Interfacial Interaction Enhanced Rheological Behavior in PAM/CTAC/Salt Aqueous Solution—A Coarse-Grained Molecular Dynamics Study
Abstract
:1. Introduction
2. Computational Methods
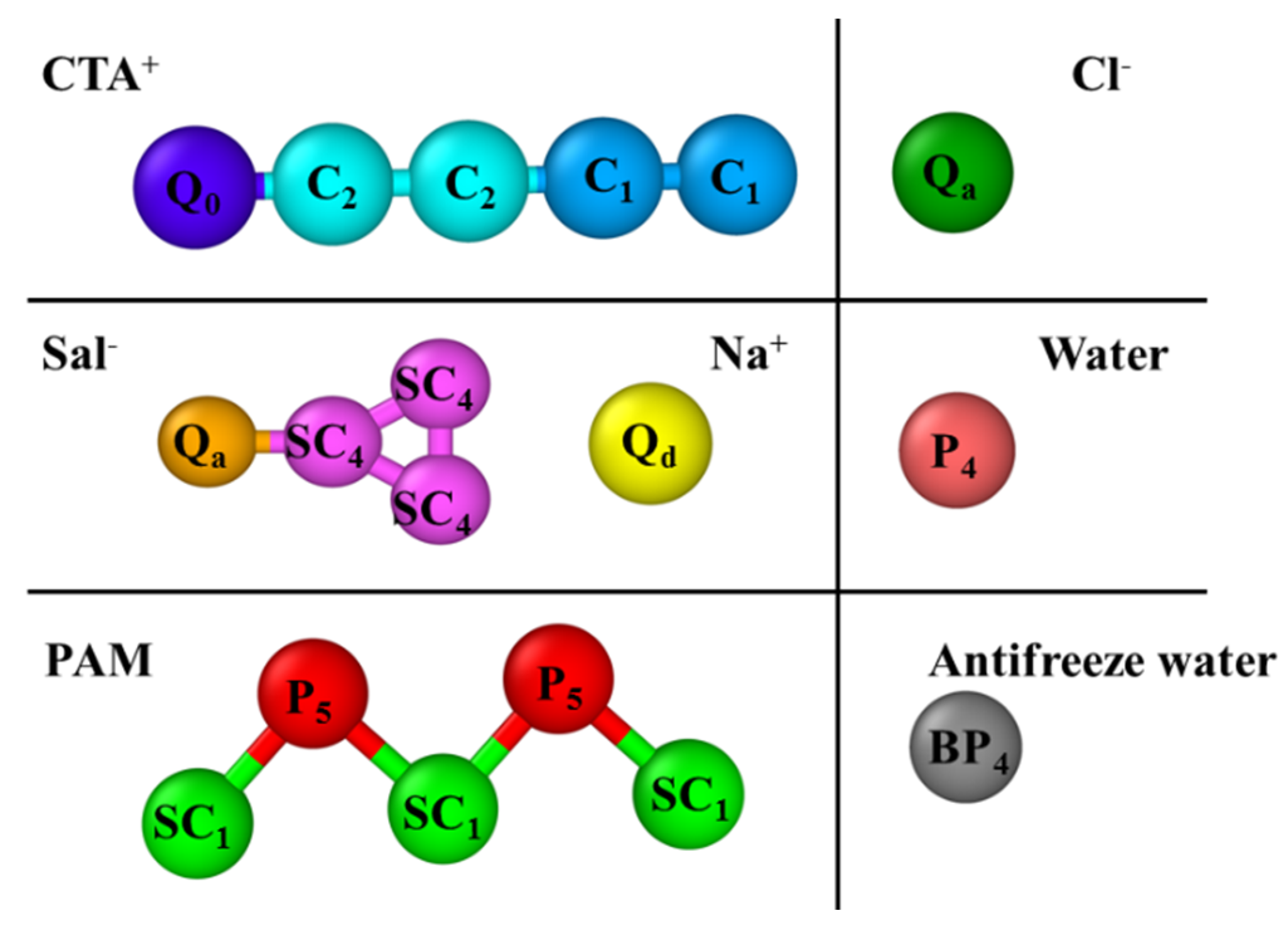
2.1. Interactions
2.2. Bonded Interaction and Nonbonded Interaction
2.3. Simulations
3. Results and Discussion
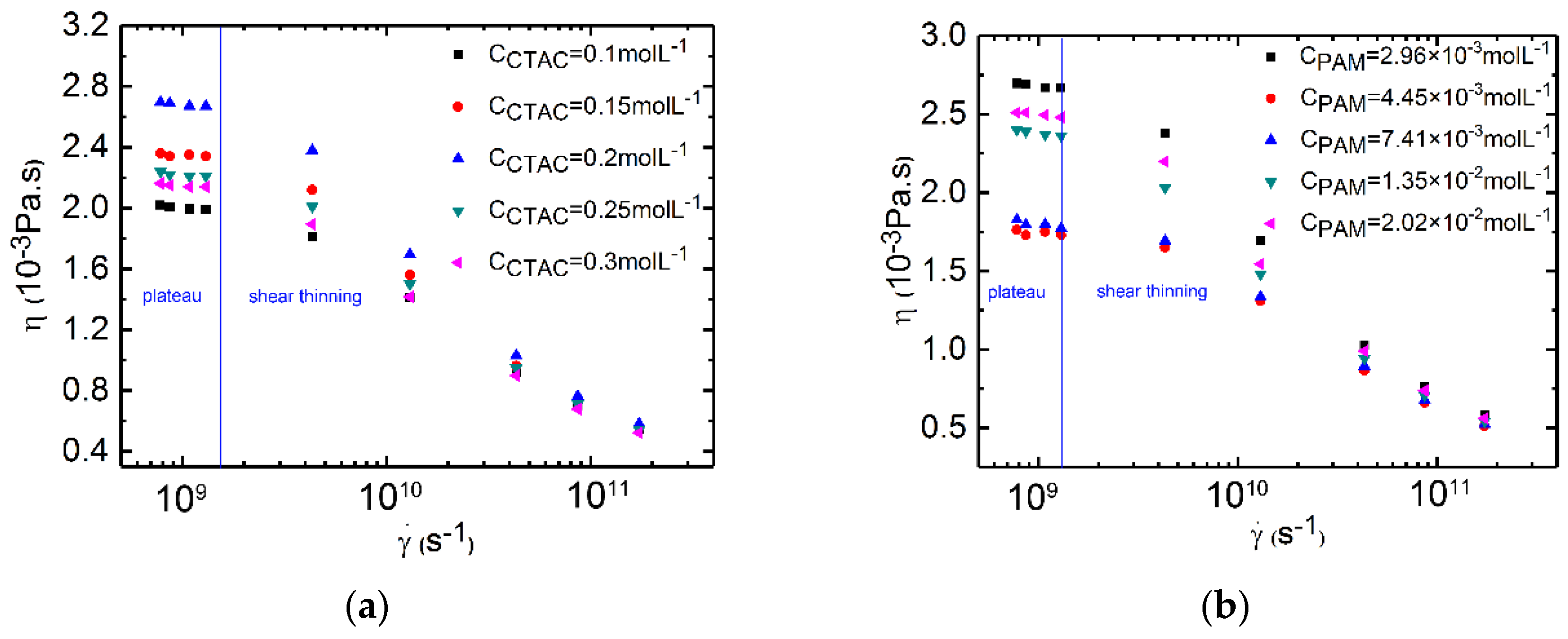
3.1. Study of Shear Viscosity for PAM/CTAC/Salt Multiphase Aqueous System
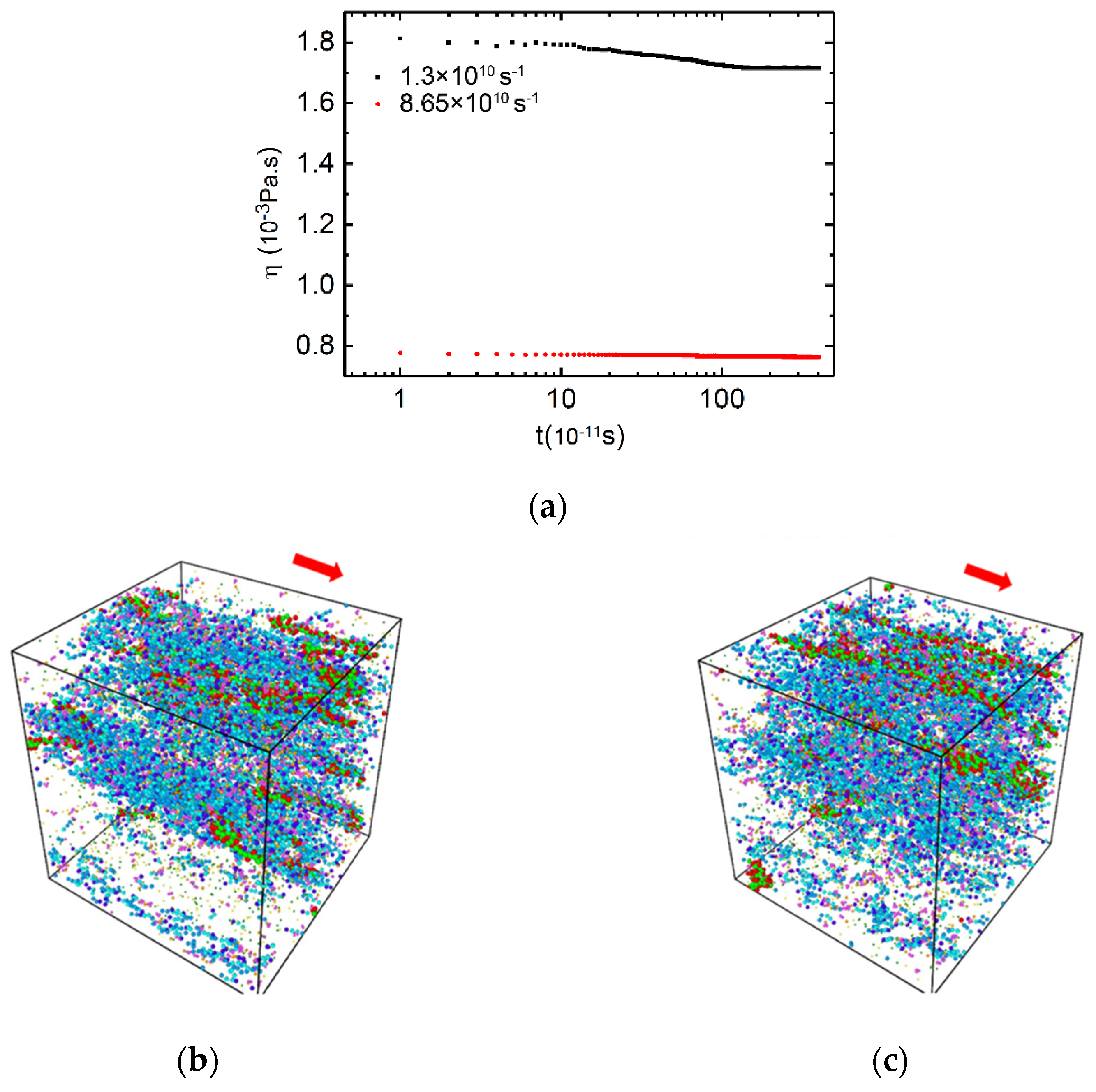
3.2. Time Dependent Shear Viscosity and Structure Evolution for PAM/CTAC/Salt Multiphase Aqueous System
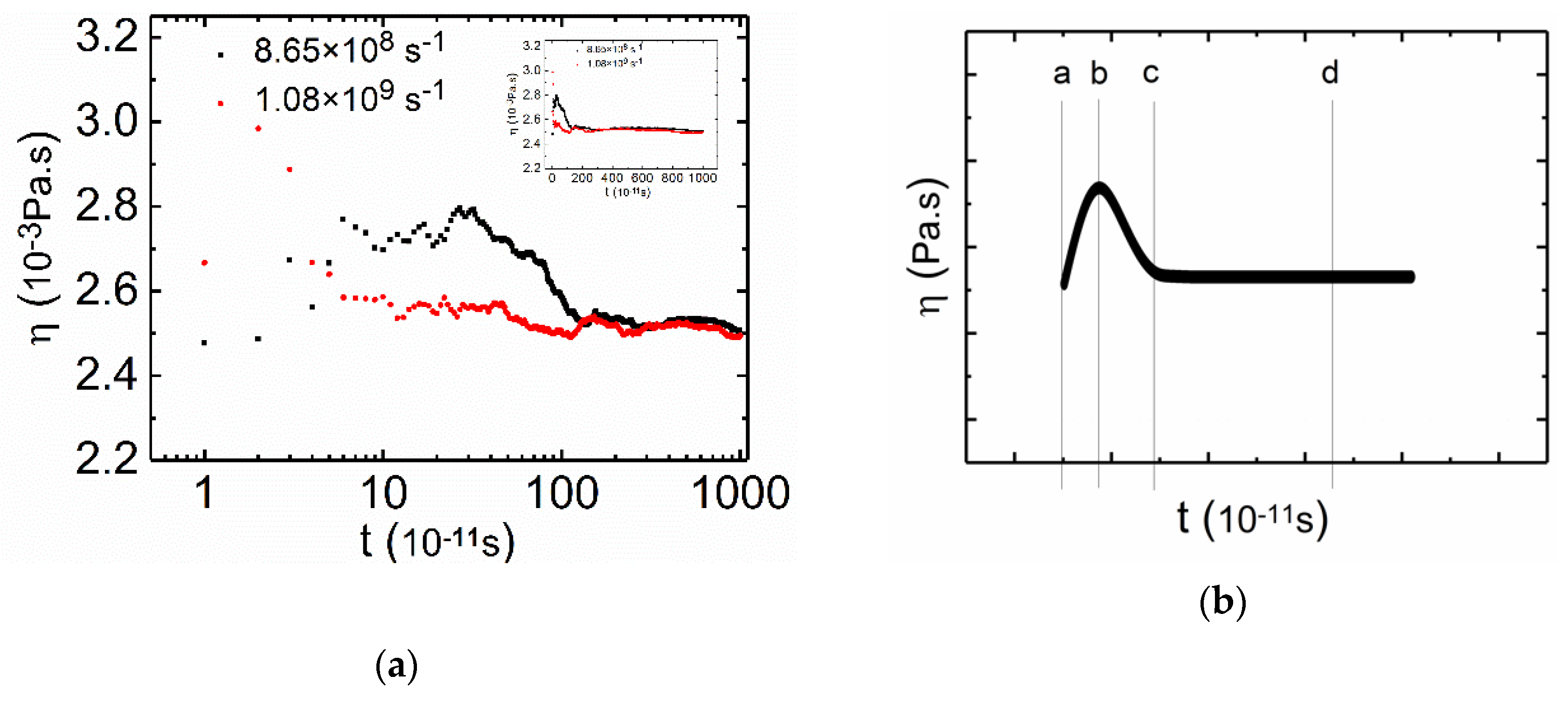
3.2.1. Medium and High Shear Rates
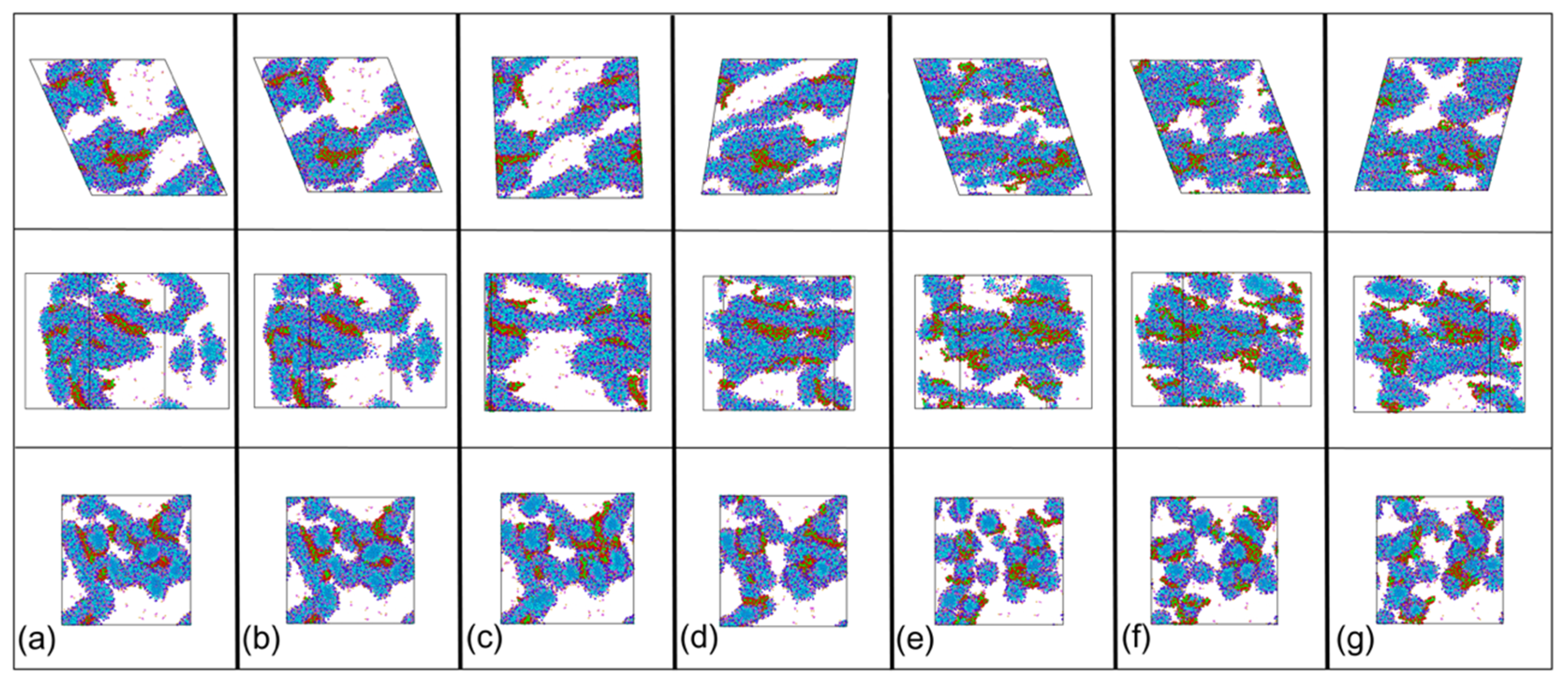
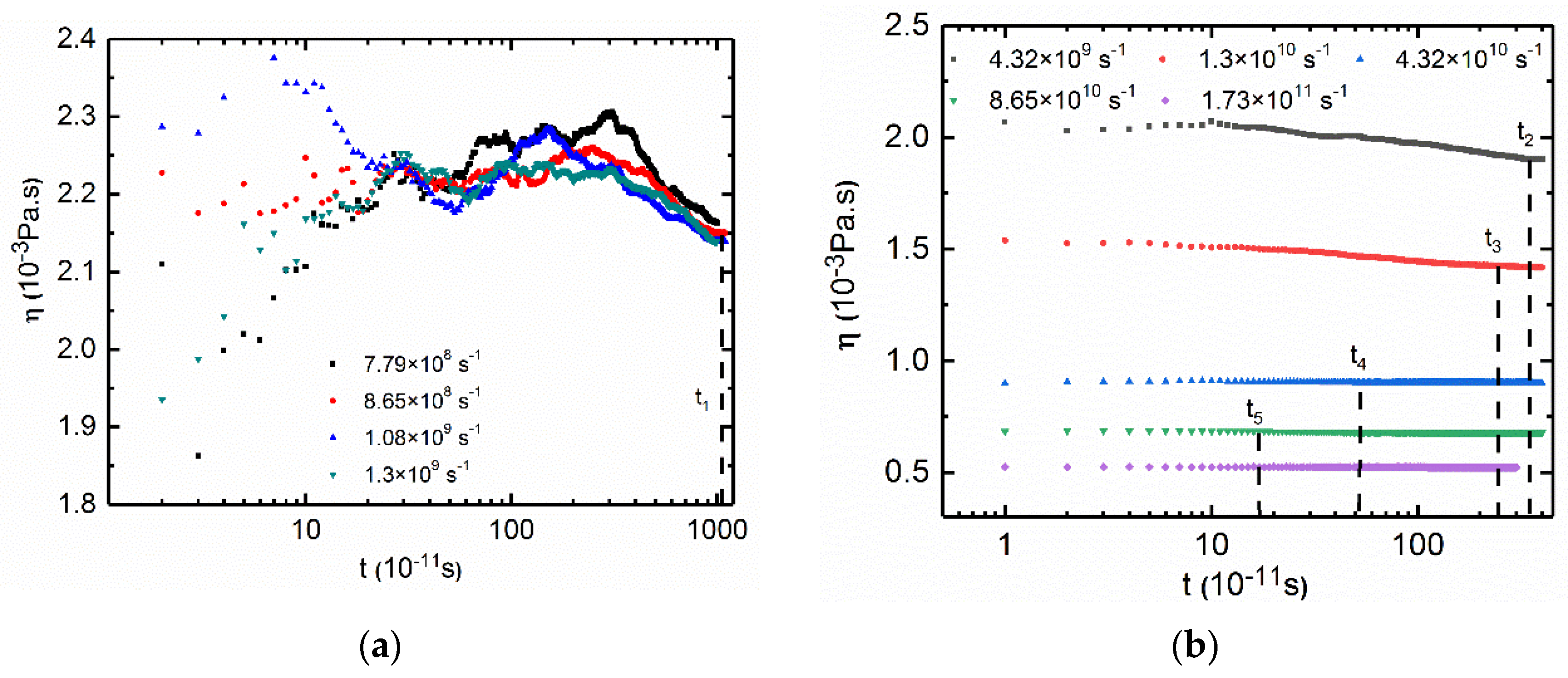
3.2.2. Low Shear Rates for PAM Based Solutions
3.2.3. Low Shear Rates for CTAC Based Solutions
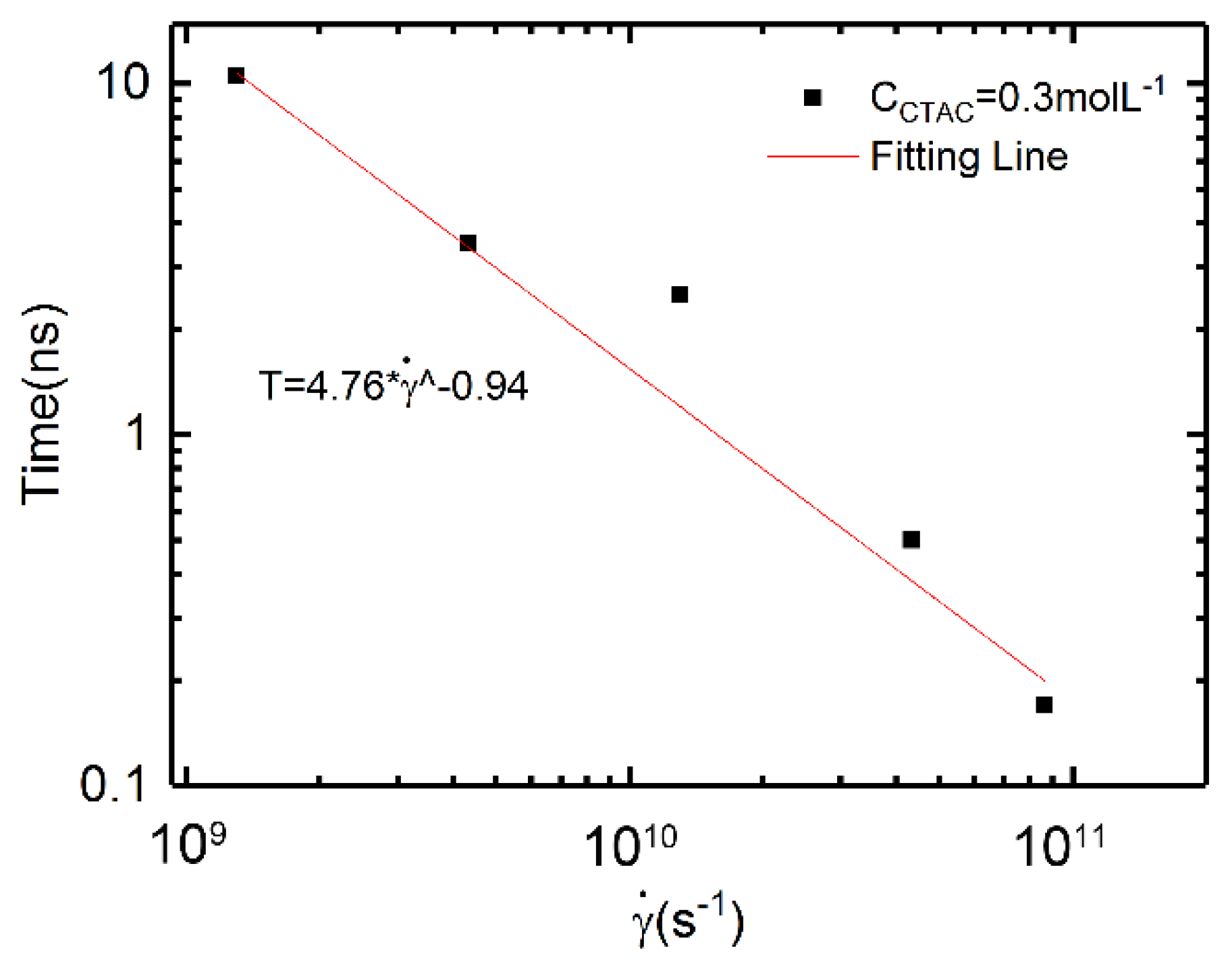
3.3. Relation between Shear Rates and Characteristic Time
3.4. Shear Viscosity Versus the Concentration of CTAC for Polymer/Surfactant Solutions
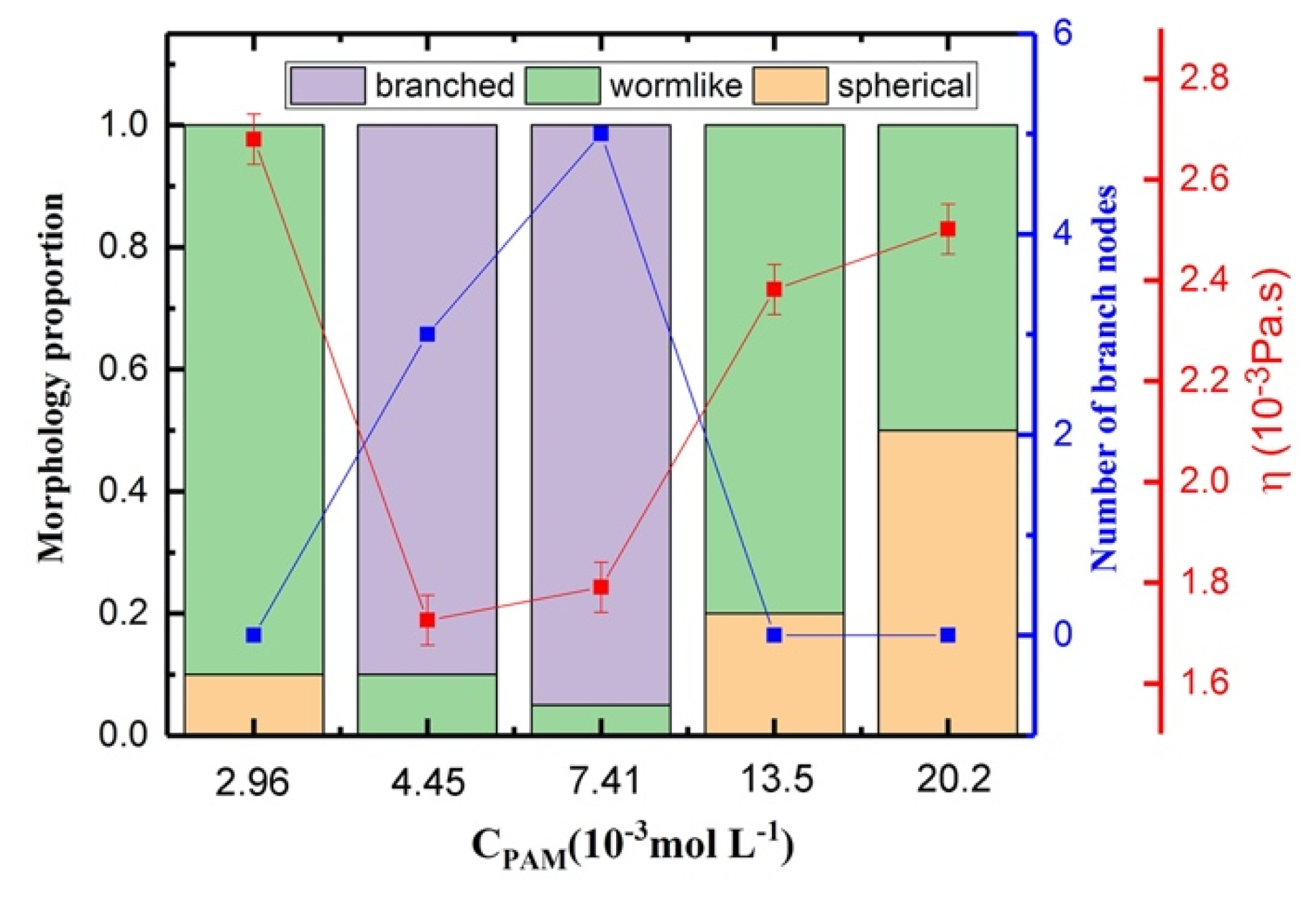
3.5. Shear Viscosity Versus the Concentration of PAM for Polymer/Surfactant Solutions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Burger, E.; Chorn, L.; Perkins, T. Studies of drag reduction conducted over a broad range of pipeline conditions when flowing Prudhoe Bay crude oil. J. Rheol. 1980, 24, 603–626. [Google Scholar] [CrossRef]
- Lucas, E.F.; Mansur, C.R.E.; Spinelli, L.; Queiros, Y.G.C. Polymer science applied to petroleum production. Pure Appl. Chem. 2009, 81, 473–494. [Google Scholar] [CrossRef]
- Huang, B.; Li, X.; Zhang, W.; Fu, C.; Wang, Y.; Fu, S. Study on Demulsification-Flocculation Mechanism of Oil-Water Emulsion in Produced Water from Alkali/Surfactant/Polymer Flooding. Polymers 2019, 11, 395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, B.; Hu, X.; Fu, C.; Cheng, H.; Wang, X.; Wang, L. Molecular Morphology and Viscoelasticity of ASP Solution under the Action of a Different Medium Injection Tool. Polymers 2019, 11, 1299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tong, L.; Akay, G. Process intensification in particle technology: Flow induced phase inversion in the intensive emulsification of epoxy polymer melts in water. J. Mater. Sci. 2002, 37, 4985–4992. [Google Scholar] [CrossRef]
