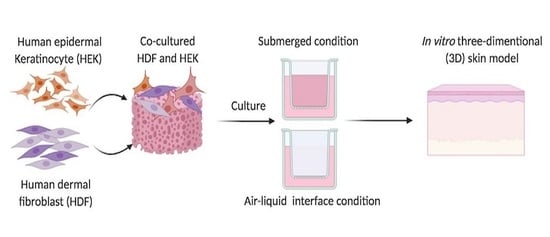
Preliminary Study of In Vitro Three-Dimensional Skin Model Using an Ovine Collagen Type I Sponge Seeded with Co-Culture Skin Cells: Submerged versus Air-Liquid Interface Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Extraction and Purification of Ovine Collagen Type I
2.2. Fabrication of OTC-I Sponge Scaffolds
2.3. Crosslinking of OTC-I Sponge Scaffolds
2.4. Characterisation of OTC-I Sponge Scaffolds
2.4.1. Gross Appearance
2.4.2. Swelling Ratio and Water Uptake Ability
2.4.3. Morphological Evaluation
2.4.4. Fourier Transform Infrared Spectrometry
2.5. Human Skin Cell Isolation and Culture
2.6. Cell-Bioscaffold Interaction
2.6.1. Cellular Toxicity
2.6.2. Cell Attachment
2.6.3. Cell Proliferation
2.7. In Vitro Reconstructed Skin Model
2.7.1. Submerged Microenvironment
2.7.2. Air-Liquid Interface (ALI) Microenvironment
2.7.3. Morphology Assessment
2.8. Statistical Analysis
3. Results
3.1. Physical Characterisation of OTC-I Bioscaffolds
3.1.1. Gross Appearance
3.1.2. Swelling Ratio and Water Uptake Ability
3.1.3. Morphological Evaluation
3.2. Chemical Characterisation of OTC-I Bioscaffolds
Fourier Transformation Infrared (FT-IR)
3.3. Cellular Biocompatibility
3.3.1. Cell Cytotoxicity
3.3.2. Cell Attachment
3.3.3. Cell Proliferation
3.4. Micromorphology under Submerged and Air-Liquid Interface Microenvironments
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- De Wever, B.; Petersohn, D.; Mewes, K.R. Overview of human three-dimensional (3D) skin models used for dermal toxicity assessment—Part 1. Househ. Pers. Care Today 2013, 8, 18–22. [Google Scholar]
- Prior, H.; Casey, W.; Kimber, I.; Whelan, M.; Sewell, F. Reflections on the progress towards non-animal methods for acute toxicity testing of chemicals. Regul. Toxicol. Pharmacol. 2019, 102, 30–33. [Google Scholar] [CrossRef] [PubMed]
- Pridgeon, C.S.; Schlott, C.; Wong, M.W.; Heringa, M.B.; Heckel, T.; Leedale, J.; Launay, L.; Gryshkova, V.; Przyborski, S.; Bearon, R.N.; et al. Innovative organotypic in vitro models for safety assessment: Aligning with regulatory requirements and understanding models of the heart, skin, and liver as paradigms. Arch. Toxicol. 2018, 92, 557–569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liguori, G.R.; Jeronimus, B.F.; de Aquinas Liguori, T.T.; Moreira, L.F.P.; Harmsen, M.C. Ethical Issues in the Use of Animal Models for Tissue Engineering: Reflections on Legal Aspects, Moral Theory, Three Rs Strategies, and Harm–Benefit Analysis. Tissue Eng. Part C Methods 2017, 23, 850–862. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duval, K.; Grover, H.; Han, L.-H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- Pedram Rad, Z.; Mokhtari, J.; Abbasi, M. Fabrication and characterization of PCL/zein/gum arabic electrospun nanocomposite scaffold for skin tissue engineering. Mater. Sci. Eng. C 2018, 93, 356–366. [Google Scholar] [CrossRef]
- Klicks, J.; von Molitor, E.; Ertongur-Fauth, T.; Rudolf, R.; Hafner, M. In vitro skin three-dimensional models and their applications. J. Cell. Biotechnol. 2017, 3, 21–39. [Google Scholar] [CrossRef] [Green Version]
- Bray, L.J.; Binner, M.; Holzheu, A.; Friedrichs, J.; Freudenberg, U.; Hutmacher, D.W.; Werner, C. Multi-parametric hydrogels support 3D in vitro bioengineered microenvironment models of tumour angiogenesis. Biomaterials 2015, 53, 609–620. [Google Scholar] [CrossRef] [Green Version]
