Synergistic Action of Montmorillonite with an Intumescent Formulation: The Impact of the Nature and the Strength of Acidic Sites on the Flame-Retardant Properties of Polypropylene Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Montmorillonite’s Acidic Activation
2.3. Montmorillonite´s Chemical Composition: X-ray Fluorescence (XRF)
2.4. Montmorillonite’s X-ray Diffraction (XRD)
2.5. Montmorillonite’s Textural Properties
2.6. Montmorillonite’s Particle Size
2.7. Montmorillonite’s Acidity Quantification
2.8. Processing of the Composites
2.9. Polymer Composites Scanning Electron Microscopy (SEM)
2.10. Polymer Composites Thermogravimetric Analysis (TGA)
2.11. Polymer Composites Flammability Tests
2.11.1. Underwriters Laboratory UL-94
2.11.2. Limit Oxygen Index (LOI)
3. Results and Discussion
3.1. Montmorillonite’s Chemical Composition: X-ray Fluorescence (XRF)
3.2. Montmorillonite’s X-ray Diffraction (XRD)
3.3. Montmorillonite’s Textural Properties
3.4. Montmorillonite’s Particle Size
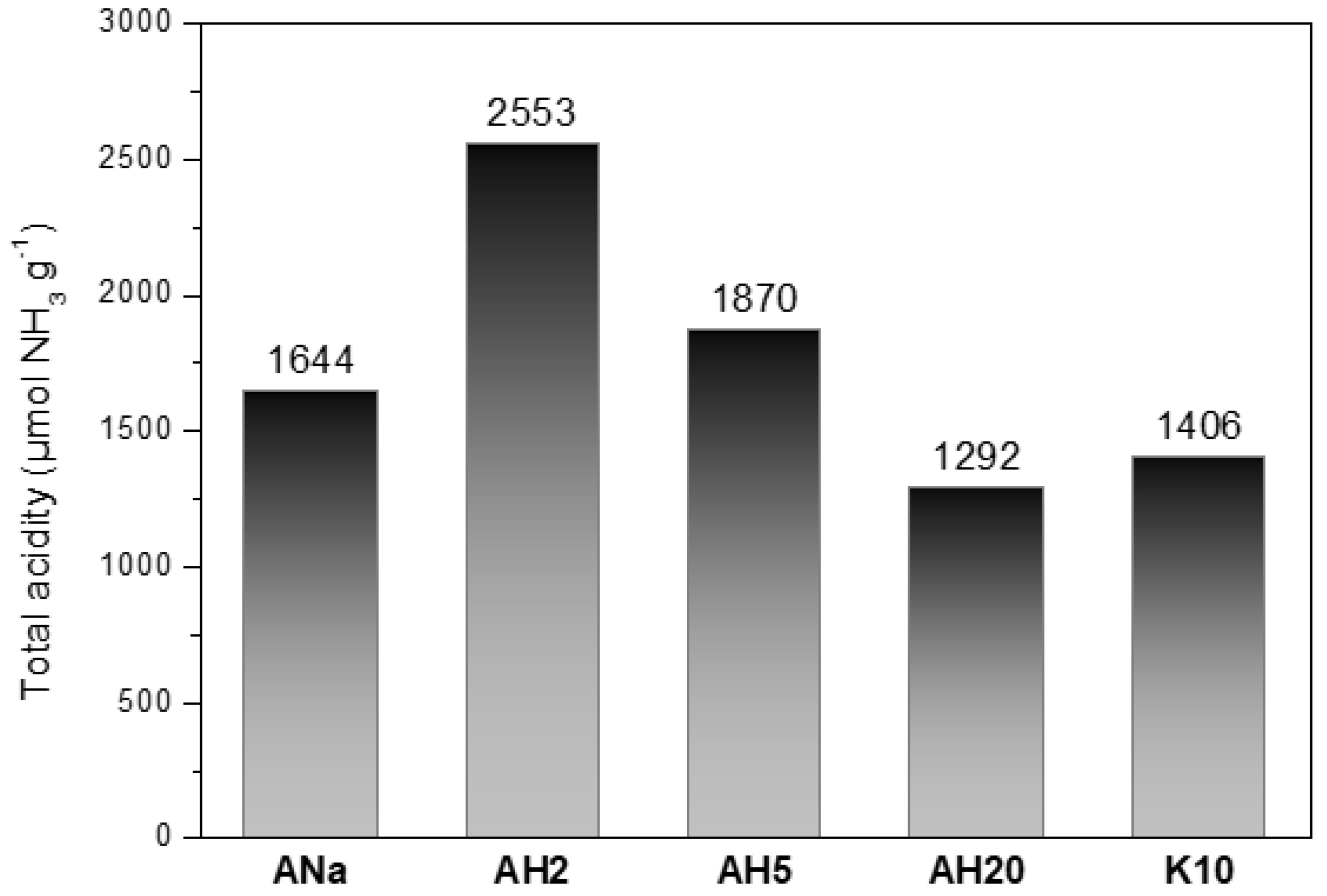
3.5. Montmorillonites Total Acidity: Temperature-Programmed Desorption (TPD)-NH3
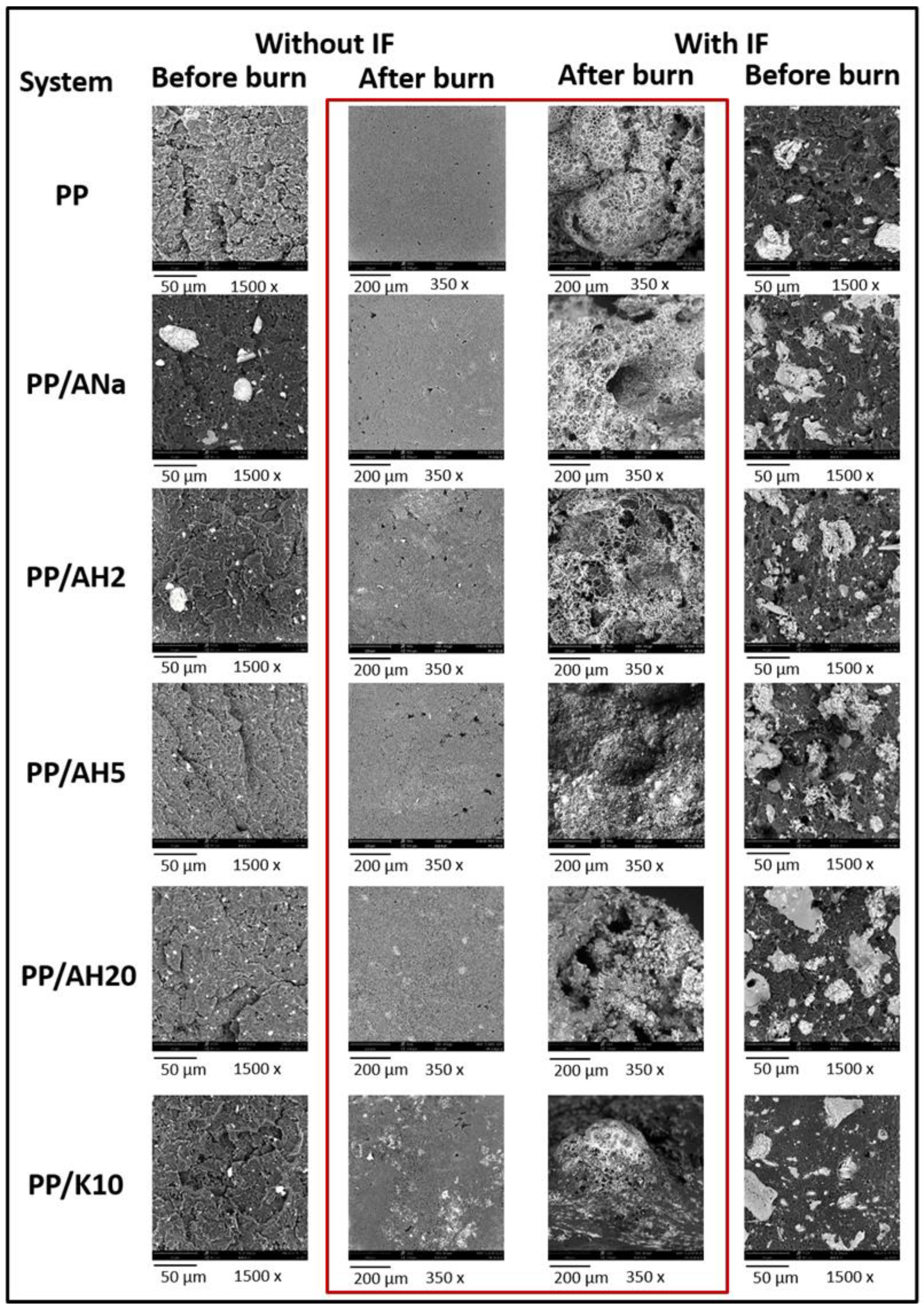
3.6. Char’s Morphology: Scanning Electron Microscopy (SEM)
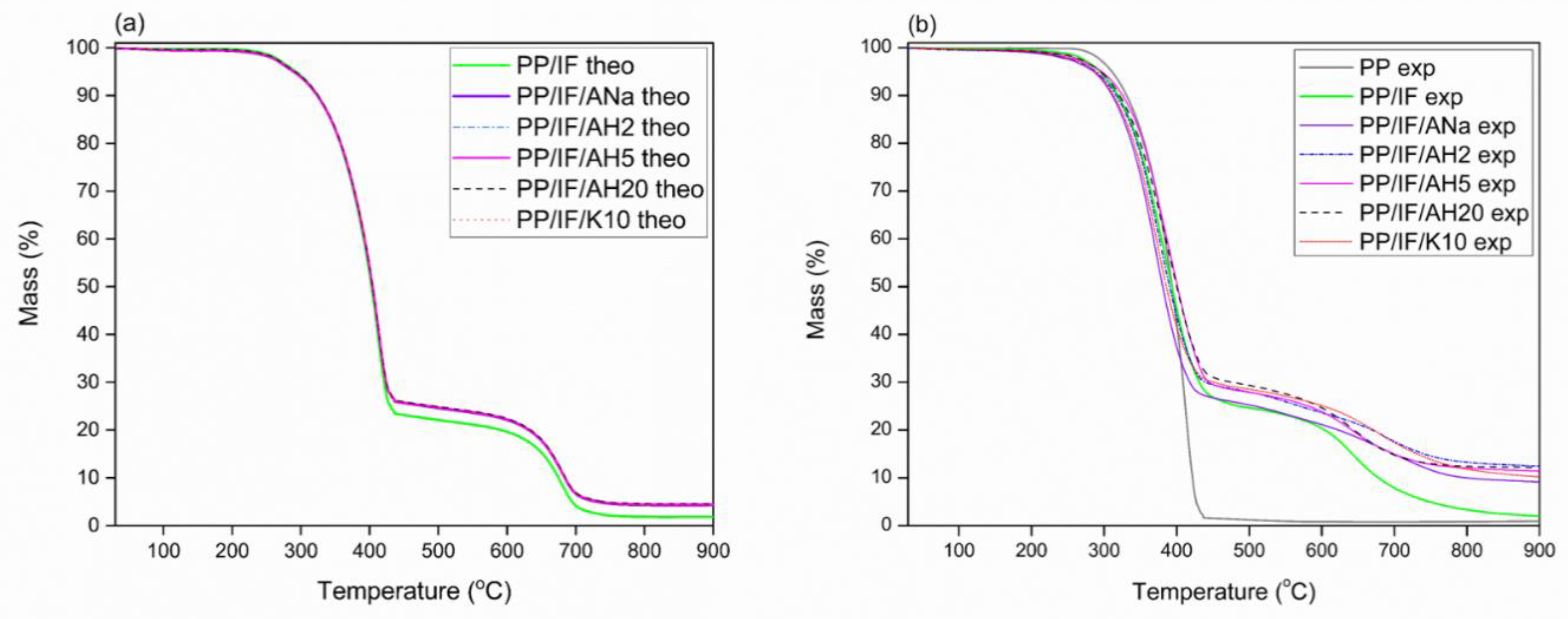
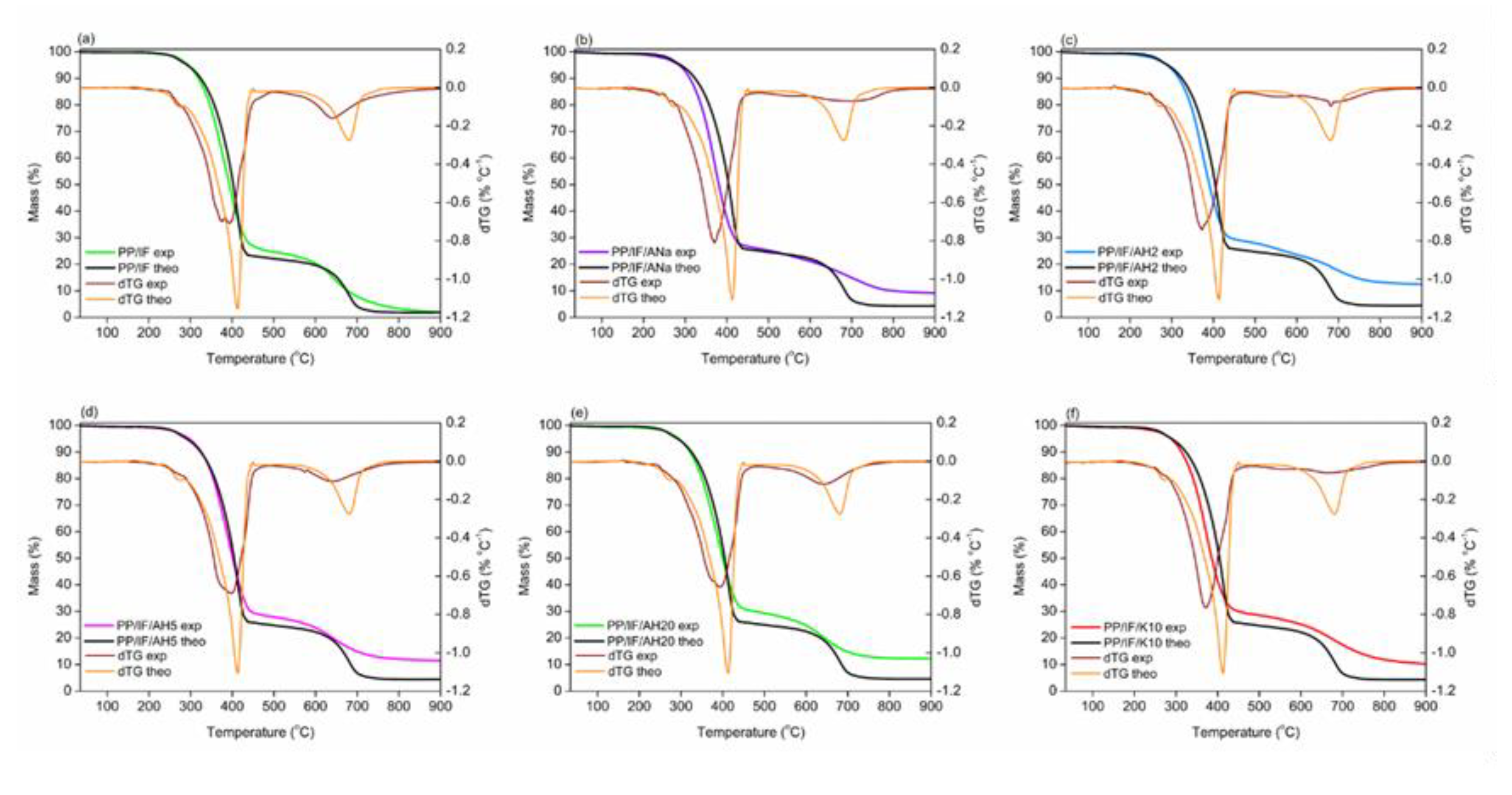
3.7. Thermal Behavior and Stability: TGA
3.8. Flammability Tests
3.8.1. UL-94
3.8.2. Limit Oxygen Index (LOI)
3.8.3. Montmorillonite’s Brønsted and Lewis Acidity: Fourier Transform Infrared Spectroscopy with Pyridine Adsorption (FTIR-Pyr)
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ahmed, L.; Zhang, B.; Shen, R.; Agnew, R.J.; Park, H.; Cheng, Z.; Mannan, M.S.; Wang, Q. Fire reaction properties of polystyrene-based nanocomposites using nanosilica and nanoclay as additives in cone calorimeter test. J. Therm. Anal. Calorim. 2018, 132, 1853–1865. [Google Scholar] [CrossRef]
