Influence of Chromophoric Electron-Donating Groups on Photoinduced Solid-to-Liquid Transitions of Azopolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Characterization
2.3. Synthesis of Monomer
2.4. Synthesis of Azopolymers
3. Results and Discussion
3.1. Nuclear Magnetic Spectrum of Monomers
3.2. Characterization of Azopolymers
3.3. Photoisomerization Properties of Azopolymers
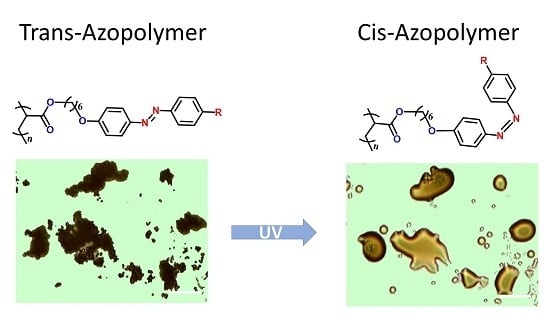
3.4. Photoinduced Solid-to-Liquid Properties of Azopolymers
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hartley, G.S. The cis-form of azobenzene. Nature 1937, 140, 281. [Google Scholar] [CrossRef]
- Delaire, J.A.; Nakatani, K. Linear and nonlinear optical properties of photochromic molecules and materials. Chem. Rev. 2000, 100, 1817–1846. [Google Scholar] [CrossRef]
- Bandara, H.M.D.; Burdette, S.C. Photoisomerization in different classes of azobenzene. Chem. Soc. Rev. 2012, 41, 1809–1825. [Google Scholar] [CrossRef] [PubMed]
- Rau, H.; Lueddecke, E. On the rotation-inversion controversy on photoisomerization of azobenzenes. experimental proof of inversion. J. Am. Chem. Soc. 1982, 104, 1616–1620. [Google Scholar] [CrossRef]
- Dürr, H.; Bouas-Laurent, H. Photochromism: Molecules and Systems; Elsevier: Amsterdam, The Netherland, 2003; pp. 165–192. [Google Scholar]
- Saltiel, J.; Cires, L.; Turek, A. CRC Handbook of Organic Photochemistry and Photobiology, 2nd ed.; CRC: Boca Raton, FL, USA, 2004. [Google Scholar]
- Ikeda, T.; Tsutsumi, O. Optical switching and image storing by means of azobenzene liquid-crystal films. Science 1996, 425, 1873–1875. [Google Scholar]
- Colquhoun, H.M. Self-repairing polymers: Materials that heal themselves. Nat. Chem. 2012, 4, 435–436. [Google Scholar] [CrossRef]
- Yu, Y.L.; Nakano, M.; Ikeda, T. Directed bending of a polymer film by light-miniaturizing a simple photomechanical system could expand its range of applications. Nature 2003, 425, 145. [Google Scholar] [CrossRef]
- Zhang, L.; Liang, H.; Jacob, J.; Naumov, P. Photogated humidity-driven motility. Nat. Commun. 2015, 6, 7429. [Google Scholar] [CrossRef]
- Rochon, P.; Batalla, E.; Natansohn, A. Optically induced surface gratings on azoaromatic polymer films. Appl. Phys. Lett. 1995, 66, 136–138. [Google Scholar] [CrossRef]
- Kim, D.Y.; Tripathy, S.K.; Li, L.; Kumar, J. Laser-induced holographic surface relief gratings on nonlinear optical polymer films. Appl. Phys. Lett. 1995, 66, 1166–1168. [Google Scholar] [CrossRef] [Green Version]
- Koskela, J.E.; Vapaavuori, J.; Hautala, J.; Priimagi, A.; Faul, C.F.J.; Kaivola, M.; Ras, R.H.A. Surface-relief gratings and stable birefringence inscribed using light of broad spectral range in supramolecular polymer-bisazobenzene complexes. J. Phys. Chem. C 2012, 116, 2363–2370. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, J. Photoresponsive molecular switches for biotechnology. J. Photoch. Photobio. C 2012, 13, 299–309. [Google Scholar] [CrossRef]
- Haberhauer, G.; Kallweit, C.; Wolper, C.; Blaser, D. An azobenzene unit embedded in a cyclopeptide as a type-specific and spatially directed switch. Angew. Chem. Int. Ed. Engl. 2013, 52, 7879–7882. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wu, S. Red-light-responsive supramolecular valves for photocontrolled drug release from mesoporous nanoparticles. Langmuir 2016, 32, 632–636. [Google Scholar] [CrossRef]
- Wang, D.; Wagner, M.; Butt, H.J.; Wu, S. Supramolecular hydrogels constructed by red-light-responsive host-guest interactions for photo-controlled protein release in deep tissue. Soft Matter 2015, 11, 7656–7662. [Google Scholar] [CrossRef] [Green Version]
- Gelebart, A.H.; Liu, D.; Mulder, D.J.; Leunissen, K.H.J.; Van Gerven, J.; Schenning, A.P.H.J.; Broer, D.J. Photoresponsive sponge-like coating for on-demand liquid release. Adv. Funct. Mater. 2018, 28, 1705942. [Google Scholar] [CrossRef] [Green Version]
