Preparation of Ethylene Glycol Dimethacrylate (EGDMA)-Based Terpolymer as Potential Sorbents for Pharmaceuticals Adsorption
Abstract
:1. Introduction
2. Experimental
2.1. Materials
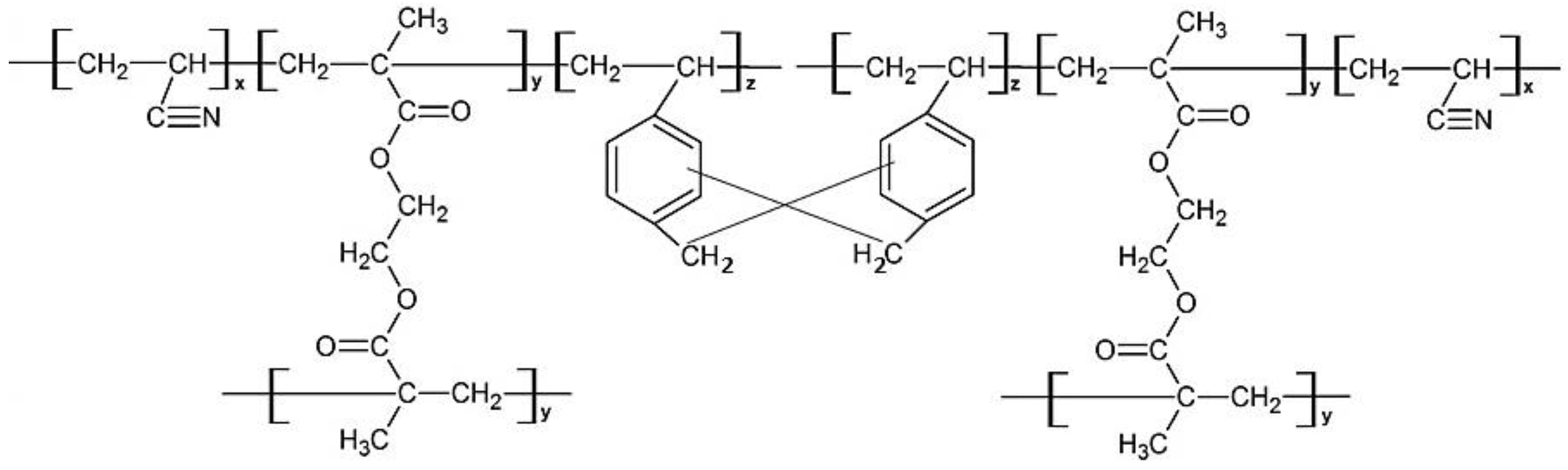
2.2. Synthesis of Poly(AN-co-EGDMA-co-VBC)
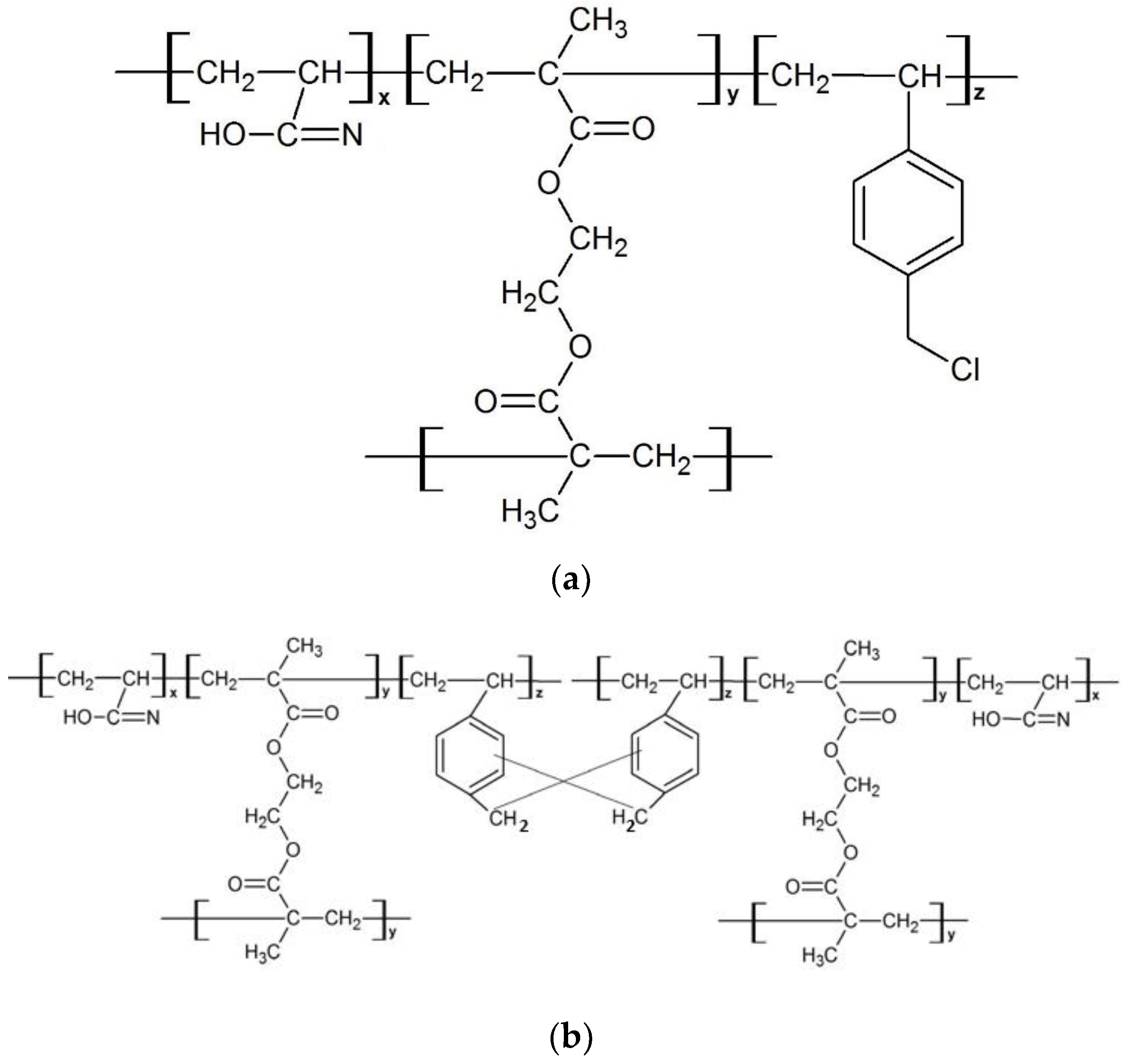
2.3. Synthesis of Hypercrosslinked Poly(AN-co-EGDMA-co-VBC)
2.4. Batch Adsorption Study
2.5. Characterizations
3. Results and Discussion
3.1. Yield of Polymerization
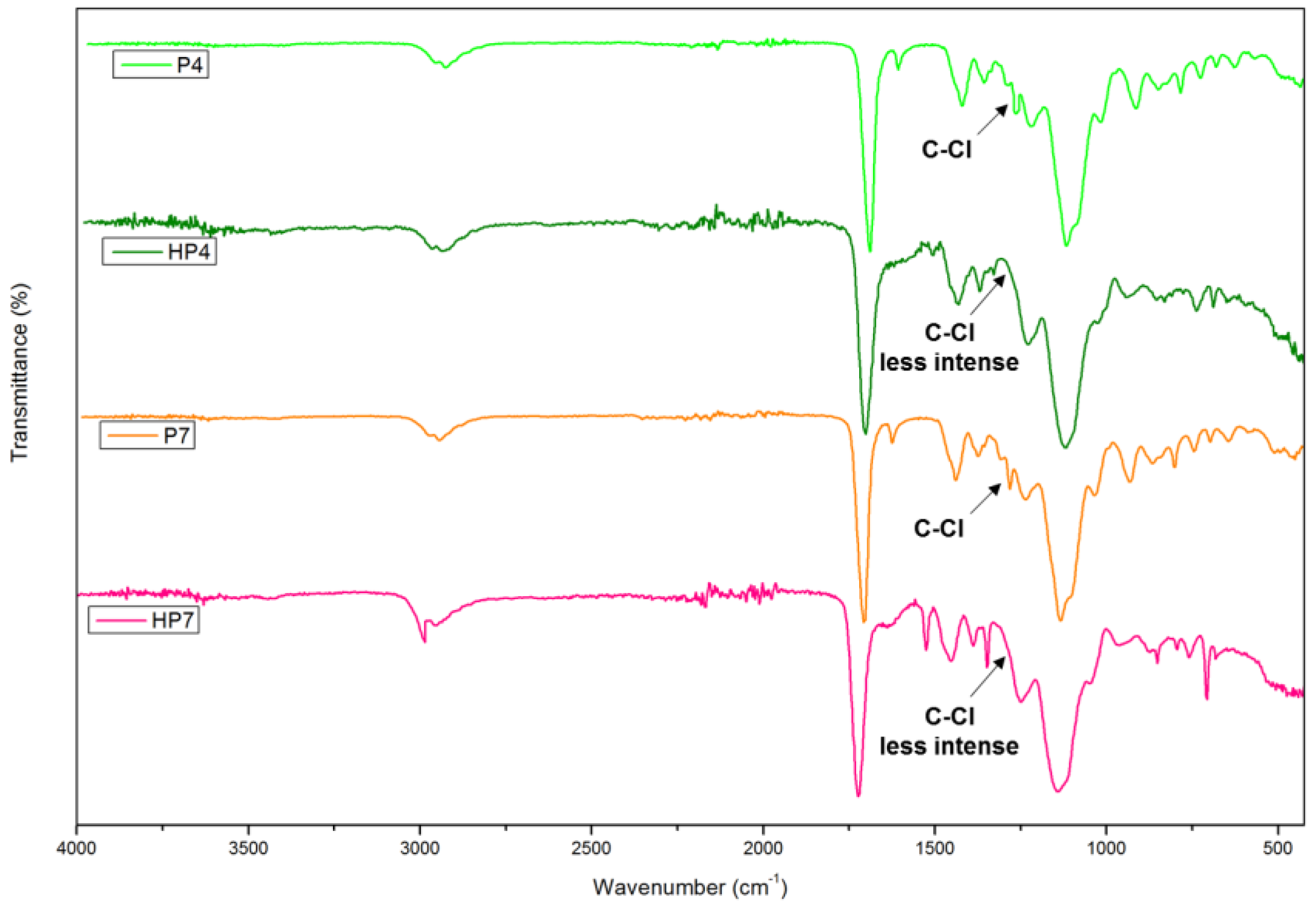
3.2. Physiochemical Characterization
3.3. Adsorption Study
3.3.1. Effect of Initial Concentration
3.3.2. Adsorption Isotherm
3.3.3. Effect of Contact Time
3.3.4. Adsorption Kinetics
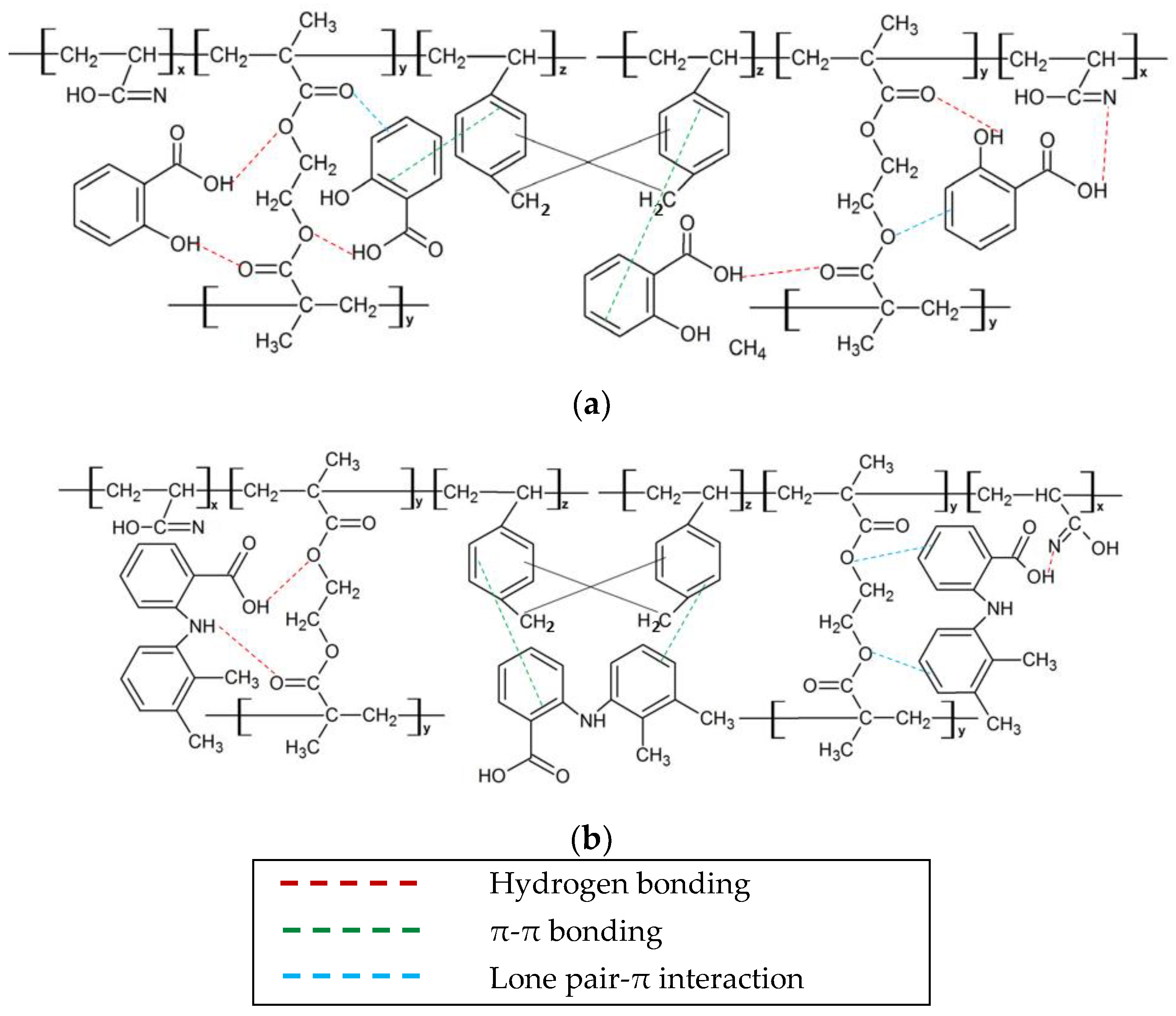
3.4. Adsorption Mechanism
3.5. Proposed Mechanism of Salicylic Acid and Mefenamic Acid Adsorption by HXL Poly(AN-co-EGDMA-co-VBC)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ensano, B.; Borea, L.; Naddeo, V.; Belgiorno, V.; Luna, M.D.; Ballesteros, F. Removal of Pharmaceuticals from Wastewater by Intermittent Electrocoagulation. Water 2017, 9, 85. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, J.; Boxall, A.; Kolpin, D. A Novel Method to Characterise Levels of Pharmaceutical Pollution in Large-Scale Aquatic Monitoring Campaigns. Appl. Sci. 2019, 9, 1368. [Google Scholar] [CrossRef] [Green Version]
- Phonsiri, V.; Choi, S.; Nguyen, C.; Tsai, Y.-L.; Coss, R.; Kurwadkar, S. Monitoring occurrence and removal of selected pharmaceuticals in two different wastewater treatment plants. SN Appl. Sci. 2019, 1. [Google Scholar] [CrossRef] [Green Version]
- Carbone, C.; Musumeci, T.; Pignatello, R. Non-steroidal anti-inflammatory drugs. Drug Biomembr. Interact. Stud. 2013, 281–303. [Google Scholar] [CrossRef]
- Al-Odaini, N.A.; Zakaria, M.P.; Yaziz, M.I.; Surif, S.; Abdulghani, M. The occurrence of human pharmaceuticals in wastewater effluents and surface water of Langat River and its tributaries, Malaysia. Int. J. Environ. Anal. Chem. 2013, 93, 245–264. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, D.; Li, Y.; Shi, J. Magnetic, core-shell structured and surface molecularly imprinted polymers for the rapid and selective recognition of salicylic acid from aqueous solutions. Appl. Surf. Sci. 2018, 435, 178–186. [Google Scholar] [CrossRef]
