Influence of Fluorine Substitution on the Optical, Thermal, Electrochemical and Structural Properties of Carbazole-Benzothiadiazole Dicarboxylic Imide Alternate Copolymers
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Measurements
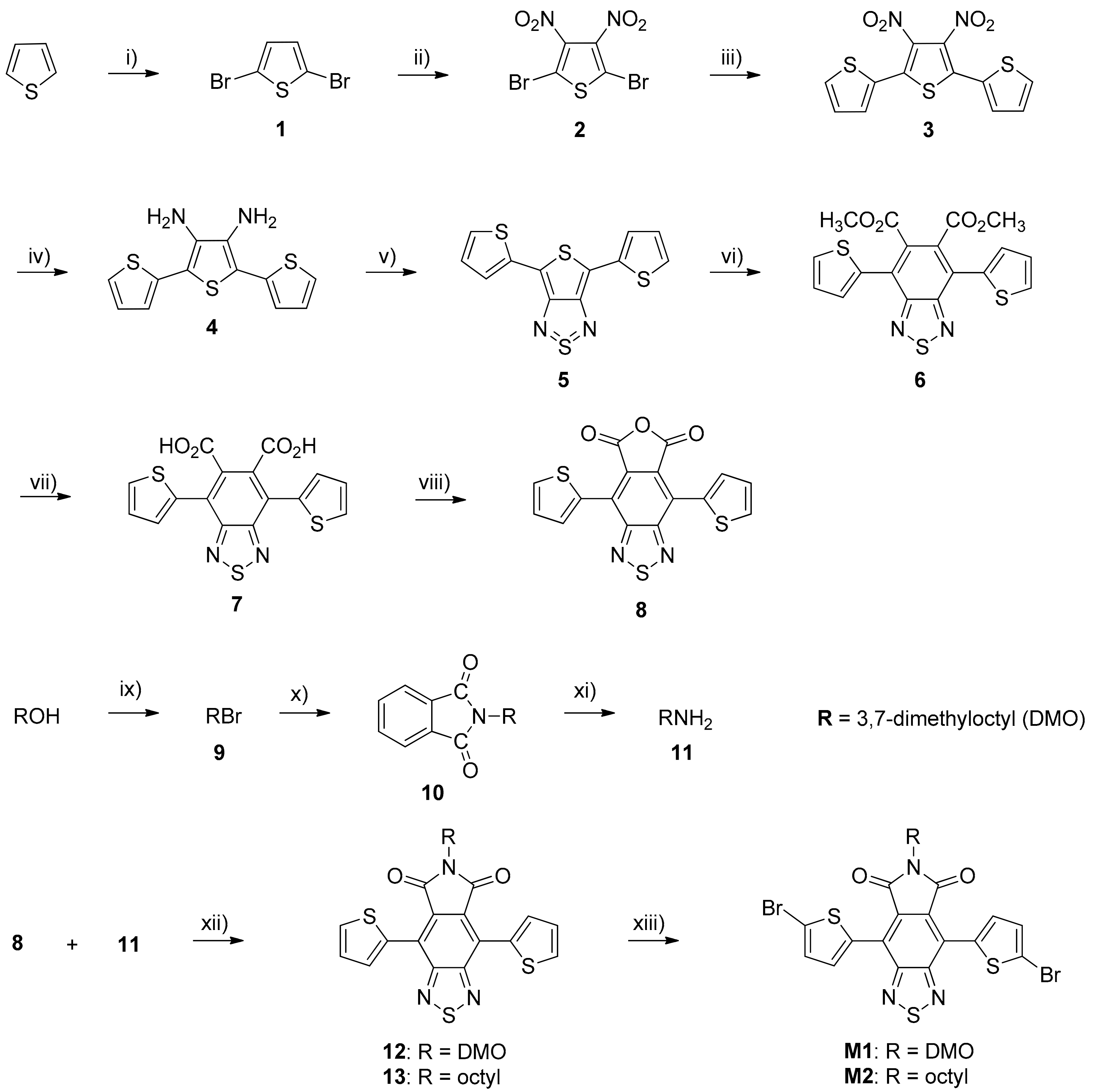
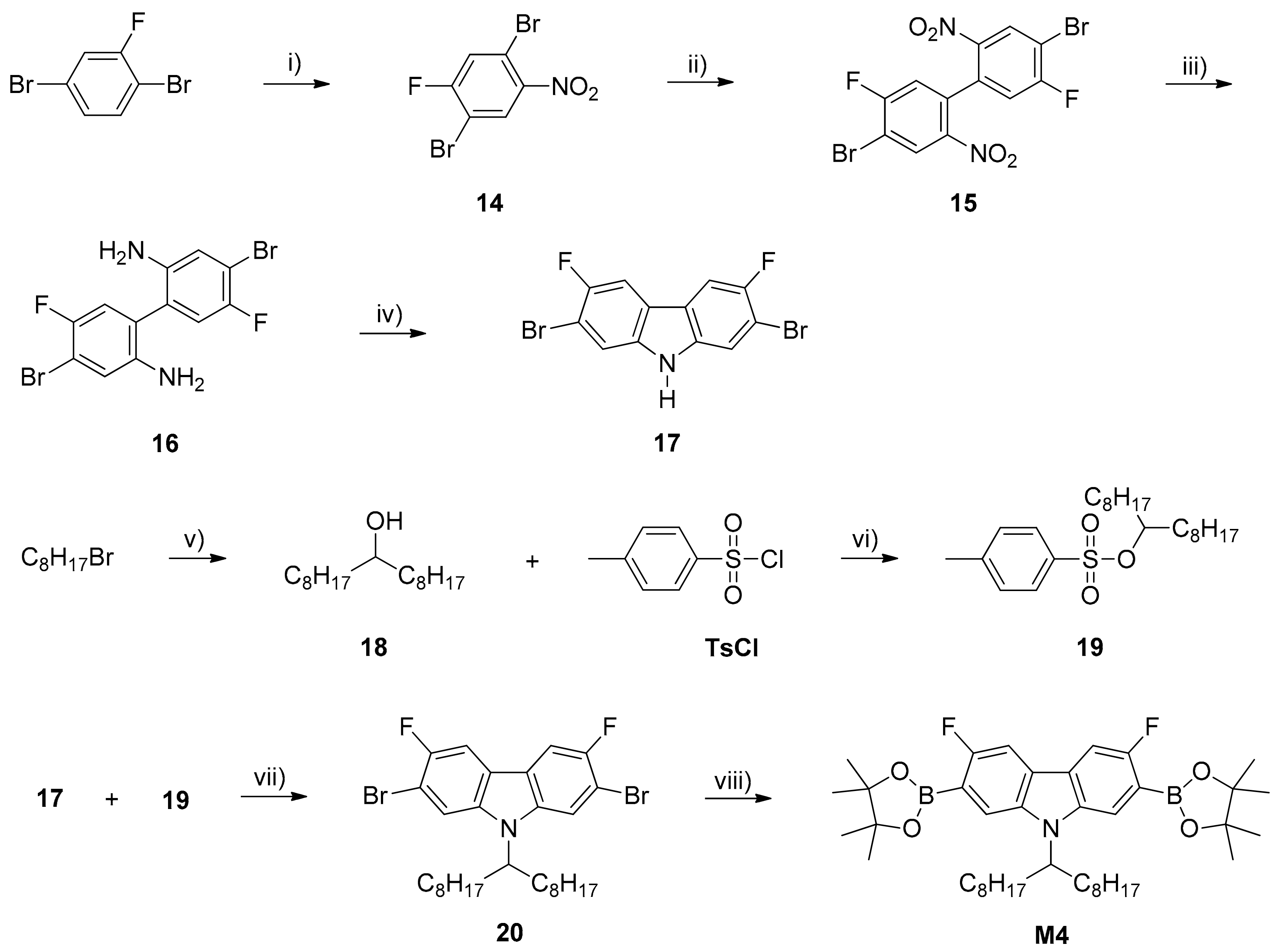
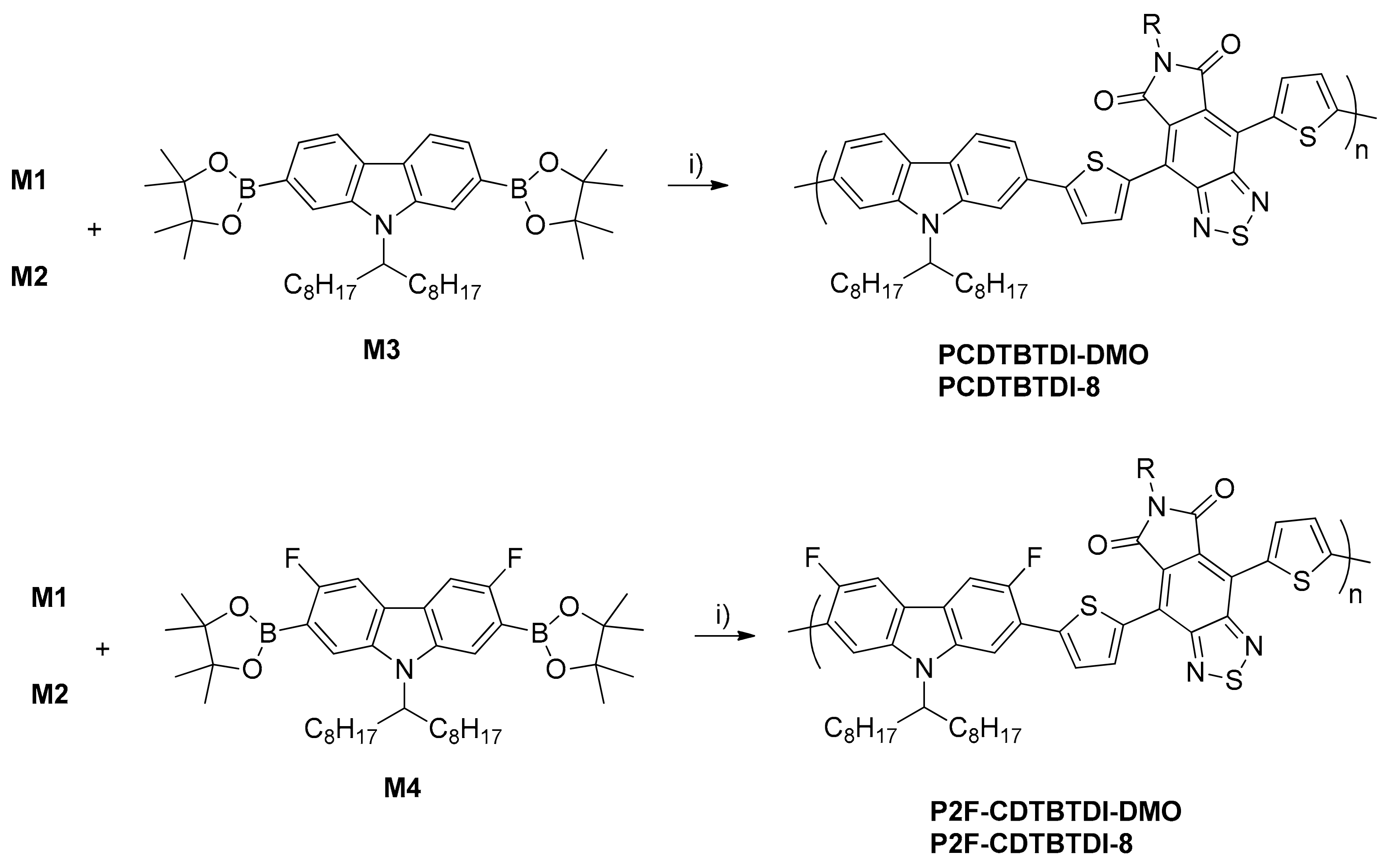
2.3. Monomers and Polymers Synthesis
3. Results and Discussion
3.1. Synthesis of Monomers and Polymers
3.2. Molecular Weights and Yield
3.3. Optical, Electrochemical, Thermal and Structural Study
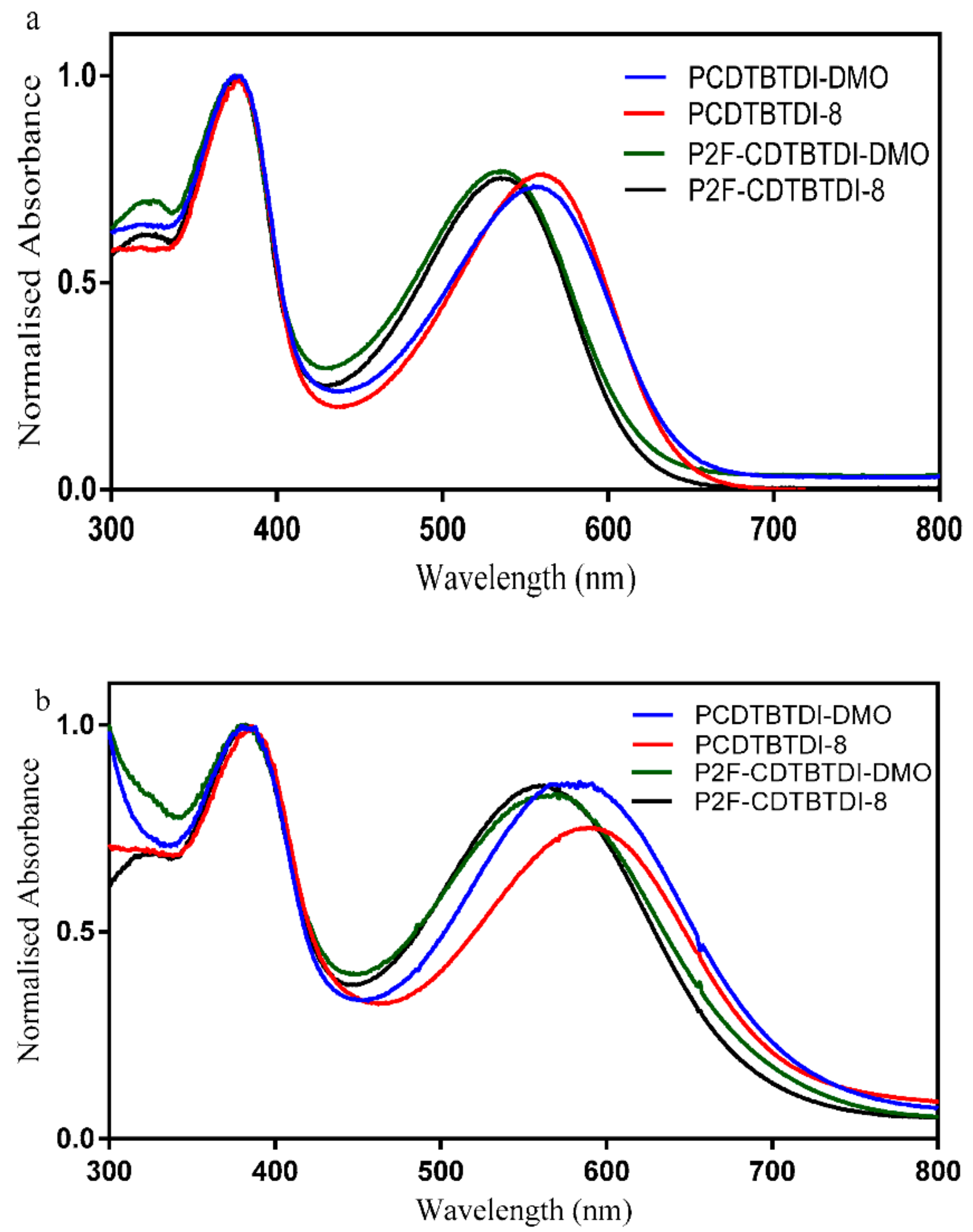
3.3.1. Optical Properties
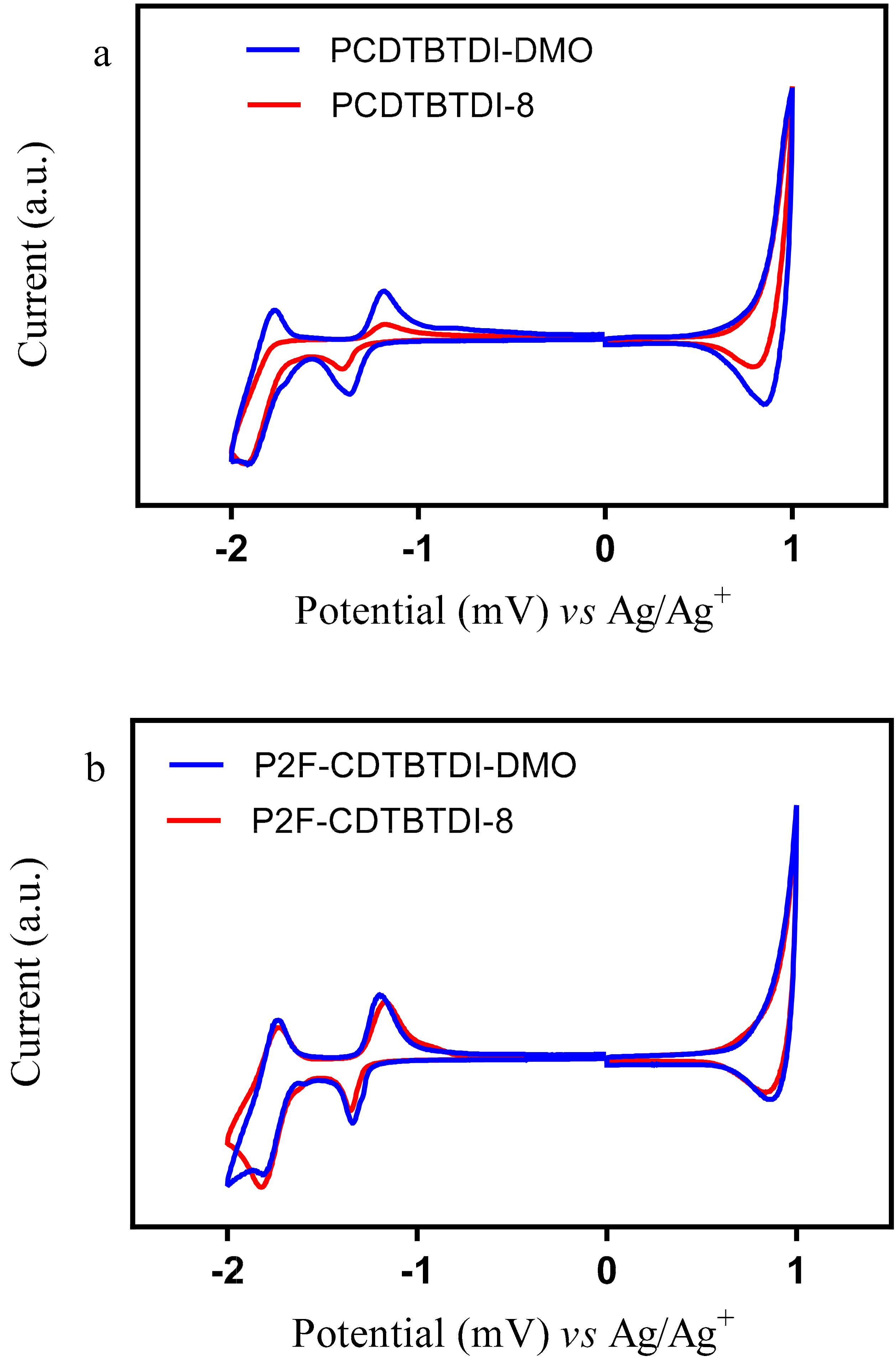
3.3.2. Electrochemical Properties
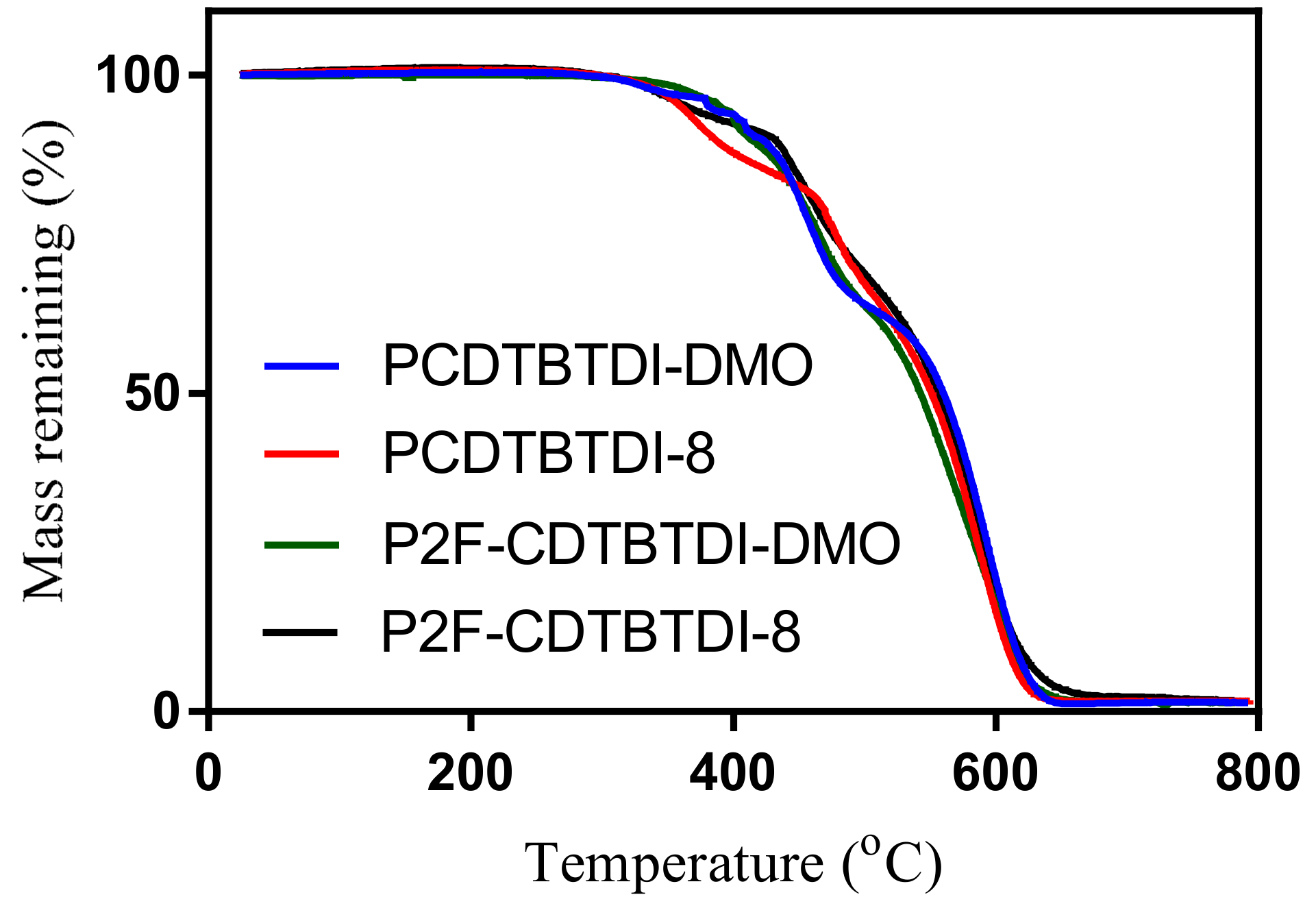
3.3.3. Thermal Properties
3.3.4. Powder X-ray Diffraction
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Murad, A.; Iraqi, A.; Aziz, S.; Abdullah, S.; Brza, M. Conducting Polymers for Optoelectronic Devices and Organic Solar Cells: A Review. Polymers 2020, 12, 2627. [Google Scholar] [CrossRef] [PubMed]
- Zulkifli, A.-M.; Said, N.; Aziz, S.; Dannoun, E.; Hisham, S.; Shah, S.; AbuBakar, A.; Zainal, Z.-H.; Tajuddin, H.-A.; Hadi, J.-M.; et al. Characteristics of Dye-Sensitized Solar Cell Assembled from Modified Chitosan-Based Gel Polymer Electrolytes Incorporated with Potassium Iodide. Molecules 2020, 25, 4115. [Google Scholar] [CrossRef]
- Chang, Y.; Lau, T.; Chow, P.-C.-Y.; Wu, N.; Su, D.; Zhang, W.; Meng, H.; Ma, C.; Liu, T.; Li, K.; et al. A 16.4% efficiency organic photovoltaic cell enabled using two donor polymers with their side-chains oriented differently by a ternary strategy. J. Mater. Chem. A 2020, 8, 3676–3685. [Google Scholar] [CrossRef]
- Cheng, P.; Yang, Y. Narrowing the Band Gap: The Key to High-Performance Organic Photovoltaics. Chem. Res. 2020, 53, 1218–1228. [Google Scholar] [CrossRef]
- Luceño-Sánchez, J.-A.; Díez-Pascual, A.-M.; Capilla, R.-P. Materials for Photovoltaics: State of Art and Recent Developments. Int. J. Mol. Sci. 2019, 20, 976. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.-J.; Yang, S.-H.; Hsu, C.-S. Synthesis of Conjugated Polymers for Organic Solar Cell Applications. Chem. Rev. 2009, 109, 5868–5923. [Google Scholar] [CrossRef]
- Husaina, A.-F.; Hasana, W.-Z.; Shafiea, S.; Hamidonb, M.-N.; Pandeyc, S.-S. A review of transparent solar photovoltaic technologies. Renew. Sustain. Energy Rev. 2018, 94, 779–791. [Google Scholar] [CrossRef]