- Benin, V.; Cui, X.; Morgan, A.B.; Seiwert, K. Synthesis and flammability testing of epoxy functionalized phosphorous-based flame retardants. J. Appl. Polym. Sci. 2015, 132, 42296. [Google Scholar] [CrossRef]
- Byard, B.; Wang, K.; Morgan, A.B.; Benin, V. New polyether diols as flame retardants for polyurethane: Derivatives of epoxy-functionalized phosphonates and phosphates. Fire Mater. 2017, 42, 3–17. [Google Scholar] [CrossRef]
- Wang, K.; Morgan, A.B.; Benin, V. Preparation and studies of new phosphorus-containing diols as potential flame retardants. Fire Mater. 2017, 41, 973–982. [Google Scholar] [CrossRef]
- Alongi, J.; Carosio, F.; Malucelli, G. Current emerging techniques to impart flame retardancy to fabrics: An overview. Polym. Degrad. Stab. 2014, 106, 138–149. [Google Scholar] [CrossRef]
- Liang, S.; Neisius, N.M.; Gaan, S. Recent developments in flame retardant polymeric coatings. Prog. Org. Coat. 2013, 76, 1642–1665. [Google Scholar] [CrossRef]
- Apaydin, K.; Laachachi, A.; Ball, V.; Jimenez, M.; Bourbigot, S.; Toniazzo, V.; Ruch, D. Polyallylamine–montmorillonite as super flame retardant coating assemblies by layer-by layer deposition on polyamide. Polym. Degrad. Stab. 2013, 98, 627–634. [Google Scholar] [CrossRef]
- Ahmed, L.; Zhang, B.; Hawkins, S.; Mannan, M.S.; Cheng, Z. Study of thermal and mechanical behaviors of flame retardant polystyrene-based nanocomposites prepared via in-situ polymerization method. J. Loss Prev. Process. Ind. 2017, 49, 228–239. [Google Scholar] [CrossRef]
- Velencoso, M.M.; Battig, A.; Markwart, J.C.; Schartel, B.; Wurm, F.R. Molecular Firefighting—How Modern Phosphorus Chemistry Can Help Solve the Challenge of Flame Retardancy. Angew. Chem. Int. Ed. 2018, 57, 10450–10467. [Google Scholar] [CrossRef] [Green Version]
