Stimuli-Responsive Nanodiamond–Polyelectrolyte Composite Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Block Copolymer Complexing Agent
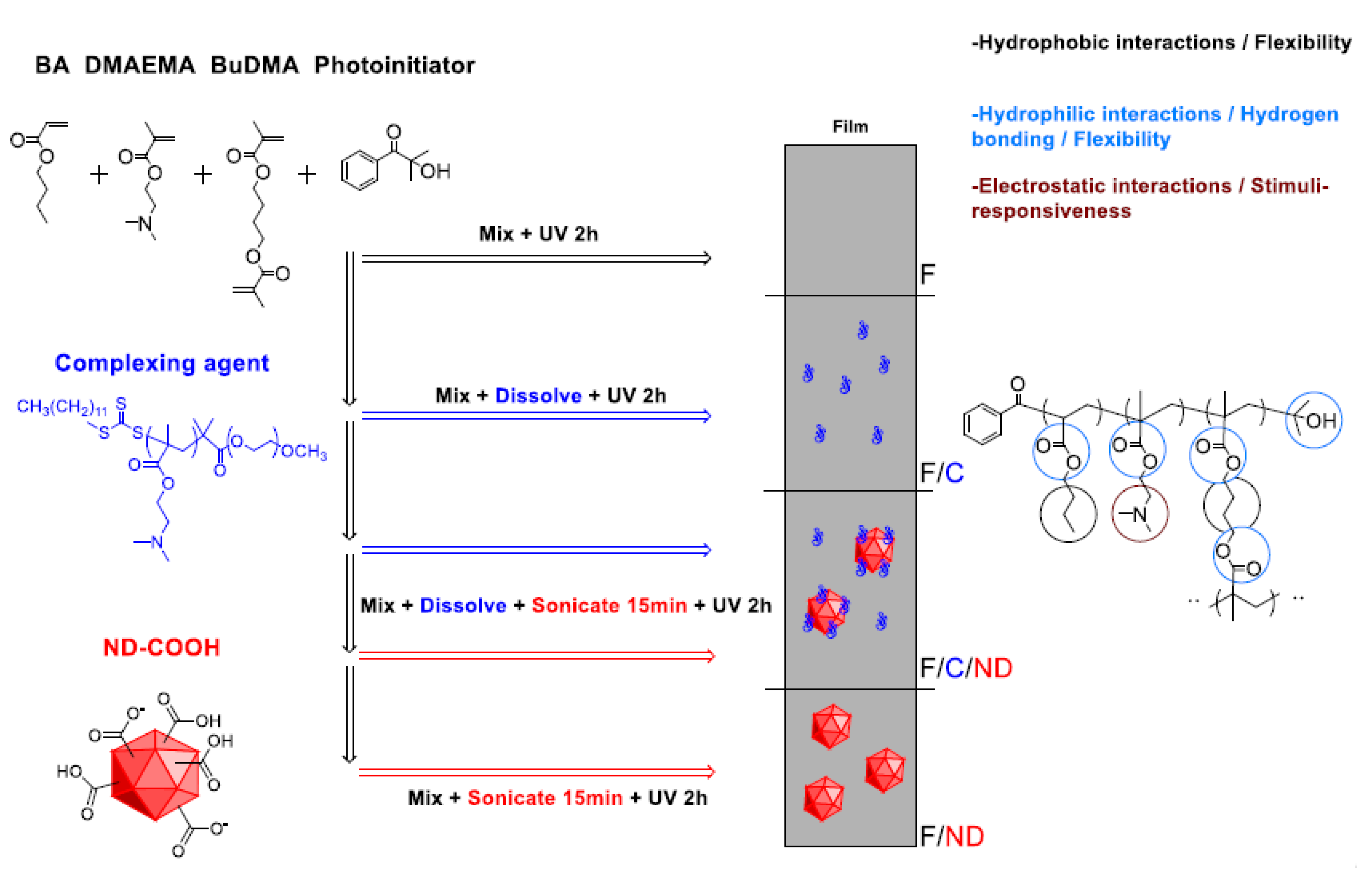
2.3. Film Preparation (Scheme 1)
2.4. Characterization Methods
3. Results and Discussion
3.1. Film Preparation
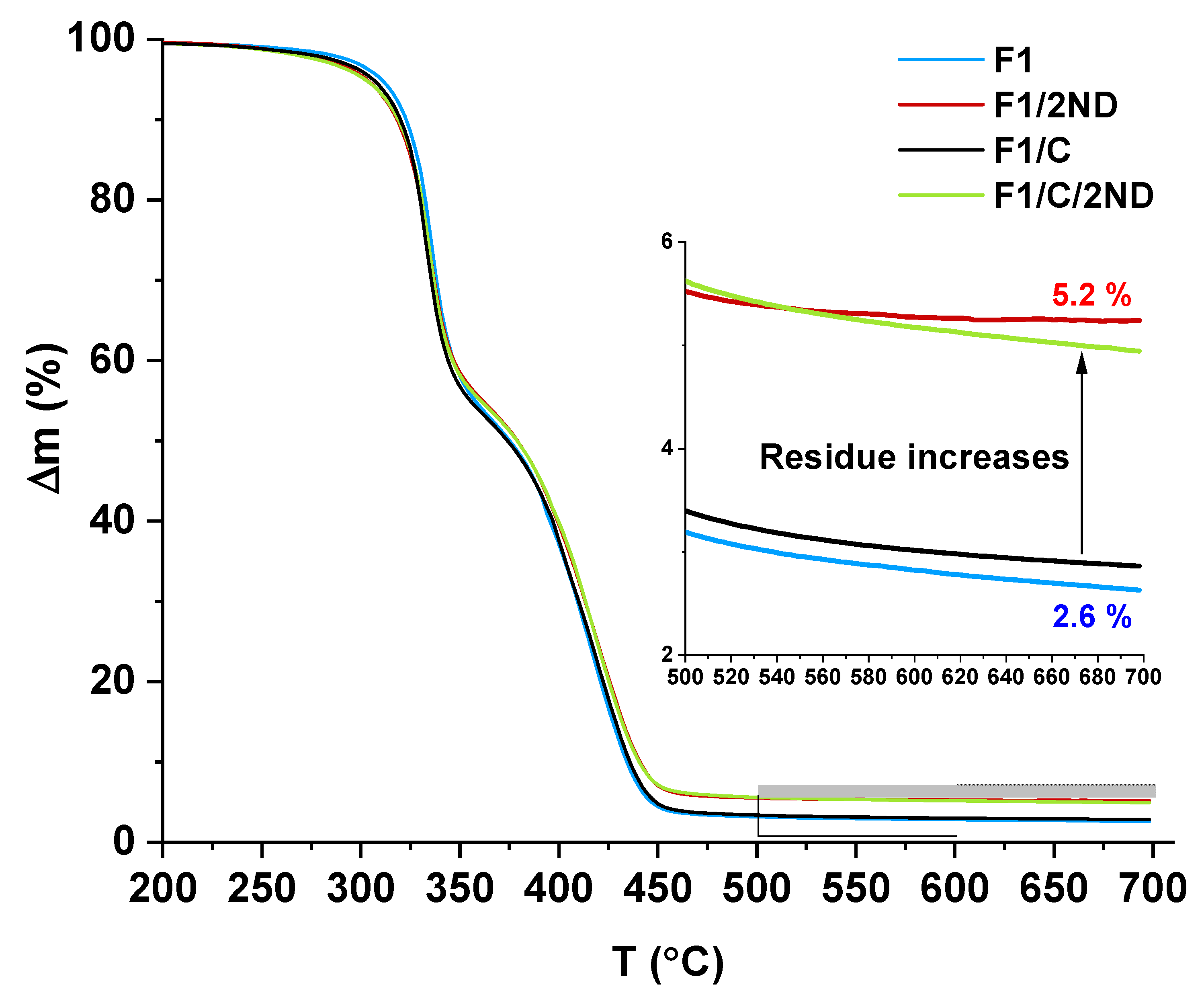
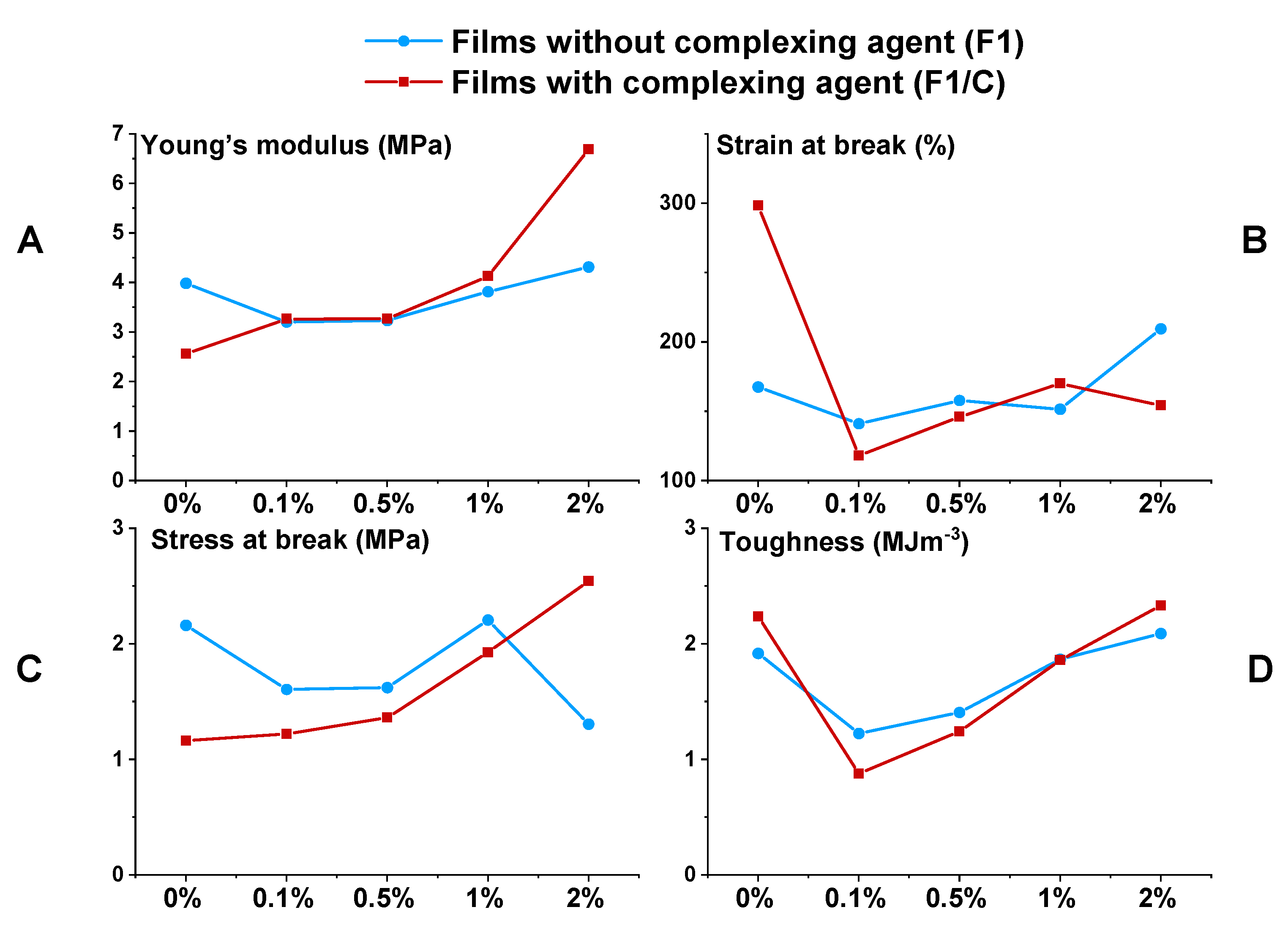
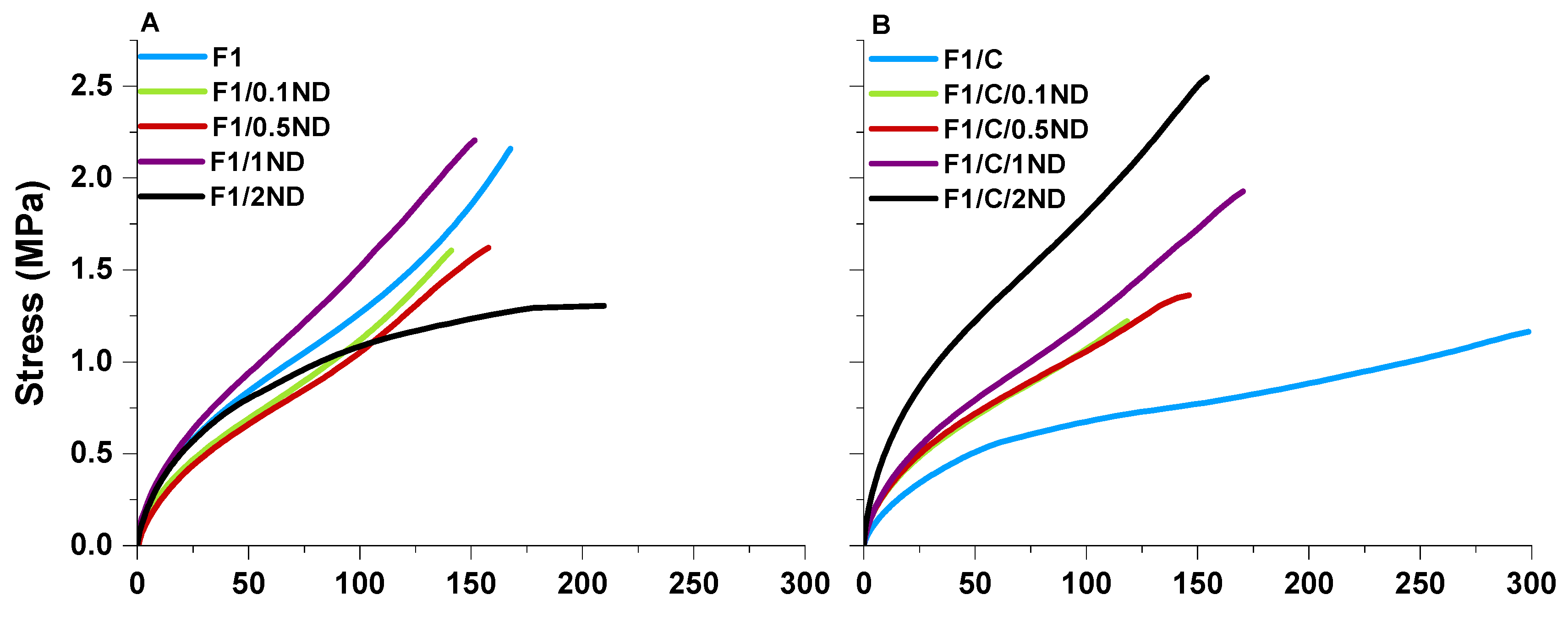
3.2. Film Properties and the Effects of the Fillers
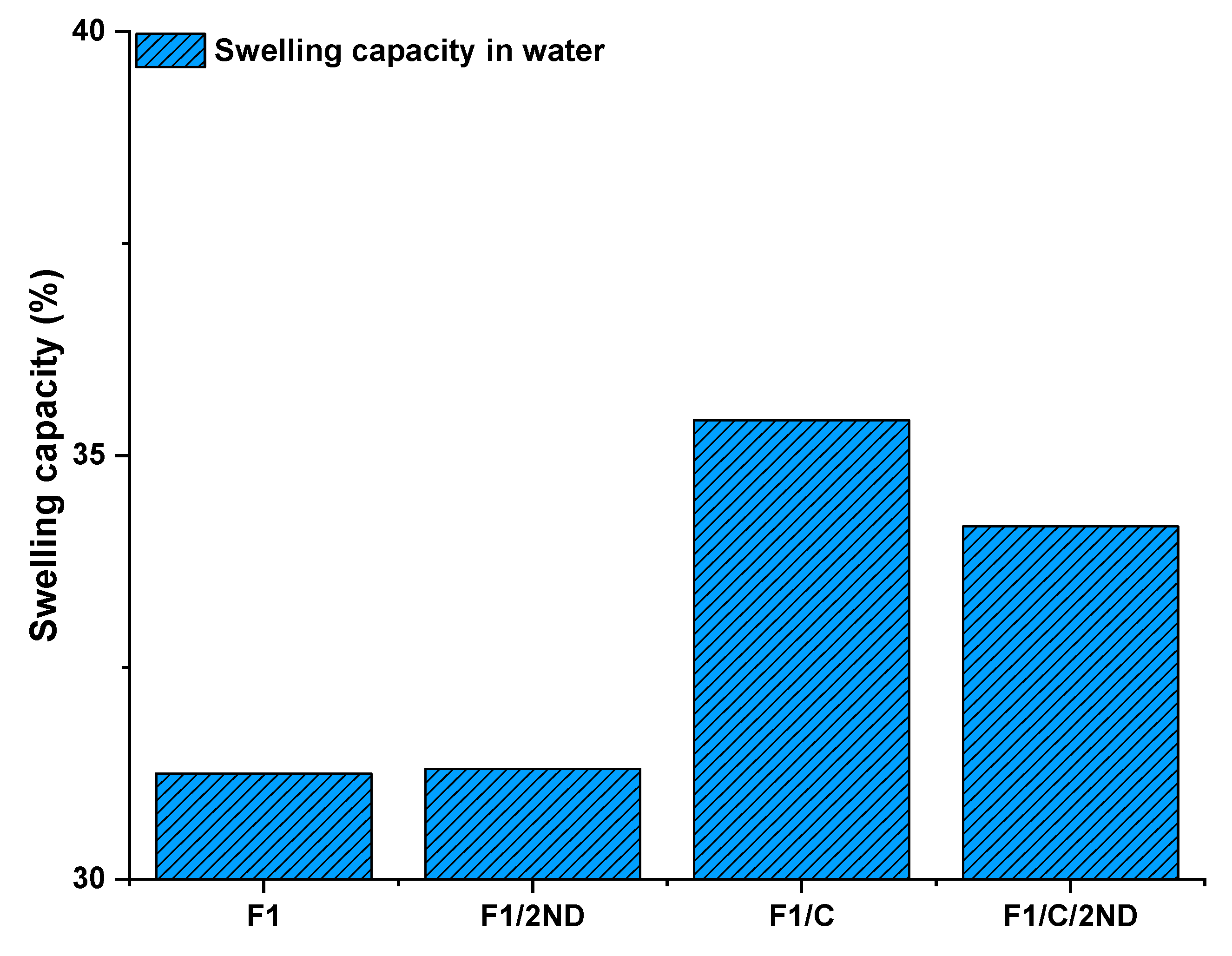
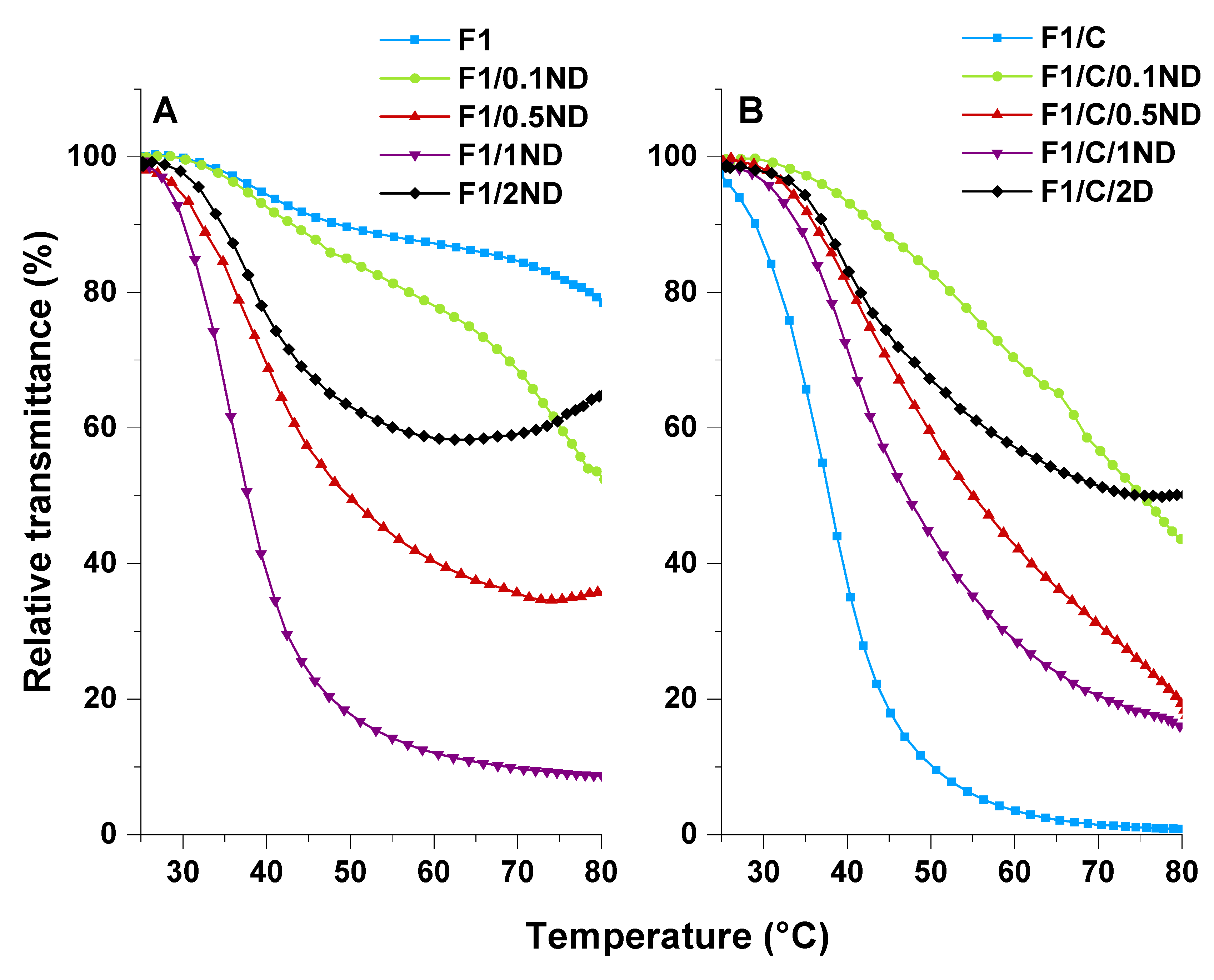
3.3. Swelling Capacity and Stimuli-Responsive Behaviour of the Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Fu, S.; Sun, Z.; Huang, P.; Li, Y.; Hu, N. Some basic aspects of polymer nanocomposites: A critical review. Nano Mater. Sci. 2019, 1, 2–30. [Google Scholar] [CrossRef]
- Kovačević, V.; Leskovac, M.; Lučić Blagojević, S.; Vrsaljko, D. Complex Adhesion Effects of Inorganic Nanofillers vs Microfillers in Polymer Composites. Macromol. Symp. 2005, 221, 11–22. [Google Scholar] [CrossRef]
- Bhattacharya, M. Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Rafiee, R.; Shahzadi, R. Mechanical Properties of Nanoclay and Nanoclay Reinforced Polymers: A Review. Polym. Compos. 2019, 40, 431–445. [Google Scholar] [CrossRef]