- Savikhin, V.; Jagadamma, L.-K.; Purvis, L.-J.; Robertson, I.; Oosterhout, S.-D.; Douglas, C.-J.; Samuel, D.-W.; Toney, M.-F. Morphological, Chemical, and Electronic Changes of the Conjugated Polymer PTB7 with Thermal Annealing. iScience 2018, 2, 182–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.-G.; Rao, K.-S. Physics and chemistry of CdTe/CdS thin film heterojunction photovoltaic devices: Fundamental and critical aspects. Energy Environ. Sci. 2014, 7, 45–102. [Google Scholar] [CrossRef]
- Kumar, K.-P.; Murali, M.-G.; Udayakumar, D. Synthesis and study of optical properties of linear and hyperbranched conjugated polymers containing thiophene and triphenylamine units. Des. Monomers Polym. 2014, 17, 7–18. [Google Scholar] [CrossRef] [Green Version]
- Koyuncu, F.; Sefer, E.; Koyuncu, S.; Ozdemir, E. A new low band gap electrochromic polymer containing 2, 5-bis-dithienyl-1H-pyrrole and 2, 1, 3-benzoselenadiazole moiety with high contrast ratio. Polymer 2011, 52, 5772–5779. [Google Scholar] [CrossRef]
- Yi, H.; Iraqi, A.; Stevenson, M.; Elliott, C.J.; Lidzey, D.G. A New Class of Blue-Emitting Materials for LED Applications: Triarylamine N-Functionalised 2, 7-Linked Carbazole Polymers. Macromol. Rapid Commun. 2007, 28, 1155–1160. [Google Scholar] [CrossRef]
- Wakim, S.; Blouin, N.; Gingras, E.; Tao, Y.; Leclerc, M. Poly (2, 7-carbazole) derivatives as semiconductors for organic thin-film transistors. Macromol. Rapid Commun. 2007, 28, 1798–1803. [Google Scholar] [CrossRef]
- Drolet, N.; Morin, J.F.; Leclerc, N.; Wakim, S.; Tao, Y.; Leclerc, M. 2, 7-carbazolenevinylene-based oligomer thin-film transistors: High mobility through structural ordering. Adv. Funct. Mater. 2005, 15, 1671–1682. [Google Scholar] [CrossRef]
- Li, J.; Dierschke, F.; Wu, J.; Grimsdale, A.C.; Müllen, K. Poly (2, 7-carbazole) and perylene tetracarboxydiimide: A promising donor/acceptor pair for polymer solar cells. J. Mater. Chem. 2006, 16, 96–100. [Google Scholar] [CrossRef]
- Bian, L.; Zhu, E.; Tang, J.; Tang, W.; Zhang, F. Recent progress in the design of narrow bandgap conjugated polymers for high-efficiency organic solar cells. Prog. Polym. Sci. 2012, 37, 1292–1331. [Google Scholar] [CrossRef]
- Blouin, N.; Leclerc, M. Poly (2, 7-carbazole) s: Structure–property relationships. Acc. Chem. Res. 2008, 41, 1110–1119. [Google Scholar] [CrossRef]