- Myska, J.; Mik, V. Application of a drag reducing surfactant in the heating circuit. Energy Build. 2003, 35, 813–819. [Google Scholar] [CrossRef]
- Sellin, R.H.J.; Ollis, M. Polymer Drag Reduction in Large Pipes and Sewers: Results of Recent Field Trials. J. Rheol. 1980, 24, 667–684. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, W.; Zhou, L.; Wang, J.; Liu, L.; Wang, S.; Wang, Y. Soft Particles of Gemini Surfactant/Conjugated Polymer for Enhanced Anticancer Activity of Chemotherapeutics. ACS Appl. Mater. Interfaces 2018, 10, 37–41. [Google Scholar] [CrossRef]
- Tangso, K.J.; Lindberg, S.; Hartley, P.G.; Knott, R.; Spicer, P.; Boyd, B.J. Formation of Liquid-Crystalline Structures in the Bile Salt–Chitosan System and Triggered Release from Lamellar Phase Bile Salt–Chitosan Capsules. ACS Appl. Mater. Interfaces 2014, 6, 12363–12371. [Google Scholar] [CrossRef]
- Groot, R.D. Mesoscopic Simulation of Polymer−Surfactant Aggregation. Langmuir 2000, 16, 7493–7502. [Google Scholar] [CrossRef]
- Winnik, F.M.; Regismond, S.T.A. Fluorescence methods in the study of the interactions of surfactants with polymers. Colloids Surf. A Physicochem. Eng. Asp. 1996, 118, 1–39. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, H.; Yuan, S.; Liu, C.; Xu, Z. Molecular dynamics study of the adsorption of anionic surfactant in a nonionic polymer brush. J. Mol. Model. 2014, 20, 2267. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, H.; Liu, C.; Yuan, S. Coarse-grained molecular dynamics simulation of self-assembly of polyacrylamide and sodium dodecylsulfate in aqueous solution. J. Colloid Interface Sci. 2012, 386, 205. [Google Scholar] [CrossRef] [PubMed]
- Shang, B.Z.; Zuowei, W.; Larson, R.G. Molecular dynamics simulation of interactions between a sodium dodecyl sulfate micelle and a poly (ethylene oxide) polymer. J. Phys. Chem. B 2008, 112, 2888–2900. [Google Scholar] [CrossRef]
- Tanaka, F. Polymer−Surfactant Interaction in Thermoreversible Gels. Macromolecules 1998, 31, 384–393. [Google Scholar] [CrossRef]