- Priimagi, A.; Shevchenko, A. Azopolymer-based micro-and nanopatterning for photonic applications. J. Polym. Sci. Part B J. Polym. Sci. Pol. Phys. 2014, 52, 163–182. [Google Scholar] [CrossRef]
- Ikeda, T.; Mamiya, J.I.; Yu, Y. Photomechanics of liquid-crystalline elastomers and other polymers. Angew. Chem. Int. Ed. 2007, 46, 506–528. [Google Scholar] [CrossRef]
- Palagi, S.; Mark, A.G.; Reigh, S.Y.; Melde, K.; Qiu, T.; Zeng, H.; Parmeggiani, C.; Martella, D.; Sanchez-Castillo, A.; Kapernaum, N. Structured light enables biomimetic swimming and versatile locomotion of photoresponsive soft microrobots. Nat. Mater. 2016, 15, 647–653. [Google Scholar] [CrossRef] [Green Version]
- Xu, B.; Zhu, C.; Qin, L.; Wei, J.; Yu, Y. Light-directed liquid manipulation in flexible bilayer microtubes. Small 2019, 15, 1901847. [Google Scholar] [CrossRef]
- Xu, W.; Sun, S.; Wu, S. Photoinduced reversible solid-to-liquid transitions for photoswitchable materials. Angew. Chem. Int. Ed. 2019, 58, 9712–9740. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Xue, C.; Weis, P.; Suzuki, Y.; Huang, S.; Koynov, K.; Auernhammer, G.K.; Berger, R.; Butt, H.J.; Wu, S. Photoswitching of glass transition temperaturesof azobenzene-containing polymers induces reversible solid-to-liquid transitions. Nat. Chem. 2017, 9, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Ito, S.; Yamashita, A.; Akiyama, H.; Kihara, H.; Yoshida, M. Azobenzene-based (meth) acrylates: Controlled radical polymerization, photoresponsive solid−liquid phase transition behavior, and application to reworkable adhesives. Macromolecules 2018, 51, 3243–3253. [Google Scholar] [CrossRef]
- Ichimura, K.; Oh, S.K.; Nakagawa, M. Light-driven motion of liquids on a photoresponsive surface. Science 2000, 288, 1624–1626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Kelch, S.; Lendlein, A. Polymers move in response to light. Adv. Mater. 2006, 18, 1471–1475. [Google Scholar] [CrossRef]
- Natansohn, A.; Rochon, P. Photoinduced motions in azo-containing polymers. Chem. Rev. 2002, 102, 4139–4175. [Google Scholar] [CrossRef]
- Bai, Y.; Hu, J.; Shen, W.; Hu, X. Recent advances and potential applications of the photo-induced macroscopic response of self-assembled azopolymer in aqueous media. Polym. Int. 2014, 63, 1539–1544. [Google Scholar] [CrossRef]
- Weis, P.; Hess, A.; Kircher, G.; Huang, S.; Auernhammer, G.K.; Koynov, K.; Butt, H.J.; Wu, S. Effects of spacers on photoinduced reversible solid-to-liquidtransitions of azobenzene-containing polymers. Chem. Eur. J. 2019, 25, 10946–10953. [Google Scholar] [CrossRef]




| Azopolymers a) | P-1-Azo | P-2-Azo | P-3-Azo | P-4-Azo | P-5-Azo |
|---|---|---|---|---|---|
| Mn (g/mol) | 4.2 × 103 | 6.1 × 103 | 4.4 × 103 | 5.8 × 103 | 6.2 × 103 |
| Mw (g/mol) | 5.4 × 103 | 8.5 × 103 | 5.7 × 103 | 7.2 × 103 | 7.7 × 103 |
| Mz (g/mol) | 7.2 × 103 | 11 × 103 | 7.0 × 103 | 8.8 × 103 | 9.2 × 103 |
| PDI | 1.29 | 1.39 | 1.31 | 1.24 | 1.24 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, J.; Niu, B.; Guo, S.; Zhao, X.; Li, X.; Peng, J.; Deng, W.; Wu, S.; Liu, Y. Influence of Chromophoric Electron-Donating Groups on Photoinduced Solid-to-Liquid Transitions of Azopolymers. Polymers 2020, 12, 901. https://doi.org/10.3390/polym12040901
Xu J, Niu B, Guo S, Zhao X, Li X, Peng J, Deng W, Wu S, Liu Y. Influence of Chromophoric Electron-Donating Groups on Photoinduced Solid-to-Liquid Transitions of Azopolymers. Polymers. 2020; 12(4):901. https://doi.org/10.3390/polym12040901
Chicago/Turabian StyleXu, Jian, Bin Niu, Song Guo, Xiaolei Zhao, Xiaoli Li, Jinwen Peng, Weixing Deng, Si Wu, and Yuanli Liu. 2020. "Influence of Chromophoric Electron-Donating Groups on Photoinduced Solid-to-Liquid Transitions of Azopolymers" Polymers 12, no. 4: 901. https://doi.org/10.3390/polym12040901