- Zheng, C.; Yan, L.; Rech, J.J.; Hu, J.; Zhang, Q.; You, W. Green-Solvent-Processed Conjugated Polymers for Organic Solar Cells: The Impact of Oligoethylene Glycol Side Chains. ACS Appl. Polym. Mater. 2019, 4, 804–814. [Google Scholar]
- Iraqi, A.; Wataru, I. Preparation and Properties of 2, 7-Linked N-Alkyl-9 H-carbazole Main-Chain Polymers. Chem. Mater. 2004, 16, 442–448. [Google Scholar] [CrossRef]
- Tang, W.; Lin, T.; Ke, L.; Chen, Z.K. Synthesis, photophysics, theoretical modeling, and electroluminescence of novel 2, 7-carbazole-based conjugated polymers with sterically hindered structures. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7725–7738. [Google Scholar] [CrossRef]
- Iraqi, A.; Pickup, D.F.; Yi, H. Effects of Methyl Substitution of Poly (9-alkyl-9 H-carbazole-2, 7-diyl) s at the 3, 6-Positions on Their Physical Properties. Chem. Mater. 2006, 18, 1007–1015. [Google Scholar] [CrossRef]
- Zotti, G.; Schiavon, G.; Zecchin, S.; Morin, J.-F.; Leclerc, M. Electrochemical, conductive, and magnetic properties of 2, 7-carbazole-based conjugated polymers. Macromolecules 2002, 35, 2122–2128. [Google Scholar] [CrossRef]
- Kun, H.; Yi, H.; Johnson, R.G.; Iraqi, A. Fluoro-protected carbazole main-chain polymers as a new class of stable blue emitting polymers. Polym. Adv. Technol. 2008, 19, 299–307. [Google Scholar] [CrossRef]
- Blouin, N.; Michaud, A.; Leclerc, M. A low-bandgap poly (2, 7-carbazole) derivative for use in high-performance solar cells. Adv. Mater. 2007, 19, 2295–2300. [Google Scholar] [CrossRef]
- Wakim, S.; Beaupré, S.; Blouin, N.; Aich, B.-R.; Rodman, S.; Gaudiana, R.; Tao, Y.; Leclerc, M. Highly efficient organic solar cells based on a poly (2, 7-carbazole) derivative. J. Mater. Chem. 2009, 19, 5351–5358. [Google Scholar] [CrossRef]
- Chu, T.-Y.; Alem, S.; Verly, P.G.; Wakim, S.; Lu, J.; Tao, Y.; Beaupre, S.; Leclerc, M.; Belanger, F.; Desilets, D. Highly efficient polycarbazole-based organic photovoltaic devices. Appl. Phys. Lett. 2009, 95, 63304. [Google Scholar] [CrossRef]
- Park, S.H.; Roy, A.; Beaupre, S.; Cho, S.; Coates, N.; Moon, J.S.; Moses, D.; Leclerc, M.; Lee, K.; Heeger, A.J. Bulk heterojunction solar cells with internal quantum efficiency approaching 100%. Nat. Photonics 2009, 3, 297–302. [Google Scholar] [CrossRef]