- Walter, R.; Rička, J.; Quellet, C.; Nyffenegger, R.; Binkert, T. Coil−Globule Transition of Poly(N-isopropylacrylamide): A Study of Polymer−Surfactant Association. Macromolecules 1996, 29, 4019–4028. [Google Scholar] [CrossRef]
- Hai, M.; Han, B.; Yan, H. Investigation on Interaction between Sodium Dodecyl Sulfate and Polyacrylamide by Electron Spin Resonance and Ultraviolet Spectrum. The J. Phys. Chem. B 2001, 105, 4824–4826. [Google Scholar] [CrossRef]
- Pojják, K.; Mészáros, R. Association between branched poly(ethyleneimine) and sodium dodecyl sulfate in the presence of neutral polymers. J. Colloid Interface Sci. 2011, 355, 410–416. [Google Scholar] [CrossRef]
- Nambam, J.S.; Philip, J. Competitive adsorption of polymer and surfactant at a liquid droplet interface and its effect on flocculation of emulsion. J. Colloid Interface Sci. 2012, 366, 88–95. [Google Scholar] [CrossRef]
- Mya, K.Y.; Sirivat, A.; Jamieson, A.M. Structure of Polymer−Surfactant Complexes by Static Light Scattering. Macromolecules 2001, 34, 5260–5266. [Google Scholar] [CrossRef]
- Brown, W.; Fundin, J.; Miguel, M.D.G. Poly(ethylene oxide)-sodium dodecyl sulfate interactions studied using static and dynamic light scattering. Macromolecules 1992, 25, 7192–7198. [Google Scholar] [CrossRef] [Green Version]
- Fundin, J.; Brown, W. Polymer/Surfactant Interactions. Sodium Poly(styrene sulfonate) and CTAB Complex Formation. Light Scattering Measurements in Dilute Aqueous Solution. Macromolecules 1994, 27, 5024–5031. [Google Scholar] [CrossRef]
- Fundin, J.; Brown, W.; Vethamuthu, M.S. Poly((N,N,N-trimethylammonio)ethyl acrylate chloride salt) (PCMA)−SDS Complex Formation in Dilute Aqueous Solution. Light Scattering and Time-Resolved Fluorescence Quenching Measurements. Macromolecules 1996, 29, 1195–1203. [Google Scholar] [CrossRef]
- Fundin, J.; Hansson, P.; Brown, W.; Lidegran, I. Poly(acrylic acid)−Cetyltrimethylammonium Bromide Interactions Studied Using Dynamic and Static Light Scattering and Time-Resolved Fluorescence Quenching. Macromolecules 1997, 30, 1118–1126. [Google Scholar] [CrossRef]
- Norwood, D.P.; Minatti, E.; Reed, W.F. Surfactant/Polymer Assemblies. 1. Surfactant Binding Properties. Macromolecules 1998, 31, 2957–2965. [Google Scholar] [CrossRef]