- Zaikov, G.E.; Lomakin, S. Ecological issue of polymer flame retardancy. J. Appl. Polym. Sci. 2002, 86, 2449–2462. [Google Scholar] [CrossRef]
- Ribeiro, S.P.D.S.; Estevao, L.R.M.; Nascimento, R.S.V. Brazilian clays as synergistic agents in an ethylenic polymer matrix containing an intumescent formulation. J. Therm. Anal. Calorim. 2007, 87, 661–665. [Google Scholar] [CrossRef]
- Bourbigot, S.; Le Bras, M.; Delobel, R. Carbonization mechanisms resulting from intumescence association with the ammonium polyphosphate-pentaerythritol fire retardant system. Carbon 1993, 31, 1219–1230. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Trossarelli, L. Study of the mechanism of intumescence in fire retardant polymers: Part I—Thermal degradation of ammonium polyphosphate-pentaerythritol mixtures. Polym. Degrad. Stab. 1984, 6, 243–252. [Google Scholar] [CrossRef]
- Camino, G.; Costa, L.; Trossarelli, L.; Costanzi, F.; Pagliari, A. Study of the mechanism of intumescence in fire retardant polymers: Part VI—Mechanism of ester formation in ammonium polyphosphate-pentaerythritol mixtures. Polym. Degrad. Stab. 1985, 12, 213–228. [Google Scholar] [CrossRef]
- Delobel, R.; Le Bras, M.; Ouassou, N.; Descressain, R. Fire retardance of polypropylene by diammonium pyrophosphate-pentaerythritol: Spectroscopic characterization of the protective coatings. Polym. Degrad. Stab. 1990, 30, 41–56. [Google Scholar] [CrossRef]
- Ribeiro, S.P.D.S.; Estevão, L.R.D.M.; Novák, C.; Nascimento, R.S.V. Clays basal spacings effect on fire retardancy of polymers by TG/DTA. J. Therm. Anal. Calorim. 2011, 106, 535–539. [Google Scholar] [CrossRef]
- Yang, W.; Hu, Y.; Tai, Q.; Lu, H.; Song, L.; Yuen, R.K. Fire and mechanical performance of nanoclay reinforced glass-fiber/PBT composites containing aluminum hypophosphite particles. Compos. Part A Appl. Sci. Manuf. 2011, 42, 794–800. [Google Scholar] [CrossRef]