- Khalaf, S.; Al-Rimawi, F.; Khamis, M.; Nir, S.; Bufo, S.A.; Scrano, L.; Karaman, R. Efficiency of Membrane Technology Activated Charcoal and a Clay Micelle Complex for the Removal of Ibuprofen and Mefenamic Acid. Int. Case Stud. J. 2015, 4, 38–78. [Google Scholar]
- Sadeghi, H.B.; Panahi, H.A.; Mahabadi, M.; Moniri, E. Preconcentration and Determination of Mefenamic Acid in Pharmaceutical and Biological Fluid Samples by Polymer-grafted Silica Gel Solid-phase Extraction Following High Performance Liquid Chromatography. Iran. J. Pharm. Res. 2015, 14, 765–773. [Google Scholar]
- Zhou, F.; Man, R.; Huang, J. Hyper-cross-linked polymers functionalized with primary amine and its efficient adsorption of salicylic acid from aqueous solution. J. Chem. Thermodyn. 2019, 131, 387–392. [Google Scholar] [CrossRef]
- Gan, Y.; Chen, G.; Sang, Y.; Zhou, F.; Man, R.; Huang, J. Oxygen-rich hyper-cross-linked polymers with hierarchical porosity for aniline adsorption. Chem. Eng. J. 2019, 368, 29–36. [Google Scholar] [CrossRef]
- Wang, X.; Ou, H.; Huang, J. One-pot synthesis of hyper-cross-linked polymers chemically modified with pyrrole, furan, and thiophene for phenol adsorption from aqueous solution. J. Colloid Interface Sci. 2019, 538, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; He, J.; Huang, J. Amino-modified hyper-cross-linked polymers with hierarchical porosity for adsorption of salicylic acid from aqueous solution. J. Chem. Thermodyn. 2019, 131, 1–8. [Google Scholar] [CrossRef]
- Wang, X.; Mao, X.; Huang, J. Hierarchical porous hyper-cross-linked polymers modified with phenolic hydroxyl groups and their efficient adsorption of aniline from aqueous solution. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 80–87. [Google Scholar] [CrossRef]
- Subri, N.N.S.; Cormack, P.A.G.; Jamil, S.N.A.M.; Abdullah, L.C.; Daik, R. Synthesis of poly(acrylonitrile-co-divinylbenzene-co-vinylbenzyl chloride)-derived hypercrosslinked polymer microspheres and a preliminary evaluation of their potential for the solid-phase capture of pharmaceuticals. J. Appl. Polym. Sci. 2018, 135, 45677. [Google Scholar] [CrossRef] [Green Version]
- Zhou, F.; Man, R.; Huang, J. Alkoxy-Modified Hyper-Cross-Linked Polymers with Hierarchical Porosity and Their Adsorption of Salicylic Acid from Aqueous Solution. Ind. Eng. Chem. Res. 2018, 57, 12420–12428. [Google Scholar] [CrossRef]