- Edmondson, R.; Broglie, J.J.; Adcock, A.F.; Yang, L. Three-dimensional cell culture systems and their applications in drug discovery and cell-based biosensors. Assay Drug Dev. Technol. 2014, 12, 207–218. [Google Scholar] [CrossRef] [Green Version]
- Meng, Q.; Shen, C. Construction of low contracted 3D skin equivalents by genipin cross-linking. Exp. Dermatol. 2018, 27, 1098–1103. [Google Scholar] [CrossRef]
- Rademacher, F.; Simanski, M.; Gläser, R.; Harder, J. Skin microbiota and human 3D skin models. Exp. Dermatol. 2018, 27, 489–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Niehues, H.; Bouwstra, J.A.; El Ghalbzouri, A.; Brandner, J.M.; Zeeuwen, P.L.J.M.; van den Bogaard, E.H. 3D skin models for 3R research: The potential of 3D reconstructed skin models to study skin barrier function. Exp. Dermatol. 2018, 27, 501–511. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, L.; Amann, P.M.; Marquardt, Y.; Heise, R.; Czaja, K.; Gerber, P.A.; Steiner, T.; Hölzle, F.; Baron, J.M. Molecular effects of fractional ablative erbium:YAG laser treatment with multiple stacked pulses on standardized human three-dimensional organotypic skin models. Lasers Med. Sci. 2017, 32, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Castro-Muñoz, R.; González-Valdez, J. New trends in biopolymer-based membranes for pervaporation. Molecules 2019, 24, 3584. [Google Scholar] [CrossRef] [Green Version]
- Solovieva, E.V.; Fedotov, A.Y.; Mamonov, V.E.; Komlev, V.; Panteleyev, A.A. Fibrinogen-modified sodium alginate as a scaffold material for skin tissue engineering. Biomed. Mater. 2018, 13, 025007. [Google Scholar] [CrossRef] [Green Version]
- Tchemtchoua, V.T.; Atanasova, G.; Aqil, A.; Filée, P.; Garbacki, N.; Vanhooteghem, O.; Deroanne, C.; Noel, A.; Jérome, C.; Nusgens, B.; et al. Development of a Chitosan Nanofibrillar Scaffold for Skin Repair and Regeneration. Biomacromolecules 2011, 12, 3194–3204. [Google Scholar] [CrossRef]
- Janik, H.; Marzec, M. A review: Fabrication of porous polyurethane scaffolds. Mater. Sci. Eng. C 2015, 48, 586–591. [Google Scholar] [CrossRef]
- Shahin, H.; Elmasry, M.; Steinvall, I.; Söberg, F.; El-Serafi, A. Vascularization is the next challenge for skin tissue engineering as a solution for burn management. Burn. Trauma 2020, 8. [Google Scholar] [CrossRef]
- Khan, F.; Tanaka, M. Designing smart biomaterials for tissue engineering. Int. J. Mol. Sci. 2018, 19, 17. [Google Scholar] [CrossRef] [Green Version]
- Nishio, A.; Kubo, H.; Hashimoto, Y.; Kakudo, K. Comparison of chondrocyte differentiation ability using three-dimensional culture in an atelocollagen sponge on dedifferentiated fat cells and adipose-derived stem cells from the human buccal fat pad. J. Oral Maxillofac. Surg. 2014, 72, e211. [Google Scholar] [CrossRef]
- Fauzi, M.B.; Lokanathan, Y.; Aminuddin, B.S.; Ruszymah, B.H.I.; Chowdhury, S.R. Ovine tendon collagen: Extraction, characterisation and fabrication of thin films for tissue engineering applications. Mater. Sci. Eng. C 2016, 68, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Fauzi, M.B.; Lokanathan, Y.; Nadzir, M.M.; Aminuddin, S.; Ruszymah, B.H.I.; Chowdhury, S.R. Attachment, proliferation, and morphological properties of human dermal fibroblasts on ovine tendon collagen scaffolds: A comparative study. Malays. J. Med. Sci. 2017, 24, 33. [Google Scholar] [CrossRef]