- Okada, A.; Usuki, A. Twenty Years of Polymer-Clay Nanocomposites. Macromol. Mater. Eng. 2006, 291, 1449–1476. [Google Scholar] [CrossRef]
- Turcheniuk, K.; Mochalin, V.N. Biomedical applications of nanodiamond (Review). Nanotechnology 2017, 28, 1–28. [Google Scholar] [CrossRef]
- Arnaut, J.C. Nanodiamonds: Advanced Material Analysis, Properties and Applications, 6th ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2017. [Google Scholar]
- Shenderova, O.; Gruen, D. Ultrananocrystalline Diamond: Synthesis, Properties and Applications, 2nd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Vul, A.Y.; Shenderova, O. Detonation Nanodiamonds: Science and Applications, 1st ed.; Taylor & Francis: New York, NY, USA, 2014. [Google Scholar]
- Shvidchenko, A.V.; Eidelman, E.D.; Vul, A.Y.; Kuznetsov, N.M.; Stolyarova, D.Y.; Belousov, S.I.; Chvalun, S.N. Colloids of detonation nanodiamond particles for advanced applications. Adv. Colloid Interface Sci. 2019, 268, 64–81. [Google Scholar] [CrossRef] [PubMed]
- Mochalin, V.N.; Shenderova, O.; Ho, D.; Gogotsi, Y. The properties and applications of nanodiamonds. Nat. Nanotechnol. 2012, 7, 11–23. [Google Scholar] [CrossRef]
- Alishahi, E.; Shadlou, S.; Doagou-R, S.; Ayatollahi, M.R. Effects of Carbon Nanoreinforcements of Different Shapes on the Mechanical Properties of Epoxy-Based Nanocomposites. Macromol. Mater. Eng. 2013, 298, 670–678. [Google Scholar] [CrossRef]