- Zhang, T.; Zhou, F.; Huang, J.; Man, R. Ethylene glycol dimethacrylate modified hyper-cross-linked resins: Porogen effect on pore structure and adsorption performance. Chem. Eng. J. 2018, 339, 278–287. [Google Scholar] [CrossRef]
- Zhang, T.; Huang, J. Tunable synthesis of the polar modified hyper-cross-linked resins and application to the adsorption. J. Colloid Interface Sci. 2017, 505, 383–391. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, T.; Huo, J.; Huang, J.; Liu, Y.-N. Tunable porosity and polarity of polar post-cross-linked resins and selective adsorption. J. Colloid Interface Sci. 2017, 487, 231–238. [Google Scholar] [CrossRef]
- Fu, Z.; Huang, J. Polar hyper-cross-linked resin with abundant micropores/mesopores and its enhanced adsorption toward salicylic acid: Equilibrium, kinetics, and dynamic operation. Fluid Phase Equilibria 2017, 438, 1–9. [Google Scholar] [CrossRef]
- Xiao, G.; Wen, R.; Liu, A.; He, G.; Wu, D. Adsorption performance of salicylic acid on a novel resin with distinctive double pore structure. J. Hazard. Mater. 2017, 329, 77–83. [Google Scholar] [CrossRef]
- Shao, L.; Li, Y.; Zhang, T.; Liu, M.; Huang, J. Controllable Synthesis of Polar Modified Hyper-Cross-Linked Resins and Their Adsorption of 2-Naphthol and 4-Hydroxybenzoic Acid from Aqueous Solution. Ind. Eng. Chem. Res. 2017, 56, 2984–2992. [Google Scholar] [CrossRef]
- Urban, J.; Svec, F.; Fréchet Jean, M.J. Efficient Separation of Small Molecules Using a Large Surface Area Hypercrosslinked Monolithic Polymer Capillary Column. Anal. Chem. 2010, 82, 1621–1623. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, W.; Sun, W.; Xu, C.; Wang, C.; Jia, Y.; Qin, X.; Xie, C.; Yu, S.; Xian, M. Effective adsorption toward p-aminobenzoic acid from aqueous solution by a L-malic acid modified hyper-crosslinked resin: Equilibria and kinetics. J. Taiwan Inst. Chem. Eng. 2018, 89, 105–112. [Google Scholar] [CrossRef]
- Yahya, M.Z.; Abdullah, N. Preparation and Characterization of Hypercrosslinked Poly (HEMA-co-EGDMA-co-VBC). Indian J. Sci. Technol. 2017, 10, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Tan, L.; Tan, B. Hypercrosslinked porous polymer materials: Design, synthesis, and applications. Chem. Soc. Rev. 2017, 46, 3322–3356. [Google Scholar] [CrossRef]