- Seo, J.H.; Gutacker, A.; Sun, Y.; Wu, H.; Huang, F.; Cao, Y.; Scherf, U.; Heeger, A.J.; Bazan, G.C. Improved high-efficiency organic solar cells via incorporation of a conjugated polyelectrolyte interlayer. J. Am. Chem. Soc. 2011, 133, 8416–8419. [Google Scholar] [CrossRef]
- He, Z.; Zhong, C.; Huang, X.; Wong, W.Y.; Wu, H.; Chen, L.; Su, S.; Cao, Y. Simultaneous enhancement of open-circuit voltage, short-circuit current density, and fill factor in polymer solar cells. Adv. Mater. 2011, 23, 4636–4643. [Google Scholar] [CrossRef]
- Wang, D.H.; Kim, J.K.; Seo, J.H.; Park, I.; Hong, B.H.; Park, J.H.; Heeger, A.J. Transferable Graphene Oxide by Stamping Nanotechnology: Electron-Transport Layer for Efficient Bulk-Heterojunction Solar Cells. Angew. Chem. Int. Ed. 2013, 52, 2874–2880. [Google Scholar] [CrossRef]
- Zhou, E.; Cong, J.; Yamakawa, S.; Wei, Q.; Nakamura, M.; Tajima, K.; Yang, C.; Hashimoto, K. Synthesis of Thieno [3, 4-b] pyrazine-Based and 2, 1, 3-Benzothiadiazole-Based Donor− Acceptor Copolymers and their Application in Photovoltaic Devices. Macromolecules 2010, 43, 2873–2879. [Google Scholar] [CrossRef]
- Zhang, L.; He, C.; Chen, J.; Yuan, P.; Huang, L.; Zhang, C.; Cai, W.; Liu, Z.; Cao, Y. Bulk-heterojunction solar cells with benzotriazole-based copolymers as electron donors: Largely improved photovoltaic parameters by using PFN/Al bilayer cathode. Macromolecules 2010, 43, 9771–9778. [Google Scholar] [CrossRef]
- Yi, H.; Al-Faifi, S.; Iraqi, A.; Watters, D.C.; Kingsley, J.; Lidzey, D.G. Carbazole and thienyl benzo [1, 2, 5] thiadiazole based polymers with improved open circuit voltages and processability for application in solar cells. J. Mater. Chem. 2011, 21, 13649–13656. [Google Scholar] [CrossRef]
- Alghamdi, A.A.; Watters, D.C.; Yi, H.; Al-Faifi, S.; Almeataq, M.S.; Coles, D.; Kingsley, J.; Lidzey, D.G.; Iraqi, A. Selenophene vs. thiophene in benzothiadiazole-based low energy gap donor–acceptor polymers for photovoltaic applications. J. Mater. Chem. A 2013, 1, 5165–5171. [Google Scholar] [CrossRef]
- Pearson, A.J.; Watters, D.C.; Yi, H.; Sarjadi, M.S.; Reynolds, L.X.; Marchisio, P.P.; Kingsley, J.; Haque, S.A.; Iraqi, A.; Lidzey, D.G. Impact of dithienyl or thienothiophene units on the optoelectronic and photovoltaic properties of benzo [1, 2, 5] thiadiazole based donor–acceptor copolymers for organic solar cell devices. RSC Adv. 2014, 4, 43142–43149. [Google Scholar] [CrossRef] [Green Version]