- Gannoruwa, A.; Sumita, M.; Kawahara, S. Highly enhanced mechanical properties in natural rubber prepared with a nanodiamond nanomatrix structure. Polymer 2017, 126, 40–47. [Google Scholar] [CrossRef]
- Karami, P.; Salkhi Khasraghi, S.; Hashemi, M.; Rabiei, S.; Shojaei, A. Polymer/nanodiamond composites—A comprehensive review from synthesis and fabrication to properties and applications. Adv. Colloid Interface Sci. 2019, 269, 122–151. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rhee, K.Y.; Hui, D.; Park, S.J. A critical review of nanodiamond based nanocomposites: Synthesis, properties and applications. Compos. Part B Eng. 2018, 143, 19–27. [Google Scholar] [CrossRef]
- Nunn, N.; Torelli, M.; McGuire, G.; Shenderova, O. Nanodiamond: A high impact nanomaterial. Curr. Opin. Solid State Mater. Sci. 2017, 21, 1–9. [Google Scholar] [CrossRef]
- Ullah, M.; Kausar, A.; Siddiq, M.; Subhan, M.; Abid Zia, M. Reinforcing Effects of Modified Nanodiamonds on the Physical Properties of Polymer-Based Nanocomposites: A Review. Polym. Plast. Technol. Eng. 2015, 54, 861–879. [Google Scholar] [CrossRef]
- Maitra, U.; Prasad, K.E.; Ramamurty, U.; Rao, C.N.R. Mechanical properties of nanodiamond-reinforced polymer-matrix composites. Solid State Commun. 2009, 149, 1693–1697. [Google Scholar] [CrossRef]