- Fontanals, N.; Cormack, P.A.; Sherrington, D.C. Hypercrosslinked polymer microspheres with weak anion-exchange character. J. Chromatogr. A 2008, 1215, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fontanals, N.; Cortés, J.; Galià, M.; Marcé, R.M.; Cormack, P.A.G.; Borrull, F.; Sherrington, D.C. Synthesis of Davankov-type hypercrosslinked resins using different isomer compositions of vinylbenzyl chloride monomer, and application in the solid-phase extraction of polar compounds. J. Polym. Sci. Part A Polym. Chem. 2005, 43, 1718–1728. [Google Scholar] [CrossRef]
- Fontanals, N.; Galià, M.; Cormack, P.A.; Marcé, R.M.; Sherrington, D.C.; Borrull, F. Evaluation of a new hypercrosslinked polymer as a sorbent for solid-phase extraction of polar compounds. J. Chromatogr. A 2005, 1075, 51–56. [Google Scholar] [CrossRef]
- Tsyurupa, M.; Davankov, V. Porous structure of hypercrosslinked polystyrene: State-of-the-art mini-review. React. Funct. Polym. 2006, 66, 768–779. [Google Scholar] [CrossRef]
- Fontanals, N.; Marcé, R.; Borrull, F. New hydrophilic materials for solid-phase extraction. TrAC Trends Anal. Chem. 2005, 24, 394–406. [Google Scholar] [CrossRef]
- Galicia-Aguilar, J.A.; Santamaría-Juárez, J.D.; López-Badillo, M.; Sánchez-Cantú, M.; Varela-Caselis, J.L. Synthesis and characterization of AN/EGDMA-based adsorbents for phenol adsorption. React. Funct. Polym. 2017, 117, 112–119. [Google Scholar] [CrossRef]
- Jose, J.; John, M.; Mathew, B. Effect of the Nature of Crosslinking Agent on the Catalase-Like Activity of Polystyrene-Bound Glycine–Metal Complexes. J. Macromol. Sci. Part A 2003, 40, 863–879. [Google Scholar] [CrossRef]
- Huang, J.; Zhu, J.; Snyder, S.A.; Morris, A.J.; Turner, S.R. Nanoporous highly crosslinked polymer networks with covalently bonded amines for CO2 capture. Polymer 2018, 154, 55–61. [Google Scholar] [CrossRef]
- Liu, W.; Zhu, X.; Yang, X.; Li, K.; Yang, Z. Preparation of highly cross-linked hydrophilic porous microspheres poly(N,N′-methylenebisacrylamide) and poly(N,N′-methylenebisacrylamide-co-acrylic acid) with an application on the removal of cadmium. Polym. Adv. Technol. 2018, 29, 2724–2734. [Google Scholar] [CrossRef]