- Gao, Z.; Wasylishen, R.E.; Kwak, J.C.T. NMR studies in surfactant and polymer-surfactant systems: Micelle formation of sodium ω-phenyldecanoate and interaction with poly(ethylene oxide). J. Colloid Interface Sci. 1990, 137, 137–146. [Google Scholar] [CrossRef]
- Lin, J.-H.; Hou, S.-S. Effects of Organic Salts on Polymer–Surfactant Interactions: Roles of Bu4NBr and Pr4NBr in PVP–SDS Complexation. Macromolecules 2014, 47, 6418–6429. [Google Scholar] [CrossRef]
- Chen, J.; Gong, X.; Yang, H.; Yao, Y.; Xu, M.; Chen, Q.; Cheng, R. NMR Study on the Effects of Sodium n-Dodecyl Sulfate on the Coil-to-Globule Transition of Poly(N-isopropylacrylamide) in Aqueous Solutions. Macromolecules 2011, 44, 6227–6231. [Google Scholar] [CrossRef]
- Tzeng, J.-K.; Hou, S.-S. Interactions between Poly(N-vinylformamide) and Sodium Dodecyl Sulfate as Studied by Fluorescence and Two-Dimensional NOE NMR Spectroscopy. Macromolecules 2008, 41, 1281–1288. [Google Scholar] [CrossRef]
- Wu, S.; Zeng, F.; Zhu, H.; Luo, S.; Ren, B.; Tong, Z. Mesomorphous Structure and Macroscopic Patterns Formed by Polymer and Surfactant from Organic Solutions. Macromolecules 2005, 38, 9266–9274. [Google Scholar] [CrossRef]
- Shibaev, A.V.; Makarov, A.V.; Kuklin, A.I.; Iliopoulos, I.; Philippova, O.E. Role of Charge of Micellar Worms in Modulating Structure and Rheological Properties of Their Mixtures with Nonionic Polymer. Macromolecules 2018, 51, 213–221. [Google Scholar] [CrossRef]
- Liu, C.-Y.; Chen, H.-L. Undulating the Lamellar Interface of Polymer–Surfactant Complex by Dendrimer. Macromolecules 2017, 50, 6501–6508. [Google Scholar] [CrossRef]
- Oliva-Ramírez, M.; López-Santos, C.; Yubero, F.; González-Elipe, A.R. Nanostructural Analysis of Porous Oblique Angle Deposited (OAD) Multilayer Systems by Grazing-Incidence Small-Angle X-Ray Scattering. Adv. Mater. Interfaces 2018, 5, 1800530. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhou, L.; Zhou, C.; Zhao, W.; Wang, J.; Liu, L.; Wang, S.; Wang, Y. Preparation of Gemini Surfactant/Conjugated Polymer Aggregates for Enhanced Fluorescence and Bioimaging Application. ACS Appl. Mater. Interfaces 2017, 9, 23544–23554. [Google Scholar] [CrossRef] [PubMed]