- Price, S.C.; Stuart, A.C.; Yang, L.; Zhou, H.; You, W. Fluorine substituted conjugated polymer of medium band gap yields 7% efficiency in polymer–fullerene solar cells. J. Am. Chem. Soc. 2011, 133, 4625–4631. [Google Scholar] [CrossRef]
- Wen, S.; Wang, C.; Ma, P.; Zhao, Y.-X.; Li, C.; Ruan, S. Synthesis and photovoltaic properties of dithieno [3, 2-b: 2′, 3′-d] silole-based conjugated copolymers. J. Mater. Chem. A 2015, 3, 13794–13800. [Google Scholar] [CrossRef]
- Zhou, H.; Yang, L.; Stuart, A.C.; Price, S.C.; Liu, S.; You, W. Development of fluorinated benzothiadiazole as a structural unit for a polymer solar cell of 7% efficiency. Angew. Chem. 2011, 123, 3051–3054. [Google Scholar] [CrossRef]
- Wang, N.; Chen, Z.; Wei, W.; Jiang, Z. Fluorinated benzothiadiazole-based conjugated polymers for high-performance polymer solar cells without any processing additives or post-treatments. J. Am. Chem. Soc. 2013, 135, 17060–17068. [Google Scholar] [CrossRef]
- Peng, Q.; Liu, X.; Su, D.; Fu, G.; Xu, J.; Dai, L. Novel Benzo [1, 2-b: 4, 5-b′] dithiophene–Benzothiadiazole Derivatives with Variable Side Chains for High-Performance Solar Cells. Adv. Mater. 2011, 23, 4554–4558. [Google Scholar] [CrossRef]
- Zhang, Y.; Zou, J.; Cheuh, C.-C.; Yip, H.-L.; Jen, A.K.-Y. Significant improved performance of photovoltaic cells made from a partially fluorinated cyclopentadithiophene/benzothiadiazole conjugated polymer. Macromolecules 2012, 45, 5427–5435. [Google Scholar] [CrossRef]
- Albrecht, S.; Janietz, S.; Schindler, W.; Frisch, J.; Kurpiers, J.; Kniepert, J.; Inal, S.; Pingel, P.; Fostiropoulos, K.; Koch, N. Fluorinated copolymer PCPDTBT with enhanced open-circuit voltage and reduced recombination for highly efficient polymer solar cells. J. Am. Chem. Soc. 2012, 134, 14932–14944. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Li, W.; Duan, Y.; Li, C.; Dong, H.; Zhu, J.; Hu, W.; Bo, Z. Conjugated polymers with 2, 7-linked 3, 6-difluorocarbazole as donor unit for high efficiency polymer solar cells. Polym. Chem. 2013, 4, 2773–2782. [Google Scholar] [CrossRef]
- Wei, H.; Chao, Y.H.; Kang, C.; Li, C.; Lu, H.; Gong, X.; Dong, H.; Hu, W.; Hsu, C.S.; Bo, Z. High-Efficiency Large-Bandgap Material for Polymer Solar Cells. Macromol. Rapid Commun. 2015, 36, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Rasmussen, S.C. Synthesis and structural characterization of 2, 5-dihalo-3, 4-dinitrothiophenes. J. Chem. Crystallogr. 2007, 37, 387–398. [Google Scholar] [CrossRef]