- El-Bahy, S.M.; El-Bahy, Z.M. Immobilization of 2-amino pyridine onto poly(acrylonitrile-co-N,N′-methylenebisacrylamide) nanoparticles for the removal of Hg(II), Cd(II) and Cr(III): Batch and column techniques. J. Environ. Chem. Eng. 2017, 5, 3560–3571. [Google Scholar] [CrossRef]
- El-Bahy, S.M.; El-Bahy, Z.M. Synthesis and characterization of polyamidoxime chelating resin for adsorption of Cu(II), Mn(II) and Ni(II) by batch and column study. J. Environ. Chem. Eng. 2016, 4, 276–286. [Google Scholar] [CrossRef]
- Kulygin, O.; Silverstein, M.S. Porous poly(2-hydroxyethyl methacrylate) hydrogels synthesized within high internal phase emulsions. Soft Matter 2007, 3, 1525. [Google Scholar] [CrossRef]
- Gokmen, M.T.; Prez, F.E.D. Porous polymer particles—A comprehensive guide to synthesis, characterization, functionalization and applications. Prog. Polym. Sci. 2012, 37, 365–405. [Google Scholar] [CrossRef] [Green Version]
- Özer, E.T.; Osman, B.; Kara, A.; Beşirli, N.; Gücer, Ş.; Sözeri, H. Removal of diethyl phthalate from aqueous phase using magnetic poly(EGDMA–VP) beads. J. Hazard. Mater. 2012, 229–230, 20–28. [Google Scholar] [CrossRef]
- Bagheri, B.; Abdouss, M.; Aslzadeh, M.M.; Shoushtari, A.M. Efficient Removal of Cr3+, Pb2+ and Hg2+ Ions from Industrial Effluents by Hydrolyzed/Thioamidated Polyacrylonitrile Fibres. Iran. Polym. J. 2010, 19, 911–925. [Google Scholar]
- Hwang, K.S.; Choi, W.J.; Kim, J.-H.; Lee, J.-Y. Preparation of hypercrosslinked poly(DVB-VBC) particles with high surface area and structured meso- and micropores. Macromol. Res. 2015, 23, 1051–1058. [Google Scholar] [CrossRef]
- Wang, R.; Zhang, Y.; Ma, G.; Su, Z. Preparation of uniform poly(glycidyl methacrylate) porous microspheres by membrane emulsification–polymerization technology. J. Appl. Polym. Sci. 2006, 102, 5018–5027. [Google Scholar] [CrossRef]
- Li, W.-H.; Stöver, H.D.H. Monodisperse Cross-Linked Core-Shell Polymer Microspheres by Precipitation Polymerization. Macromolecules 2000, 33, 4354–4360. [Google Scholar] [CrossRef]
- Norhayati, A.; Zuhaili, Y.M.; Rabiatuladawiah, M. Synthesis and characterization of poly(HEMA-co-EGDMA-co-VBC) by modified suspension polymerization: Effects of polymerization parameters reaction on chemical and thermal properties of polymer. Mater. Today Proc. 2018, 5, 22010–22019. [Google Scholar] [CrossRef]