- Cao, Q.; Zuo, C.; Li, L.; He, H. Self-assembled nanostructures of bottle-brush polyelectrolytes with oppositely charged surfactants: A computational simulation study. Soft Matter 2011, 7, 6522–6528. [Google Scholar] [CrossRef]
- Yuan, S.M.; Yan, H.; Lv, K.; Liu, C.B.; Yuan, S.L. Surface behavior of a model surfactant: A theoretical simulation study. J. Colloid Interface Sci. 2010, 348, 159–166. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Guo, X.L.; Yuan, S.L.; Liu, C.B. Molecular Dynamics Study of the Effect of Calcium Ions on the Monolayer of SDC and SDSn Surfactants at the Vapor/Liquid Interface. Langmuir 2011, 27, 5762–5771. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Song, K.; Yuan, S.-L.; Liu, C.-B. Microscopic Wetting of Self-Assembled Monolayers with Different Surfaces: A Combined Molecular Dynamics and Quantum Mechanics Study. Langmuir 2011, 27, 8611–8620. [Google Scholar] [CrossRef]
- Zhang, J.; Cui, J.; Cheng, Y.; Wang, W.; He, X.; Mei, X. A Molecular Dynamics Study on Self-Assembly of Single-Walled Carbon Nanotubes: From Molecular Morphology and Binding Energy. Adv. Mater. Interfaces 2019, 6, 1900983. [Google Scholar] [CrossRef]
- Castillo-Tejas, J.; Alvarado, J.F.J.; Carro, S.; Pérez-Villaseñor, F.; Bautista, F.; Manero, O. Rheology of wormlike micelles from non-equilibrium molecular dynamics. J. Non-Newton. Fluid Mech. 2011, 166, 194–207. [Google Scholar] [CrossRef]
- Castillo-Tejas, J.; Castrejón-González, O.; Carro, S.; González-Coronel, V.; Alvarado, J.F.J.; Manero, O. Associative polymers. Part III: Shear rheology from molecular dynamics. Colloids Surf. A Physicochem. Eng. Asp. 2016, 491, 37–49. [Google Scholar] [CrossRef]
- Castrejón-González, E.O.; Baños, V.E.M.; Alvarado, J.F.J.; Rico-Ramírez, V.; Castillo-Tejas, J.; Jiménez-Islas, H. Rheological model for micelles in solution from molecular dynamics. J. Mol. Liq. 2014, 198, 84–93. [Google Scholar] [CrossRef]
- Xu, Z.; Pablo, J.J.D.; Kim, S. Transport properties of polymer melts from nonequilibrium molecular dynamics. J. Chem. Phys. 1995, 102, 5836–5844. [Google Scholar] [CrossRef] [Green Version]
- Marrink, S.J.; Vries, A.H.D.; Mark, A.E. Coarse Grained Model for Semiquantitative Lipid Simulations. J. Phys. Chem. B 2004, 108, 750–760. [Google Scholar] [CrossRef] [Green Version]