- Ayatollahi, M.R.; Alishahi, E.; Doagou-R, S.; Shadlou, S. Tribological and mechanical properties of low content nanodiamond/epoxy nanocomposites. Compos. Part B Eng. 2012, 43, 3425–3430. [Google Scholar] [CrossRef]
- Morimune-Moriya, S.; Salajkova, M.; Zhou, Q.; Nishino, T.; Berglund, L.A. Reinforcement Effects from Nanodiamond in Cellulose Nanofibril Films. Biomacromolecules 2018, 19, 2423–2431. [Google Scholar] [CrossRef]
- Liang, Y.; Ozawa, M.; Krueger, A. A General Procedure to Functionalize Agglomerating Nanoparticles Demonstrated on Nanodiamond. ACS Nano 2009, 3, 2288–2296. [Google Scholar] [CrossRef]
- Jesson, D.A.; Watts, J.F. The Interface and Interphase in Polymer Matrix Composites: Effect on Mechanical Properties and Methods for Identification. Polym. Rev. 2012, 52, 321–354. [Google Scholar] [CrossRef]
- Ran, J.; Wu, L.; He, Y.; Yang, Z.; Wang, Y.; Jiang, C.; Ge, L.; Bakangura, E.; Xu, T. Ion exchange membranes: New developments and applications. J. Memb. Sci. 2017, 522, 267–291. [Google Scholar] [CrossRef]
- Yi, S.; Lin, C.; Leon, W.; Vezenov, D.; Regen, S.L. Gas Permeability of Hyperthin Polyelectrolyte Multilayers Having Matched and Mismatched Repeat Units. Langmuir 2016, 32, 12332–12337. [Google Scholar] [CrossRef] [PubMed]