- Yuan, H.G.; Kalfas, G.; Ray, W.H. Suspension Polymerization. J. Macromol. Sci. Part C Polym. Rev. 1991, 31, 215–299. [Google Scholar] [CrossRef]
- Liu, F.; Chen, S.; Gao, Y. Synthesis of porous polymer-based solid amine adsorbent: Effect of pore size and amine loading on CO2 adsorption. J. Colloid Interface Sci. 2017, 506, 236–244. [Google Scholar] [CrossRef]
- Adeyi, A.A.; Jamil, S.N.A.M.; Abdullah, L.C.; Choong, T.S.Y.; Lau, K.L.; Abdullah, M. Adsorptive Removal of Methylene Blue from Aquatic Environments Using Thiourea-Modified Poly(Acrylonitrile-co-Acrylic Acid). Materials 2019, 12, 1734. [Google Scholar] [CrossRef] [Green Version]
- Adeyi, A.A.; Jamil, S.N.A.M.; Abdullah, L.C.; Choong, T.S.Y. Adsorption of Malachite Green Dye from Liquid Phase Using Hydrophilic Thiourea-Modified Poly(acrylonitrile-co-acrylic acid): Kinetic and Isotherm Studies. J. Chem. 2019, 2019, 1–14. [Google Scholar] [CrossRef]
- Leshchinskaya, A.; Ezhova, N.; Pisarev, O. Synthesis and characterization of 2-hydroxyethyl methacrylate-ethylene glycol dimethacrylate polymeric granules intended for selective removal of uric acid. React. Funct. Polym. 2016, 102, 101–109. [Google Scholar] [CrossRef]
- Boudouaia, N.; Bengharez, Z.; Jellali, S. Preparation and characterization of chitosan extracted from shrimp shells waste and chitosan film: Application for Eriochrome black T removal from aqueous solutions. Appl. Water Sci. 2019, 9. [Google Scholar] [CrossRef] [Green Version]
- Haghseresht, F.; Lu, G.Q. Adsorption Characteristics of Phenolic Compounds onto Coal-Reject-Derived Adsorbents. Energy Fuels 1998, 12, 1100–1107. [Google Scholar] [CrossRef]
- Majeed, B.A.A.; Muhseen, R.J.; Jassim, N.J. Adsorption of Mefenamic Acid from Water by Bentonite Poly urea formaldehyde Composite Adsorbent. J. Eng. 2017, 23, 50–73. [Google Scholar]
- Fallou, H.; Cimetière, N.; Giraudet, S.; Wolbert, D.; Cloirec, P.L. Adsorption of pharmaceuticals onto activated carbon fiber cloths—Modeling and extrapolation of adsorption isotherms at very low concentrations. J. Environ. Manag. 2016, 166, 544–555. [Google Scholar] [CrossRef]