- Mh Busra, F.; Rajab, N.F.; Tabata, Y.; Saim, A.B.; BHIdrus, R.; Chowdhury, S.R. Rapid treatment of full-thickness skin loss using ovine tendon collagen type I scaffold with skin cells. J. Tissue Eng. Regen. Med. 2019, 13, 874–891. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Li, Q.; Pan, Y.; Yao, Y.; Tang, S.; Su, J.; Shin, J.-W.; Wei, J.; Zhao, J. Nanoporosity improved water absorption, in vitro degradability, mineralization, osteoblast responses and drug release of poly(butylene succinate)-based composite scaffolds containing nanoporous magnesium silicate compared with magnesium silicate. Int. J. Nanomed. 2017, 12, 3637–3651. [Google Scholar] [CrossRef] [Green Version]
- Nayak, S.; Dey, S.; Kundu, S.C. Skin Equivalent Tissue-Engineered Construct: Co-Cultured Fibroblasts/Keratinocytes on 3D Matrices of Sericin Hope Cocoons. PLoS ONE 2013, 8, e74779. [Google Scholar] [CrossRef]
- Bellas, E.; Seiberg, M.; Garlick, J.; Kaplan, D.L. In vitro 3D Full-Thickness Skin-Equivalent Tissue Model Using Silk and Collagen Biomaterials. Macromol. Biosci. 2012, 12, 1627–1636. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.-X.; Luo, X.; Gu, Y.-W.; Yang, B.; Wang, Z. Correlation of discoloration and biomechanical properties in porcine sclera induced by genipin. Int. J. Ophthalmol. 2014, 7, 621. [Google Scholar] [CrossRef]
- Sundararaghavan, H.G.; Monteiro, G.A.; Lapin, N.A.; Chabal, Y.J.; Miksan, J.R.; Shreiber, D.I. Genipin-induced changes in collagen gels: Correlation of mechanical properties to fluorescence. J. Biomed. Mater. Res. Part A 2008, 87, 308–320. [Google Scholar] [CrossRef]
- Mekhail, M.; Wong, K.K.H.; Padavan, D.T.; Wu, Y.; O’Gorman, D.B.; Wan, W. Genipin-cross-linked electrospun collagen fibers. J. Biomater. Sci. Polym. Ed. 2011, 22, 2241–2259. [Google Scholar] [CrossRef]
- Ghodbane, S.A.; Dunn, M.G. Physical and mechanical properties of cross-linked type I collagen scaffolds derived from bovine, porcine, and ovine tendons. J. Biomed. Mater. Res. Part A 2016, 104, 2685–2692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, B.S.; Kim, J.S.; Lee, J. Improvements of osteoblast adhesion, proliferation, and differentiation in vitro via fibrin network formation in collagen sponge scaffold. J. Biomed. Mater. Res. Part A 2013, 101, 2661–2666. [Google Scholar] [CrossRef] [PubMed]
- Amri, M.; Firdaus, M.; Fauzi, M.; Chowdhury, S.; Fadilah, N.; Hamirul, W.W.; Reusmaazran, M.; Aminuddin, B.; Ruszymah, B. Cytotoxic evaluation of biomechanically improved crosslinked ovine collagen on human dermal fibroblasts. Bio-Med. Mater. Eng. 2014, 24, 1715–1724. [Google Scholar] [CrossRef] [PubMed]
- Riaz, T.; Zeeshan, R.; Zarif, F.; Ilyas, K.; Muhammad, N.; Safi, S.Z.; Rahim, A.; Rizvi, S.A.A.; Rehman, I.U. FTIR analysis of natural and synthetic collagen. Appl. Spectrosc. Rev. 2018, 53, 703–746. [Google Scholar] [CrossRef]
- Wang, C.; Lau, T.T.; Loh, W.L.; Su, K.; Wang, D.A. Cytocompatibility study of a natural biomaterial crosslinker-Genipin with therapeutic model cells. J. Biomed. Mater. Res. Part B Appl. Biomater. 2011, 97, 58–65. [Google Scholar] [CrossRef]
- Fessel, G.; Cadby, J.; Wunderli, S.; Van Weeren, R.; Snedeker, J.G. Dose- and time-dependent effects of genipin crosslinking on cell viability and tissue mechanics—Toward clinical application for tendon repair. Acta Biomater. 2014, 10, 1897–1906. [Google Scholar] [CrossRef] [Green Version]