- Schwiderski, R.L.; Rasmussen, S.C. Synthesis and Characterization of Thieno [3, 4-b] pyrazine-Based Terthienyls: Tunable Precursors for Low Band Gap Conjugated Materials. J. Org. Chem. 2013, 78, 5453–5462. [Google Scholar] [CrossRef]
- Hailu, H.; Atsbeha, B.; Admassie, S.; Mammo, W.; Raju, V.; Chebude, Y. Variable denticity of a multidentate terthiophene derivative towards Ni (II) and Zn (II)–structural studies. Bull. Chem. Soc. Ethiop. 2011, 25, 221–231. [Google Scholar] [CrossRef]
- Delgado, M.R.; Hernandez, V.; Navarrete, J.L.; Tanaka, S.; Yamashita, Y. Combined spectroscopic and theoretical study of narrow band gap heterocyclic co-oligomers containing alternating aromatic donor and o-quinoid acceptor units. J. Phys. Chem. B 2004, 108, 2516–2526. [Google Scholar] [CrossRef]
- Wang, L.; Cai, D.; Zheng, Q.; Tang, C.; Chen, S.-C.; Yin, Z. Low Band Gap Polymers Incorporating a Dicarboxylic Imide-Derived Acceptor Moiety for Efficient Polymer Solar Cells. ACS Macro Lett. 2013, 2, 605–608. [Google Scholar] [CrossRef]
- Nielsen, C.B.; Ashraf, R.S.; Treat, N.D.; Schroeder, B.C.; Donaghey, J.E.; White, A.J.; Stingelin, N.; McCulloch, I. 2, 1, 3-Benzothiadiazole-5, 6-Dicarboxylic Imide–A Versatile Building Block for Additive-and Annealing-Free Processing of Organic Solar Cells with Efficiencies Exceeding 8%. Adv. Mater. 2015, 27, 948–953. [Google Scholar] [CrossRef] [Green Version]
- Lan, L.; Chen, Z.; Li, Y.; Ying, L.; Huang, F.; Cao, Y. Donor–acceptor conjugated polymers based on cyclic imide substituted quinoxaline or dibenzo [a, c] phenazine for polymer solar cells. Polym. Chem. 2015, 6, 7558–7569. [Google Scholar] [CrossRef]
- Matsueda, Y.; Xu, S.; Negishi, E.-I. A novel highly enantio-and diastereoselective synthesis of vitamin E side-chain. Tetrahedron Lett. 2015, 56, 3346–3348. [Google Scholar] [CrossRef]
- Thomson, A.; O’Connor, S.; Knuckley, B.; Causey, C.P. Design, synthesis, and in vitro evaluation of an activity-based protein profiling (ABPP) probe targeting agmatine deiminases. Bioorg. Med. Chem. 2014, 22, 4602–4608. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.; Zhao, Y.; Shao, S.; Tian, H.; Xie, Z.; Geng, Y.; Wang, F. Novel NIR-absorbing conjugated polymers for efficient polymer solar cells: Effect of alkyl chain length on device performance. J. Mater. Chem. 2009, 19, 2199–2206. [Google Scholar] [CrossRef]