- Varga, M.; Elabadsa, M.; Tatár, E.; Mihucz, V.G. Removal of selected pharmaceuticals from aqueous matrices with activated carbon under batch conditions. Microchem. J. 2019, 148, 661–672. [Google Scholar] [CrossRef]
- Jayalakshmi, R.; Jeyanthi, J. Simultaneous removal of binary dye from textile effluent using cobalt ferrite-alginate nanocomposite: Performance and mechanism. Microchem. J. 2019, 145, 791–800. [Google Scholar] [CrossRef]







| Sample | AN (mol%) | EGDMA (mol%) | VBC (mol%) | AN (mL) | EGDMA (mL) | VBC (mL) |
|---|---|---|---|---|---|---|
| P1 | 100 | - | - | 4.938 | - | - |
| P4 | 25 | 70 | 5 | 0.410 | 3.308 | 0.177 |
| P7 | 20 | 75 | 5 | 0.314 | 3.390 | 0.169 |
| Sample | AN/EGDMA/VBC (mol%) | Yields (%) |
|---|---|---|
| P1 | 100/0/0 | 3 |
| P4 | 25/70/5 | 39 |
| P7 | 20/75/5 | 48 |
| Elemental Microanalysis (%) | ||||||
|---|---|---|---|---|---|---|
| Sample | AN/EGDMA/VBC (mol%) | C | H | N | O | Cl |
| P1 | 100/0/0 | 63.08 | 5.49 | 25.39 | - | - |
| P4 | 25/70/5 | 57.43 | 6.63 | 0.51 | 27.40 | 7.55 |
| HP4 | 25/70/5 | 54.13 | 6.25 | 1.17 | 32.55 | 5.48 |
| P7 | 20/75/5 | 57.70 | 6.70 | 0.40 | 29.81 | 4.95 |
| HP7 | 20/75/5 | 52.12 | 6.16 | 0.17 | 29.30 | 11.58 |
| Sample | AN/EGDMA/VBC (mol%) | Specific Surface Area (m2·g−1) | Specific Pore Volume (cm3·g−1) | Mean Pore Size (nm) |
|---|---|---|---|---|
| P1 | 100/0/0 | 2 | - | - |
| P4 | 25/70/5 | 45.6 | 0.851 | 74.7 |
| HP4 | 25/70/5 | 59.1 | 0.631 | 42.7 |
| P7 | 20/75/5 | 47.4 | 0.350 | 29.5 |
| HP7 | 20/75/5 | 1.1 | 0.118 | 447.0 |
| Langmuir Model | Freundlich Model | |||||
|---|---|---|---|---|---|---|
| KL | qm | R2 | KF | n | R2 | |
| Salicylic acid | ||||||
| HP4 | 4.420 × 10−2 | 416.7 | 0.9275 | 66.188 | 2.9709 | 0.9717 |
| P4 | 2.555 × 10−2 | 333.3 | 0.7854 | 32.779 | 2.4096 | 0.8582 |
| P1 | 3.473 × 10−2 | 149.2 | 0.7532 | 15.393 | 2.2568 | 0.8005 |
| Mefenamic acid | ||||||
| HP4 | 1.673 × 10−3 | 625.0 | 0.9950 | 2.3085 | 0.8061 | 0.9955 |
| P4 | 1.741 × 10−3 | 370.4 | 0.9762 | 4.5172 | 0.7765 | 0.9698 |
| P1 | 4.319 × 10−3 | 84.7 | 0.9120 | 6.3777 | 0.7567 | 0.8967 |
| Adsorbents | BET Surface Area (m2·g−1) | qmax (mg·g−1) | References |
|---|---|---|---|
| Salicylic acid | |||
| Magnetic molecularly imprinted polymer | - | 36.8 | [6] |
| Magnetic non-imprinted polymer | - | 6.5 | [6] |
| Hypercrosslinked DVB/VBC | 1027 | 333.3 | [15] |