- Yang, W.; Kan, Y.; Song, L.; Hu, Y.; Lu, H.; Yuen, R.K. Effect of organo-modified montmorillonite on flame retardant poly(1,4-butylene terephthalate) composites. Polym. Adv. Technol. 2010, 22, 2564–2570. [Google Scholar] [CrossRef]
- Liu, M.; Zhang, X.; Zammarano, M.; Gilman, J.W.; Kashiwagi, T. Flame retardancy of poly(styrene-co-acrylonitrile) by the synergistic interaction between clay and phosphomolybdate hydrates. Polym. Degrad. Stab. 2011, 96, 1000–1008. [Google Scholar] [CrossRef]
- Fina, A.; Cuttica, F.; Camino, G. Ignition of polypropylene/montmorillonite nanocomposites. Polym. Degrad. Stab. 2012, 97, 2619–2626. [Google Scholar] [CrossRef]
- Jha, A.; Garade, A.; Shirai, M.; Rode, C.V. Metal cation-exchanged montmorillonite clay as catalysts for hydroxyalkylation reaction. Appl. Clay Sci. 2013, 74, 141–146. [Google Scholar] [CrossRef]
- Tyagi, B.; Chudasama, C.D.; Jasra, R.V. Characterization of surface acidity of an acid montmorillonite activated with hydrothermal, ultrasonic and microwave techniques. Appl. Clay Sci. 2006, 31, 16–28. [Google Scholar] [CrossRef]
- Zanetti, M.; Camino, G.; Thomann, R.; Mülhaupt, R. Synthesis and thermal behaviour of layered silicate–EVA nanocomposites. Polymer 2001, 42, 4501–4507. [Google Scholar] [CrossRef]
- Tang, Y.; Hu, Y.; Li, B.; Liu, L.; Wang, Z.; Chen, Z.; Fan, W. Polypropylene/montmorillonite nanocomposites and intumescent, flame-retardant montmorillonite synergism in polypropylene nanocomposites. J. Polym. Sci. Part A Polym. Chem. 2004, 42, 6163–6173. [Google Scholar] [CrossRef]
- Bernardes, F.R.; Rezende, M.J.; Rodrigues, V.D.O.; Nascimento, R.S.V.; Ribeiro, S.P.D.S. Synthesis and Application of H-ZSM-5 Zeolites with Different Levels of Acidity as Synergistic Agents in Flame Retardant Polymeric Materials. Polymers 2019, 11, 2110. [Google Scholar] [CrossRef] [Green Version]