- Diep, J.; Tek, A.; Thompson, L.; Frommer, J.; Wang, R.; Piunova, V.; Sly, J.; La, Y.H. Layer-by-layer assembled core–shell star block copolymers for fouling resistant water purification membranes. Polymer 2016, 103, 468–477. [Google Scholar] [CrossRef]
- Liu, F.; Jarrett, W.L.; Urban, M.W. Glass (Tg) and Stimuli-Responsive (TSR) Transitions in Random Copolymers. Macromolecules 2010, 43, 5330–5337. [Google Scholar] [CrossRef]
- Liu, F.; Urban, M.W. Dual Temperature and pH Responsiveness of Poly(2-(N,N-dimethylamino)ethyl methacrylate-co-n-butyl acrylate) Colloidal Dispersions and Their Films. Macromolecules 2008, 41, 6531–6539. [Google Scholar] [CrossRef]
- Liu, H.; Lin, S.; Feng, Y.; Theato, P. CO2-Responsive polymer materials. Polym. Chem. 2017, 8, 12–23. [Google Scholar] [CrossRef]
- Dong, L.; Wang, Y.; Chen, M.; Shi, D.; Li, X.; Zhang, C.; Wang, H. Enhanced CO2 separation performance of P(PEGMA-co-DEAEMA-co-MMA) copolymer membrane through the synergistic effect of EO groups and amino groups. RSC Adv. 2016, 6, 59946–59955. [Google Scholar] [CrossRef]
- Zhang, Z.; Xie, G.; Xiao, K.; Kong, X.Y.; Li, P.; Tian, Y.; Wen, L.; Jiang, L. Asymmetric Multifunctional Heterogeneous Membranes for pH- and Temperature-Cooperative Smart Ion Transport Modulation. Adv. Mater. 2016, 28, 9613–9619. [Google Scholar] [CrossRef]