- Marrink, S.J.; Risselada, H.J.; Yefimov, S.; Tieleman, D.P.; de Vries, A.H. The MARTINI force field: Coarse grained model for biomolecular simulations. J. Phys. Chem. B 2007, 111, 7812–7824. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhong, T.; Quan, X.; Jian, Z. Solvent-responsiveness of PS–PEO binary mixed polymer brushes: A coarse-grained molecular dynamics study. Mol. Simul. 2017, 43, 1322–1330. [Google Scholar]
- Liu, D.; Liu, F.; Zhou, W.; Chen, F.; Wei, J. Molecular dynamics simulation of self-assembly and viscosity behavior of PAM and CTAC in salt-added solutions. J. Mol. Liq. 2018, 268, 131–139. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Q.; Wei, J. Experimental study on drag reduction performance of mixed polymer and surfactant solutions. Chem. Eng. Res. Des. 2018, 132, 460–469. [Google Scholar] [CrossRef]
- Rossi, G.; Monticelli, L.; Puisto, S.R.; Vattulainen, I.; Alanissila, T. Coarse-graining polymers with the MARTINI force-field: Polystyrene as a benchmark case. Soft Matter 2011, 7, 698–708. [Google Scholar] [CrossRef] [Green Version]
- Dhakal, S.; Sureshkumar, R. Topology, length scales, and energetics of surfactant micelles. J. Chem. Phys. 2015, 143, 024905. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Liu, D.; Zhou, W.; Chen, F.; Wei, J. Coarse-Grained Molecular Dynamics Simulations of the Breakage and Recombination Behaviors of Surfactant Micelles. Ind. Eng. Chem. Res. 2018, 57, 9018–9027. [Google Scholar] [CrossRef]
- Liu, F.; Zhou, W.; Liu, D.; Chen, F.; Wei, J. Coarse-grained molecular dynamics study on the rheological behaviors of surfactant aqueous solution. J. Mol. Liq. 2018, 265, 572–577. [Google Scholar] [CrossRef]
- Ohlendorf, D.; Interthal, W.; Hoffmann, H. Surfactant systems for drag reduction: Physico-chemical properties and rheological behaviour. Rheol. Acta 1986, 25, 468–486. [Google Scholar] [CrossRef]
- Boltenhagen, P.; Hu, Y.; Matthys, E.; Pine, D. Observation of Bulk Phase Separation and Coexistence in a Sheared Micellar Solution. Phys. Rev. Lett. 1997, 79, 2359–2362. [Google Scholar] [CrossRef] [Green Version]