- Ribeiro, S.P.D.S.; Martins, R.C.; Barbosa, G.M.; Rocha, M.A.D.F.; Landesmann, A.; Nascimento, M.A.C.; Nascimento, R.S.V. Influence of the zeolite acidity on its synergistic action with a flame-retarding polymeric intumescent formulation. J. Mater. Sci. 2019, 55, 619–630. [Google Scholar] [CrossRef]
- Rezende, M.J.; Pereira, M.S.; Santos, G.F.; Aroeira, G.O.; Albuquerque, T.C., Jr.; Suarez, P.A.; Pinto, A.C. Preparation, characterisation and evaluation of brazilian clay-based catalysts for use in esterification reactions. J. Braz. Chem. Soc. 2012, 23, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Underwriters’ Laboratories. Test for Flammability of Plastic Materials for Parts in Devices and Appliances—UL-94; Underwriters’ Laboratories: Northbrook, IL, USA, 2003. [Google Scholar]
- Steudel, A.; Batenburg, L.; Fischer, H.; Weidler, P.; Emmerich, K. Alteration of swelling clay minerals by acid activation. Appl. Clay Sci. 2009, 44, 105–115. [Google Scholar] [CrossRef]
- Barzetti, T.; Selli, E.; Moscotti, D.; Forni, L. Pyridine and ammonia as probes for FTIR analysis of solid acid catalysts. J. Chem. Soc. Faraday Trans. 1996, 92, 1401–1407. [Google Scholar] [CrossRef]
- Emeis, C. Determination of Integrated Molar Extinction Coefficients for Infrared Absorption Bands of Pyridine Adsorbed on Solid Acid Catalysts. J. Catal. 1993, 141, 347–354. [Google Scholar] [CrossRef]
- Hughes, T.R.; White, H.M. A study of the surface structure of decationized Y zeolite by quantitative infrared spectroscopy. J. Phys. Chem. 1967, 71, 2192–2201. [Google Scholar] [CrossRef]
- Datka, J.; Turek, A.M.; Jehng, J.M.; Wachs, I.E. Acidic properties of supported niobium oxide catalysts: An infrared spectroscopy investigation. J. Catal. 1992, 135, 186–199. [Google Scholar] [CrossRef]