- Schacher, F.; Ulbricht, M.; Müller, A.H.E. Self-Supporting, Double Stimuli-Responsive Porous Membranes from Polystyrene-block-poly(N,N-dimethylaminoethyl methacrylate) Diblock Copolymers. Adv. Funct. Mater. 2009, 19, 1040–1045. [Google Scholar] [CrossRef]
- Schacher, F.; Rudolph, T.; Wieberger, F.; Ulbricht, M.; Müller, A.H.E. Double Stimuli-Responsive Ultrafiltration Membranes from Polystyrene-block-poly(N,N-dimethylaminoethyl methacrylate) Diblock Copolymers. ACS Appl. Mater. Interfaces 2009, 1, 1492–1503. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, N.; Fu, C.; Li, K.; Tao, L.; Feng, L.; Wei, Y. Thermo and pH Dual-Responsive Materials for Controllable Oil/Water Separation. ACS Appl. Mater. Interfaces 2014, 6, 2026–2030. [Google Scholar] [CrossRef]
- Cheng, Y.; Huang, C.; Yang, D.; Ren, K.; Wei, J. Bilayer hydrogel mixed composites that respond to multiple stimuli for environmental sensing and underwater actuation. J. Mater. Chem. B 2018, 8170–8179. [Google Scholar] [CrossRef]
- Lee, J.W.; Lee, S.; Jang, S.; Han, K.Y.; Kim, Y.; Hyun, J.; Kim, S.K.; Lee, Y. Preparation of non-aggregated fluorescent nanodiamonds (FNDs) by non-covalent coating with a block copolymer and proteins for enhancement of intracellular uptake. Mol. Biosyst. 2013, 9, 1004–1011. [Google Scholar] [CrossRef] [PubMed]