- Bhardwaj, A.; Richter, D.; Chellamuthu, M.; Rothstein, J.P. The effect of pre-shear on the extensional rheology of wormlike micelle solutions. Rheol. Acta 2007, 46, 861–875. [Google Scholar] [CrossRef]
- Helgeson, M.E.; Vasquez, P.A.; Kaler, E.W.; Wagner, N.J. Rheology and spatially resolved structure of cetyltrimethylammonium bromide wormlike micelles through the shear banding transition. J. Rheol. 2009, 53, 727–756. [Google Scholar] [CrossRef]
- Qi, Y.; Zakin, J.L. Chemical and Rheological Characterization of Drag-Reducing Cationic Surfactant Systems. Ind. Eng. Chem. Res. 2002, 41, 6326–6336. [Google Scholar] [CrossRef]
- Sambasivam, A.; Dhakal, S.; Sureshkumar, R. Structure and rheology of self-assembled aqueous suspensions of nanoparticles and wormlike micelles. Mol. Simul. 2017, 44, 485–493. [Google Scholar] [CrossRef]
- Dehmoune, J.; Decruppe, J.P.; Greffier, O.; Xu, H. Rheometric investigation on the temporal shear thickening of dilute micellar solutions of C14-, C16-, and C18TAB/NaSal. J. Rheol. 2008, 52, 923–940. [Google Scholar] [CrossRef]
- Xu, N.; Wei, J. Time-Dependent Shear-Induced Nonlinear Viscosity Effects in Dilute CTAC/NaSal Solutions: Mechanism Analyses. Adv. Mech. Eng. 2014, 6, 179394. [Google Scholar] [CrossRef]
- Lumley, J.L. Drag Reduction by Additives. Annu. Rev. Fluid Mech. 1969, 1, 367–384. [Google Scholar] [CrossRef]
- Khan, M.B.; Khan, M.S. Experimental investigation on effect of alkali, salts on the rheological properties of polyacrylamide and mixed assembly of polymer-surfactant. Chem. Data Collect. 2018, 13, 60–72. [Google Scholar] [CrossRef]
- Shettigar, R.R.; Misra, N.M.; Patel, K. CTAB grafted PAM gel and its application in drilling fluid. J. Pet. Sci. Eng. 2018, 160, 129–135. [Google Scholar] [CrossRef]
- Xu, N.; Wei, J.; Kawaguchi, Y. Rheology test on shear viscosity of surfactant solution: Characteristic time, hysteresis phenomenon, and fitting equation. Ind. Eng. Chem. Res. 2016, 55, 5817–5924. [Google Scholar] [CrossRef]
- Ma, N.; Wei, J.; Zhang, C.; Xu, N.; Yu, Z. Rheological properties of CTAC surfactant aqueous solutions. J. Eng. Thermophys. 2012, 33, 9. [Google Scholar]