- Zaki, M.I.; Hussein, G.; Mansour, S.; El-Ammawy, H. Adsorption and surface reactions of pyridine on pure and doped ceria catalysts as studied by infrared spectroscopy. J. Mol. Catal. 1989, 51, 209–220. [Google Scholar] [CrossRef]
- Neto, R.C.R.; Schmal, M. Synthesis of CeO2 and CeZrO2 mixed oxide nanostructured catalysts for the iso-syntheses reaction. Appl. Catal. A Gen. 2013, 450, 131–142. [Google Scholar] [CrossRef]
- Zaki, M.I.; Hasan, M.A.; Al-Sagheer, F.A.; Pasupulety, L. In situ FTIR spectra of pyridine adsorbed on SiO2–Al2O3, TiO2, ZrO2 and CeO2: General considerations for the identification of acid sites on surfaces of finely divided metal oxides. Colloids Surf. A Physicochem. Eng. Asp. 2001, 190, 261–274. [Google Scholar] [CrossRef]
- Reddy, C.R.; Bhat, Y.; Nagendrappa, G.; Prakash, B.J. Brønsted and Lewis acidity of modified montmorillonite clay catalysts determined by FT-IR spectroscopy. Catal. Today 2009, 141, 157–160. [Google Scholar] [CrossRef] [Green Version]
- Busca, G. The surface acidity of solid oxides and its characterization by IR spectroscopic methods. An attempt at systematization. Phys. Chem. Chem. Phys. 1999, 1, 723–736. [Google Scholar] [CrossRef]
- Akçay, M. The surface acidity and characterization of Fe-montmorillonite probed by in situ FT-IR spectroscopy of adsorbed pyridine. Appl. Catal. A Gen. 2005, 294, 156–160. [Google Scholar] [CrossRef]
- Zaccheria, F.; Santoro, F.; Iftitah, E.D.; Ravasio, N. Brønsted and Lewis Solid Acid Catalysts in the Valorization of Citronellal. Catalysts 2018, 8, 410. [Google Scholar] [CrossRef] [Green Version]
- Kooli, F.; Jones, W. Characterization and catalytic properties of a saponite clay modified by acid activation. Clay Miner. 1997, 32, 633–643. [Google Scholar] [CrossRef]