- Tiainen, T.; Myllymäki, T.T.T.; Hatanpää, T.; Tenhu, H.; Hietala, S. Polyelectrolyte stabilized nanodiamond dispersions. Diam. Relat. Mater. 2019, 95, 185–194. [Google Scholar] [CrossRef]
- Brandrup, J.; Immergut, E.H.; Grulke, E.A. Polymer Handbook, 4th ed.; Wiley: New York, NY, USA, 2008. [Google Scholar]
- Van de Wetering, P.; Zuidam, N.J.; van Steenbergen, M.J.; van der Houwen, O.A.G.J.; Underberg, W.J.M.; Hennink, W.E. A Mechanistic Study of the Hydrolytic Stability of Poly(2-(dimethylamino)ethyl methacrylate). Macromolecules 1998, 31, 8063–8068. [Google Scholar] [CrossRef]
- Lide, D.R. CRC Handbook of Chemistry and Physics, 84th ed.; CRC Press: Boca Raton, FL, USA, 2003. [Google Scholar]
- Niskanen, J.; Karesoja, M.; Rossi, T.; Tenhu, H. Temperature and pH responsive hybrid nanoclay grafted with PDMAEMA. Polym. Chem. 2011, 2, 2027–2036. [Google Scholar] [CrossRef]

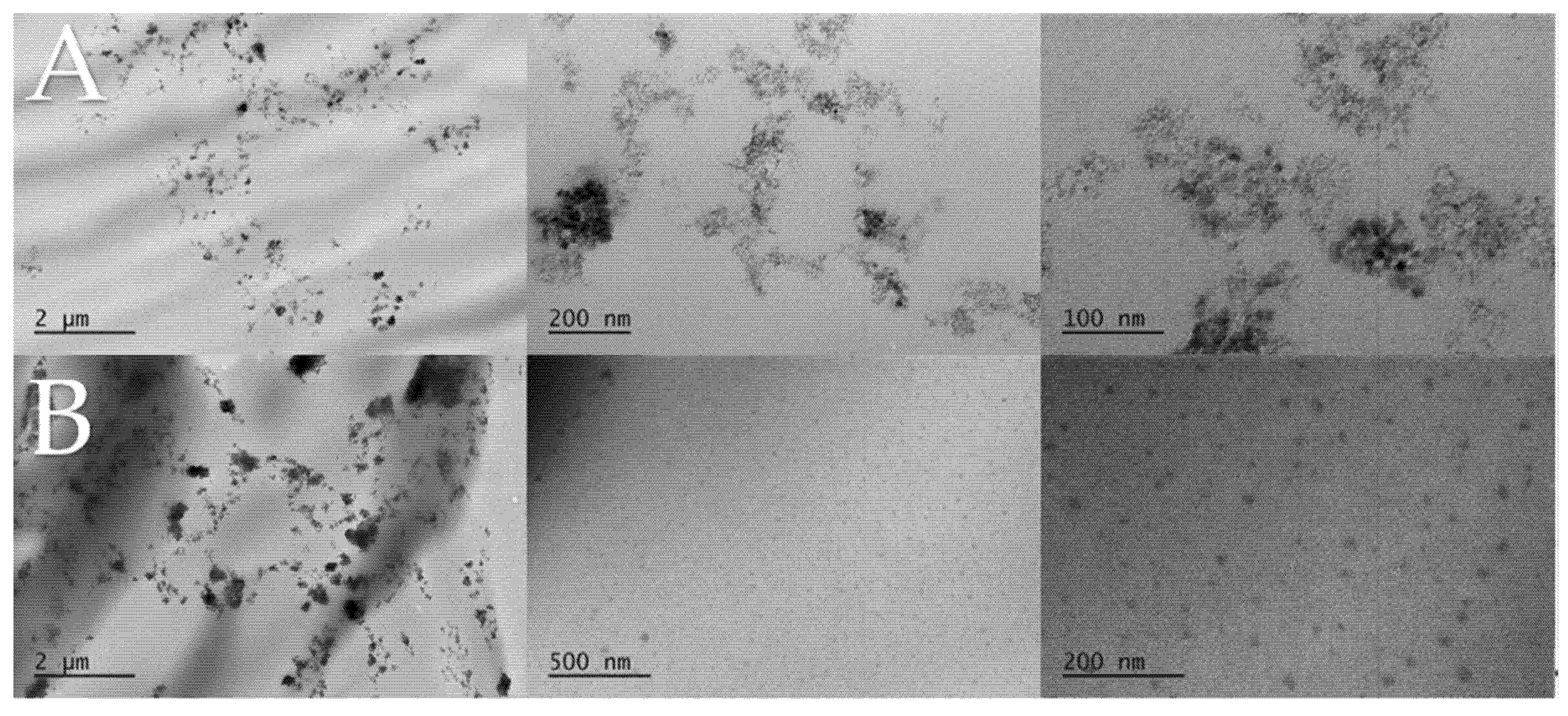


| Film Label | BA wt % | DMAEMA wt % | ND wt % | PDMAEMA-b-PEO wt % |
|---|---|---|---|---|
| F1 | 20 | 80 | 0 | 0 |
| F1/0.1ND | 20 | 80 | 0.1 | 0 |
| F1/0.5ND | 20 | 80 | 0.5 | 0 |
| F1/1ND | 20 | 80 | 1 | 0 |
| F1/2ND | 20 | 80 | 2 | 0 |
| F1/C | 20 | 77.5 | 0 | 2.5 |
| F1/C/0.1ND | 20 | 77.5 | 0.1 | 2.5 |
| F1/C/0.5ND | 20 | 77.5 | 0.5 | 2.5 |