- Kundu, P.K.; Lerner, A.; Kučanda, K.; Leitus, G.; Klajn, R. Cyclic Kinetics during Thermal Equilibration of an Axially Chiral Bis-Spiropyran. J. Am. Chem. Soc. 2014, 136, 11276–11279. [Google Scholar] [CrossRef]
- Nakamura, M.; Yamabuki, K.; Oishi, T.; Onimura, K. Synthesis and fluorescent properties of conjugated co-oligomers containing maleimide and carbazole units at the main chain. Polym. J. 2014, 46, 94–103. [Google Scholar] [CrossRef]
- Sonntag, M.; Strohriegl, P. Novel 2, 7-linked carbazole trimers as model compounds for conjugated carbazole polymers. Chem. Mater. 2004, 16, 4736–4742. [Google Scholar] [CrossRef]
- Zou, Y.; Gendron, D.; Aïch, R.B.; Najari, A.; Tao, Y.; Leclerc, M. A High-Mobility Low-Bandgap Poly(2,7-carbazole) Derivative for Photovoltaic Applications. Macromolecules 2009, 42, 2891–2894. [Google Scholar] [CrossRef] [Green Version]
- Bao, W.; Reddy Billa, M.; Kassireddy, K.; Haro, M.; Kelly, M.J.; Kitney, S.P.; Al Kalifah, M.S.; Wei, P.; Dong, D.; O’Neill, M. Carbazole nematic liquid crystals. Liq. Cryst. 2010, 37, 1289–1303. [Google Scholar] [CrossRef]
- Zhou, E.; Yamakawa, S.; Tajima, K.; Yang, C.; Hashimoto, K. Synthesis and photovoltaic properties of diketopyrrolopyrrole-based donor–acceptor copolymers. Chem. Mater. 2009, 21, 4055–4061. [Google Scholar] [CrossRef]
- Jo, J.; Chi, C.; Höger, S.; Wegner, G.; Yoon, D.Y. Synthesis and characterization of monodisperse oligofluorenes. Chem. A Eur. J. 2004, 10, 2681–2688. [Google Scholar] [CrossRef] [PubMed]
- Kenning, D.D.; Mitchell, K.A.; Calhoun, T.R.; Funfar, M.R.; Sattler, D.J.; Rasmussen, S.C. Thieno [3, 4-b] pyrazines: Synthesis, structure, and reactivity. J. Org. Chem. 2002, 67, 9073–9076. [Google Scholar] [CrossRef] [PubMed]
- McNamara, L.E.; Liyanage, N.; Peddapuram, A.; Murphy, J.S.; Delcamp, J.H.; Hammer, N.I. Donor–Acceptor–Donor Thienopyrazines via Pd-Catalyzed C–H Activation as NIR Fluorescent Materials. J. Org. Chem. 2015, 81, 32–42. [Google Scholar] [CrossRef]
- Saeki, A.; Yoshikawa, S.; Tsuji, M.; Koizumi, Y.; Ide, M.; Vijayakumar, C.; Seki, S. A versatile approach to organic photovoltaics evaluation using white light pulse and microwave conductivity. J. Am. Chem. Soc. 2012, 134, 19035–19042. [Google Scholar] [CrossRef]
- Cartwright, L.; Yi, H.; Iraqi, A. Effect of fluorination pattern and extent on the properties of PCDTBT derivatives. New J. Chem. 2016, 40, 1655–1662. [Google Scholar] [CrossRef] [Green Version]