| Methoxy modified hypercrosslinked DVB/VBC | 848 | 492.6 | [15] |
| Phenoxy modified hypercrosslinked DVB/VBC | 875 | 621.1 | [15] |
| Hypercrosslinked poly(VBC-co-DVB-co-MA) | 1047 | 584.3 | [19] |
| Modified hypercrosslinked poly(VBC-co-DVB-co-MA) | 1010 | 457.9 | [19] |
| HP4 | 59.1 | 416.7 | This work |
| P4 | 45.6 | 333.3 | This work |
| Mefenamic acid | |||
| Clay micelle complex | - | 100.0 | [7] |
| Activated charcoal | - | 90.9 | [7] |
| Poly allyl glycidyl ether /iminodiacetic acid-co-N,N-dimethylacrylamide grafted to silica gel | - | 7.0 | [8] |
| Poly urea formaldehyde-bentonite | - | 28 | [52] |
| HP4 | 59.1 | 625.0 | This work |
| P4 | 45.6 | 370.4 | This work |
| qe (exp) | Pseudo-First-Order | Pseudo-Second-Order | |||||
|---|---|---|---|---|---|---|---|
| k1 | qe | R2 | k2 | qe | R2 | ||
| Salicylic acid | |||||||
| HP4 | 169.5 | 0.1422 | 41.1 | 0.6651 | 1.934 × 10−2 | 169.5 | 0.9996 |
| P4 | 144.1 | 0.1739 | 39.9 | 0.7106 | 1.536 × 10−2 | 144.9 | 0.9988 |
| P1 | 61.9 | 0.1139 | 34.8 | 0.6895 | 4.956 × 10−3 | 66.7 | 0.9329 |
| Mefenamic acid | |||||||
| HP4 | 73.1 | 0.1292 | 27.5 | 0.7410 | 2.048 × 10−2 | 72.5 | 0.9970 |
| P4 | 52.6 | 0.1423 | 15.3 | 0.7069 | 4.395 × 10−2 | 52.4 | 0.9988 |
| P1 | 16.9 | 0.1396 | 11.5 | 0.8052 | 1.985 × 10−2 | 18.3 | 0.9652 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shaipulizan, N.S.; Md Jamil, S.N.A.; Kamaruzaman, S.; Subri, N.N.S.; Adeyi, A.A.; Abdullah, A.H.; Abdullah, L.C. Preparation of Ethylene Glycol Dimethacrylate (EGDMA)-Based Terpolymer as Potential Sorbents for Pharmaceuticals Adsorption. Polymers 2020, 12, 423. https://doi.org/10.3390/polym12020423
Shaipulizan NS, Md Jamil SNA, Kamaruzaman S, Subri NNS, Adeyi AA, Abdullah AH, Abdullah LC. Preparation of Ethylene Glycol Dimethacrylate (EGDMA)-Based Terpolymer as Potential Sorbents for Pharmaceuticals Adsorption. Polymers. 2020; 12(2):423. https://doi.org/10.3390/polym12020423
Chicago/Turabian StyleShaipulizan, Nur Syafiqah, Siti Nurul Ain Md Jamil, Sazlinda Kamaruzaman, Nur Nida Syamimi Subri, Abel Adekanmi Adeyi, Abdul Halim Abdullah, and Luqman Chuah Abdullah. 2020. "Preparation of Ethylene Glycol Dimethacrylate (EGDMA)-Based Terpolymer as Potential Sorbents for Pharmaceuticals Adsorption" Polymers 12, no. 2: 423. https://doi.org/10.3390/polym12020423






