CrossRef] [Green Version]




| S.N. | Blending Partner | Focused Study | Ref. |
|---|---|---|---|
| 1 | PP + EVA | Thermal and dynamic mechanical properties | [28] |
| 2 | Nylon 12 (PA12) | Thermal stability and mechanical properties | [26] |
| 3 | Isotactic Polypropylene | Thermal behavior | [29] |
| 4 | Epoxy Adhesives | Toughness properties | [30] |
| 5 | Phenylenesulfide (PPS) | Isokinetic and iso-conversional study of dynamic crystallization kinetics | [31] |
| 6 | PP | Dynamic crystallization and melting behavior | [32] |
| 7 | PPS | The cold crystallization kinetics and mechanical performance | [33] |
| 8 | Poly ether ether ketone (PEEK) | Thermal and mechanical performance | [34] |
| 9 | PPS + PEEK | Rheological and Tribological Properties | [33] |
| 10 | PEEK | Thermal, mechanical, and tribological properties | [35] |
| 11 | ultra-high molecular weight polyethylene (UHMWPE) | Bulletproof properties | [36] |
| 12 | PEEK | Thermal, mechanical performance, and interface investigation | [25] |
| 13 | PPS + Isotactic polypropylene (iPP) | Mechanical and hardness properties | [37] |
| 14 | PVB | thermo-mechanical and tribological properties | [38] |
| 15 | Nylon 6 (PA6) | Dynamic crystallization and melting behavior | [26] |
| Sample Code | PP (wt%) | PET (wt%) | IF-WS2 (wt%) |
|---|---|---|---|
| PP/PET0.0 | 80 | 20 | 0 |
| PP/PET0.1 | 80 | 20 | 0.1 |
| PP/PET0.5 | 80 | 20 | 0.5 |
| PP/PET1.0 | 80 | 20 | 1.0 |
| PP/PET1.5 | 80 | 20 | 1.5 |
| PP/PET2.0 | 80 | 20 | 2.0 |
| Sample Code | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|
| PP/PET0.0 | 13.06 | 3.32 |
| PP/PET0.1 | 14.79 | 4.42 |
| PP/PET0.5 | 15.92 | 4.65 |
| PP/PET1.0 | 17.22 | 4.88 |
| PP/PET1.5 | 16.39 | 4.29 |
| PP/PET2.0 | 15.69 | 4.18 |
| Sample Code | Ts (°C) | T50 (°C) | TM (°C) | Char Residue at 500 °C (wt%) |
|---|---|---|---|---|
| PP/PET0.0 | 415 | 435 | 449 | 9.4% |
| PP/PET0.1 | 424 | 447 | 468 | 6.4% |
| PP/PET0.5 | 417 | 443 | 465 | 10.3% |
| PP/PET1.0 | 421 | 445 | 464 | 11.1% |
| PP/PET1.5 | 426 | 452 | 472 | 9.9% |
| PP/PET2.0 | 433 | 454 | 473 | 6.6% |
| Sample Code | Melting Temperature |
|---|---|
| PP/PET0.0 | 146 |
| PP/PET0.1 | 146.6 |
| PP/PET0.5 | 147.2 |
| PP/PET1.0 | 148.9 |
| PP/PET1.5 | 146.2 |
| PP/PET2.0 | 147.5 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, D.; Tiwari, S.K.; Ma, Z.; Wen, J.; Liu, S.; Li, J.; Wei, F.; Thummavichai, K.; Yang, Z.; Zhu, Y.; et al. Phase Behavior and Thermo-Mechanical Properties of IF-WS2 Reinforced PP–PET Blend-Based Nanocomposites. Polymers 2020, 12, 2342. https://doi.org/10.3390/polym12102342
Chen D, Tiwari SK, Ma Z, Wen J, Liu S, Li J, Wei F, Thummavichai K, Yang Z, Zhu Y, et al. Phase Behavior and Thermo-Mechanical Properties of IF-WS2 Reinforced PP–PET Blend-Based Nanocomposites. Polymers. 2020; 12(10):2342. https://doi.org/10.3390/polym12102342
Chicago/Turabian StyleChen, Ding, Santosh K. Tiwari, Zhiyuan Ma, Jiahao Wen, Song Liu, Jiewei Li, Feng Wei, Kunyapat Thummavichai, Zhuxian Yang, Yanqiu Zhu, and et al. 2020. "Phase Behavior and Thermo-Mechanical Properties of IF-WS2 Reinforced PP–PET Blend-Based Nanocomposites" Polymers 12, no. 10: 2342. https://doi.org/10.3390/polym12102342