- Komadel, P.; Janek, M.; Madejová, J.; Weekes, A.; Breen, R. Acidity and catalytic activity of mildly acid-treated Mg-rich montmorillonite and hectorite. J. Chem. Soc. Faraday Trans. 1997, 93, 4207–4210. [Google Scholar] [CrossRef]





| System | Polypropylene (PP) | Intumescent Formulation (IF) | Clay | Mtheo (T) |
|---|---|---|---|---|
| PP/IF | 70% | 30% | - | Mtheo (T) = 0.70 MPP (T) + 0.30 MIF (T) |
| PP/IF/clay | 67% | 30% | 3% | Mtheo (T) = 0.67 MPP (T) + 0.30 MIF (T) + 0.03 Mclay (T) |
| Criteria | V-0 | V-1 | V-2 |
|---|---|---|---|
| Individual values of t1 and t2 for the 5 specimens | <10 s | <30 s | <30 s |
| (t1+t2) for the 5 specimens | <50 s | <250 s | <250 s |
| t3 values | <30 s | <60 s | <60 s |
| Combustion up to holding clamp (specimens completely burned) | No | No | No |
| Dripping of burning specimens (ignition of cotton batting) | No | No | Yes |
| MgO | Al2O3 | SiO2 | SO3 | K2O | TiO2 | Fe2O3 | CaO | Cl | Na2O | *LDC | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| ANa | 2.00 | 12.8 | 62.7 | <0.1 | 0.13 | 0.63 | 5.1 | 0.57 | 0.15 | 0.48 | 15.4 |
| AH2 | 0.96 | 10.7 | 72.0 | <0.1 | 0.12 | 0.70 | 3.0 | <0.1 | <0.1 | <0.1 | 12.5 |
| AH5 | 0.78 | 8.9 | 75.5 | 0.12 | 0.12 | 0.71 | 2.1 | <0.1 | <0.1 | <0.1 | 11.7 |
| AH20 | <0.1 | 1.4 | 90.8 | 0.31 | 0.12 | 0.75 | 0.3 | <0.1 | <0.1 | <0.1 | 6.2 |
| K10 | 1.80 | 15.6 | 60.3 | 0.12 | 1.20 | 0.47 | 3.7 | 0.26 | <0.1 | 0.30 | 16.3 |
| Sample | Specific Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Desorption Average Pore Diameter (Å) |
|---|---|---|---|
| ANa | 104 | 0.16 | 53 |
| AH2 | 145 | 0.18 | 51 |
| AH5 | 137 | 0.18 | 53 |
| AH20 | 87 | 0.23 | 139 |
| K10 | 178 | 0.22 | 51 |
| Sample | Experimental Data | Theoretical Data | ||||||
|---|---|---|---|---|---|---|---|---|
| % Mass at 900 °C | Tonset (°C) | a Tpeak1 (°C) | a Tpeak2 (°C) | % Mass at 900 °C | Tonset (°C) | Tpeak1 (°C) | Tpeak2 (°C) | |