- Cartwright, L.; Iraqi, A.; Zhang, Y.; Wang, T.; Lidzey, D.G. Impact of fluorine substitution upon the photovoltaic properties of benzothiadiazole-fluorene alternate copolymers. RSC Adv. 2015, 5, 46386–46394. [Google Scholar] [CrossRef] [Green Version]

| Polymer | % Yield | Toluene Fraction | Chloroform Fraction | ||||
|---|---|---|---|---|---|---|---|
| Mn (g mol−1) | Mw (g mol−1) | PDI | Mn (g mol−1) | Mw (g mol−1) | PDI | ||
| PCDTBTDI-DMO | 73 | 12,200 | 30,400 | 2.4 | |||
| PCDTBTDI-8 | 86 | 20,500 | 65,300 | 3.1 | |||
| P2F-CDTBTDI-DMO | 98 | 8700 | 16,300 | 1.8 | |||
| P2F-CDTBTDI-8 | 68 | 8200 | 18,400 | 2.2 | 24,200 | 46,300 | 1.9 |
| Polymer | ε (M−1 cm−1) | Solution | Film | ||
|---|---|---|---|---|---|
| λmax (nm) | λmax (nm) | λonset (nm) | Eg (eV) | ||
| PCDTBTDI-DMO | 31,900 | 557 | 583 | 701 | 1.76 |
| PCDTBTDI-8 | 29,100 | 561 | 588 | 702 | 1.76 |
| P2F-CDTBTDI-DMO | 31,400 | 536 | 568 | 693 | 1.78 |
| P2F-CDTBTDI-8 | 21,800 | 536 | 561 | 684 | 1.81 |
| Polymer | Td (°C) | Eox0 (V) | HOMO (eV) | Ered0 (V) | LUMO (eV) | Eg (elec) (eV) |
|---|---|---|---|---|---|---|
| PCDTBTDI-DMO | 381 | 0.84 | −5.56 | 1.23 | −3.48 | 2.08 |
| PCDTBTDI-8 | 359 | 0.83 | −5.55 | 1.30 | −3.41 | 2.14 |
| P2F-CDTBTDI-DMO | 392 | 0.84 | −5.56 | 1.23 | −3.48 | 2.08 |
| P2F-CDTBTDI-8 | 371 | 0.86 | −5.56 | 1.26 | −3.45 | 2.12 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
R. Murad, A.; Iraqi, A.; Aziz, S.B.; Hi, H.; N. Abdullah, S.; Brza, M.A.; Abdulwahid, R.T. Influence of Fluorine Substitution on the Optical, Thermal, Electrochemical and Structural Properties of Carbazole-Benzothiadiazole Dicarboxylic Imide Alternate Copolymers. Polymers 2020, 12, 2910. https://doi.org/10.3390/polym12122910
R. Murad A, Iraqi A, Aziz SB, Hi H, N. Abdullah S, Brza MA, Abdulwahid RT. Influence of Fluorine Substitution on the Optical, Thermal, Electrochemical and Structural Properties of Carbazole-Benzothiadiazole Dicarboxylic Imide Alternate Copolymers. Polymers. 2020; 12(12):2910. https://doi.org/10.3390/polym12122910
Chicago/Turabian StyleR. Murad, Ary, Ahmed Iraqi, Shujahadeen B. Aziz, Hunan Hi, Sozan N. Abdullah, M. A. Brza, and Rebar T. Abdulwahid. 2020. "Influence of Fluorine Substitution on the Optical, Thermal, Electrochemical and Structural Properties of Carbazole-Benzothiadiazole Dicarboxylic Imide Alternate Copolymers" Polymers 12, no. 12: 2910. https://doi.org/10.3390/polym12122910