| F1/C/1ND | 20 | 77.5 | 1 | 2.5 |
| F1/C/2ND | 20 | 77.5 | 2 | 2.5 |
| Film Label | Tg (°C) | Young’s Modulus (MPa) | Strain at Break (%) | Stress at Break (MPa) | Toughness 1 (MJ·m−3) |
|---|---|---|---|---|---|
| F1 | 5.0 | 3.9 | 167.6 | 2.2 | 1.9 |
| F1/0.1ND | 6.1 | 3.2 | 141.1 | 1.6 | 1.2 |
| F1/0.5ND | 9.1 | 3.2 | 157.8 | 1.6 | 1.4 |
| F1/1ND | 5.9 | 3.8 | 151.5 | 2.2 | 1.9 |
| F1/2ND | 5.0 | 4.3 | 209.5 | 1.3 | 2.1 |
| F1/C | 7.0 | 2.6 | 298.5 | 1.2 | 2.2 |
| F1/C/0.1ND | 7.2 | 3.3 | 118.1 | 1.2 | 0.9 |
| F1/C/0.5ND | 6.6 | 3.3 | 146.0 | 1.4 | 1.2 |
| F1/C/1ND | 7.3 | 4.1 | 170.2 | 1.9 | 1.9 |
| F1/C/2ND | 7.3 | 6.7 | 154.0 | 2.5 | 2.3 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tiainen, T.; Lobanova, M.; Karjalainen, E.; Tenhu, H.; Hietala, S. Stimuli-Responsive Nanodiamond–Polyelectrolyte Composite Films. Polymers 2020, 12, 507. https://doi.org/10.3390/polym12030507
Tiainen T, Lobanova M, Karjalainen E, Tenhu H, Hietala S. Stimuli-Responsive Nanodiamond–Polyelectrolyte Composite Films. Polymers. 2020; 12(3):507. https://doi.org/10.3390/polym12030507
Chicago/Turabian StyleTiainen, Tony, Marina Lobanova, Erno Karjalainen, Heikki Tenhu, and Sami Hietala. 2020. "Stimuli-Responsive Nanodiamond–Polyelectrolyte Composite Films" Polymers 12, no. 3: 507. https://doi.org/10.3390/polym12030507