| PP/IF | 2.0 | 275 | 378/394 | 642 | 1.8 | 290 | 414 | 680 |
| PP/IF/Ana | 9.1 | 271 | 370 | 697 | 4.3 | 311 | 414 | 680 |
| PP/IF/AH2 | 12.5 | 273 | 374 | 682 | 4.4 | 311 | 414 | 680 |
| PP/IF/AH5 | 11.4 | 290 | 399 | 642 | 4.4 | 311 | 414 | 680 |
| PP/IF/AH20 | 12.2 | 293 | 394 | 642 | 4.6 | 311 | 414 | 680 |
| PP/IF/K10 | 10.2 | 288 | 369 | 664 | 4.3 | 311 | 414 | 680 |
| Sample Without IF | LOI ± 1 (% O2) | Sample With IF | LOI ± 1 (% O2) |
|---|---|---|---|
| PP | 17 | PP/IF | 30 |
| PP/ANa | 18 | PP/IF/Ana | 35 |
| PP/AH2 | 18 | PP/IF/AH2 | 35 |
| PP/AH5 | 18 | PP/IF/AH5 | 38 |
| PP/AH20 | 18 | PP/IF/AH20 | 27 |
| PP/K10 | 18 | PP/IF/K10 | 33 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martins, R.C.; Rezende, M.J.C.; Nascimento, M.A.C.; Nascimento, R.S.V.; Ribeiro, S.P.d.S. Synergistic Action of Montmorillonite with an Intumescent Formulation: The Impact of the Nature and the Strength of Acidic Sites on the Flame-Retardant Properties of Polypropylene Composites. Polymers 2020, 12, 2781. https://doi.org/10.3390/polym12122781
Martins RC, Rezende MJC, Nascimento MAC, Nascimento RSV, Ribeiro SPdS. Synergistic Action of Montmorillonite with an Intumescent Formulation: The Impact of the Nature and the Strength of Acidic Sites on the Flame-Retardant Properties of Polypropylene Composites. Polymers. 2020; 12(12):2781. https://doi.org/10.3390/polym12122781
Chicago/Turabian StyleMartins, Raíssa Carvalho, Michelle Jakeline Cunha Rezende, Marco Antonio Chaer Nascimento, Regina Sandra Veiga Nascimento, and Simone Pereira da Silva Ribeiro. 2020. "Synergistic Action of Montmorillonite with an Intumescent Formulation: The Impact of the Nature and the Strength of Acidic Sites on the Flame-Retardant Properties of Polypropylene Composites" Polymers 12, no. 12: 2781. https://doi.org/10.3390/polym12122781