- Lin, Z.Q.; Zakin, J.L.; Zheng, Y.; Davis, H.T.; Scriven, L.E.; Talmon, Y. Comparison of the effects of dimethyl and dichloro benzoate counterions on drag reduction, rheological behaviors, and microstructures of a cationic surfactant. J. Rheol. 2001, 45, 963–981. [Google Scholar] [CrossRef]
- Xin, X.; Xu, G.; Gong, H.; Bai, Y.; Tan, Y. Interaction between sodium oleate and partially hydrolyzed polyacrylamide: A rheological study. Colloids Surf. A Physicochem. Eng. Asp. 2008, 326, 1–9. [Google Scholar] [CrossRef]
- Nilsson, S.; Thuresson, K.; Hansson, P.; Lindman, B. Mixed Solutions of Surfactant and Hydrophobically Modified Polymer. Controlling Viscosity with Micellar Size. J. Phys. Chem. B 1998, 102, 7099–7105. [Google Scholar] [CrossRef]
- Smith, G.L.; McCormick, C.L. Water-Soluble Polymers. 79. Interaction of Microblocky Twin-Tailed Acrylamido Terpolymers with Anionic, Cationic, and Nonionic Surfactants. Langmuir 2001, 17, 1719–1725. [Google Scholar] [CrossRef]
- Wang, P.; Pei, S.; Wang, M.; Yan, Y.; Sun, X.; Zhang, J. Study on the transformation from linear to branched wormlike micelles: An insight from molecular dynamics simulation. J. Colloid Interface Sci. 2017, 494, 47–53. [Google Scholar] [CrossRef]
- Dhakal, S.; Sureshkumar, R. Anomalous diffusion and stress relaxation in surfactant micelles. Phys. Rev. E 2016, 96, 012605. [Google Scholar] [CrossRef] [PubMed] [Green Version]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, D.; Li, Y.; Liu, F.; Zhou, W.; Sun, A.; Liu, X.; Chen, F.; Xu, B.B.; Wei, J. Interfacial Interaction Enhanced Rheological Behavior in PAM/CTAC/Salt Aqueous Solution—A Coarse-Grained Molecular Dynamics Study. Polymers 2020, 12, 265. https://doi.org/10.3390/polym12020265
Liu D, Li Y, Liu F, Zhou W, Sun A, Liu X, Chen F, Xu BB, Wei J. Interfacial Interaction Enhanced Rheological Behavior in PAM/CTAC/Salt Aqueous Solution—A Coarse-Grained Molecular Dynamics Study. Polymers. 2020; 12(2):265. https://doi.org/10.3390/polym12020265
Chicago/Turabian StyleLiu, Dongjie, Yong Li, Fei Liu, Wenjing Zhou, Ansu Sun, Xiaoteng Liu, Fei Chen, Ben Bin Xu, and Jinjia Wei. 2020. "Interfacial Interaction Enhanced Rheological Behavior in PAM/CTAC/Salt Aqueous Solution—A Coarse-Grained Molecular Dynamics Study" Polymers 12, no. 2: 265. https://doi.org/10.3390/polym12020265