- Malcor, J.-D.; Bax, D.; Hamaia, S.W.; Davidenko, N.; Best, S.M.; Cameron, R.E.; Farndale, R.W.; Bihan, D. The synthesis and coupling of photoreactive collagen-based peptides to restore integrin reactivity to an inert substrate, chemically-crosslinked collagen. Biomaterials 2016, 85, 65–77. [Google Scholar] [CrossRef] [Green Version]
- Powell, H.M.; Boyce, S.T. EDC cross-linking improves skin substitute strength and stability. Biomaterials 2006, 27, 5821–5827. [Google Scholar] [CrossRef]
- Bax, D.V.; Davidenko, N.; Gullberg, D.; Hamaia, S.W.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Fundamental insight into the effect of carbodiimide crosslinking on cellular recognition of collagen-based scaffolds. Acta Biomater. 2017, 49, 218–234. [Google Scholar] [CrossRef]
- Lammers, G.; Tjabringa, G.S.; Schalkwijk, J.; Daamen, W.F.; van Kuppevelt, T.H. A molecularly defined array based on native fibrillar collagen for the assessment of skin tissue engineering biomaterials. Biomaterials 2009, 30, 6213–6220. [Google Scholar] [CrossRef]
- Park, S.-N.; Park, J.-C.; Kim, H.O.; Song, M.J.; Suh, H. Characterization of porous collagen/hyaluronic acid scaffold modified by 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide cross-linking. Biomaterials 2002, 23, 1205–1212. [Google Scholar] [CrossRef]
- Sakwe, A.M.; Koumangoye, R.; Goodwin, S.J.; Ochieng, J. Fetuin-A (α2HS-glycoprotein) is a major serum adhesive protein that mediates growth signaling in breast tumor cells. J. Biol. Chem. 2010, 285, 41827–41835. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franco-Barraza, J.; Beacham, D.A.; Amatangelo, M.D.; Cukierman, E. Preparation of extracellular matrices produced by cultured and primary fibroblasts. Curr. Protoc. Cell Biol. 2016, 33, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Giavazzi, F.; Paoluzzi, M.; Macchi, M.; Bi, D.; Scita, G.; Manning, M.L.; Cerbino, F.G.R.; Marchetti, M.C. Flocking transitions in confluent tissues. Soft Matter 2018, 14, 3471–3477. [Google Scholar] [CrossRef] [PubMed]
- Bernstam, L.I.; Vaughan, F.L.; Bernstein, I.A. Keratinocytes grown at the air-liquid interface. In Vitro Cell. Dev. Biol. Anim. 1986, 22, 695–705. [Google Scholar] [CrossRef] [PubMed]
- Xian, L.J.; Chowdhury, S.R.; Bin Saim, A.; Idrus, R.B.H. Concentration-dependent effect of platelet-rich plasma on keratinocyte and fibroblast wound healing. Cytotherapy 2015, 17, 293–300. [Google Scholar] [CrossRef] [PubMed]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fauzi, M.B.; Rashidbenam, Z.; Bin Saim, A.; Binti Hj Idrus, R. Preliminary Study of In Vitro Three-Dimensional Skin Model Using an Ovine Collagen Type I Sponge Seeded with Co-Culture Skin Cells: Submerged versus Air-Liquid Interface Conditions. Polymers 2020, 12, 2784. https://doi.org/10.3390/polym12122784
Fauzi MB, Rashidbenam Z, Bin Saim A, Binti Hj Idrus R. Preliminary Study of In Vitro Three-Dimensional Skin Model Using an Ovine Collagen Type I Sponge Seeded with Co-Culture Skin Cells: Submerged versus Air-Liquid Interface Conditions. Polymers. 2020; 12(12):2784. https://doi.org/10.3390/polym12122784
Chicago/Turabian StyleFauzi, Mh Busra, Zahra Rashidbenam, Aminuddin Bin Saim, and Ruszymah Binti Hj Idrus. 2020. "Preliminary Study of In Vitro Three-Dimensional Skin Model Using an Ovine Collagen Type I Sponge Seeded with Co-Culture Skin Cells: Submerged versus Air-Liquid Interface Conditions" Polymers 12, no. 12: 2784. https://doi.org/10.3390/polym12122784