Nile Red-Poly(Methyl Methacrylate)/Silica Nanocomposite Particles Increase the Sensitivity of Cervical Cancer Cells to Tamoxifen
Abstract
:1. Introduction
2. Methods
2.1. Poly (Methyl Methacrylate) (PMMA)
2.2. Preparation PMMA-NR Fluorescent Nanoparticle
2.3. Tamoxifen Loading on PMMA-NR/Silica (PMMA-NR-Si)
2.4. Tamoxifen Release Study
2.5. Material Characterization
2.6. Cell Culture and Treatment
2.7. Cell Viability Assay
2.8. Hoechst Staining
2.9. Statistical Analysis
3. Results and Discussion
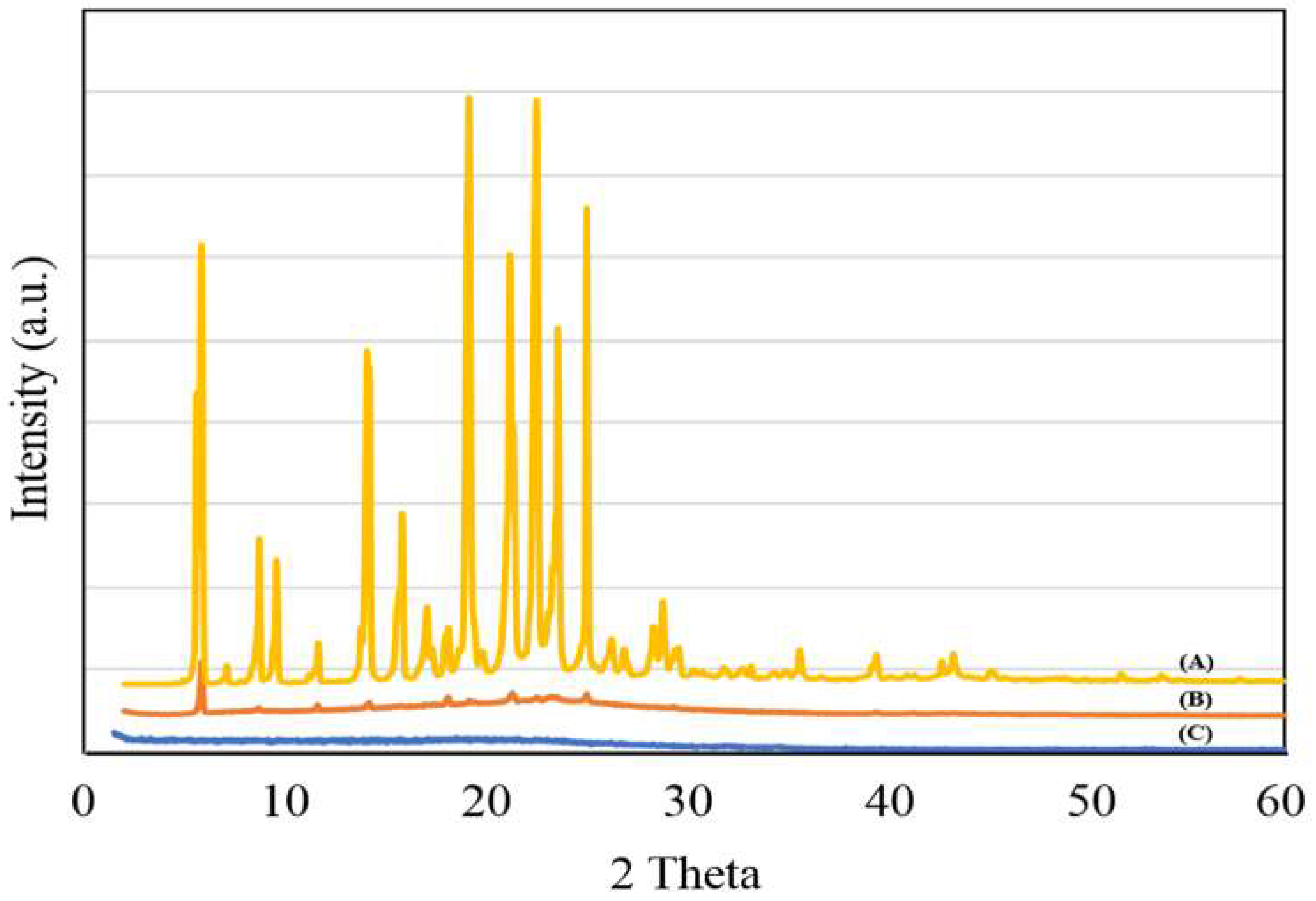
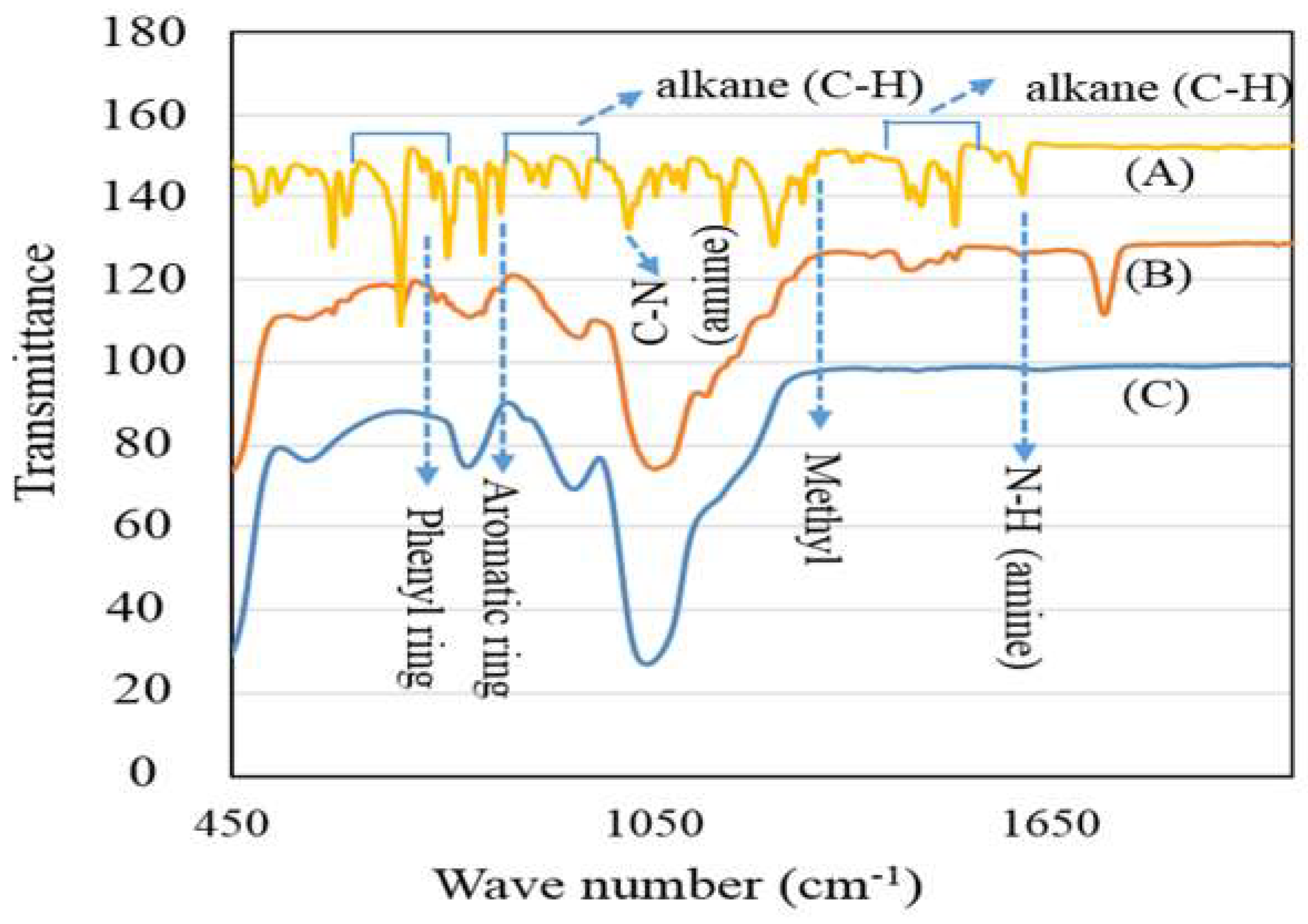
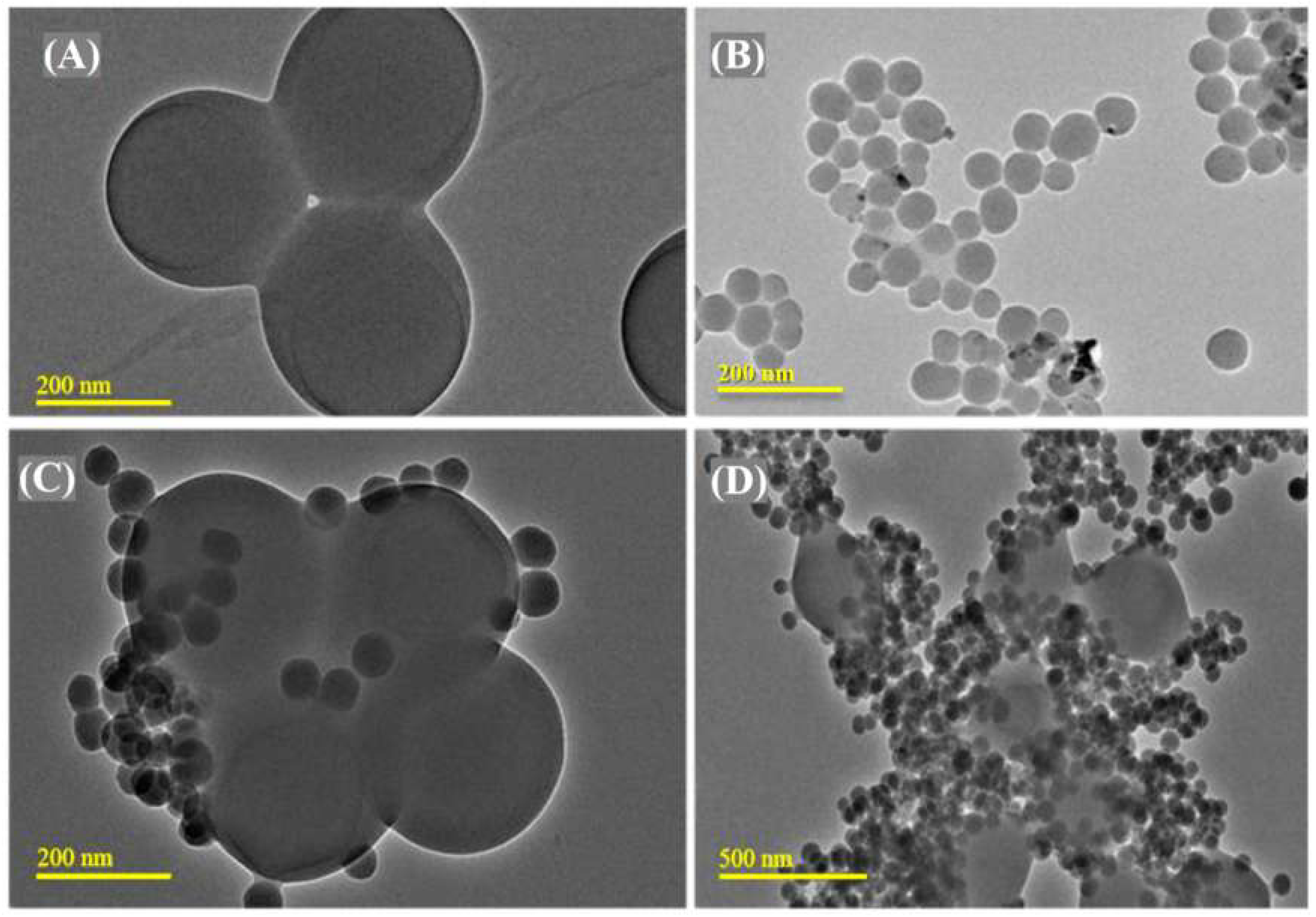
3.1. PMMA-NR Properties
3.2. In Vitro Tamoxifen Release Study
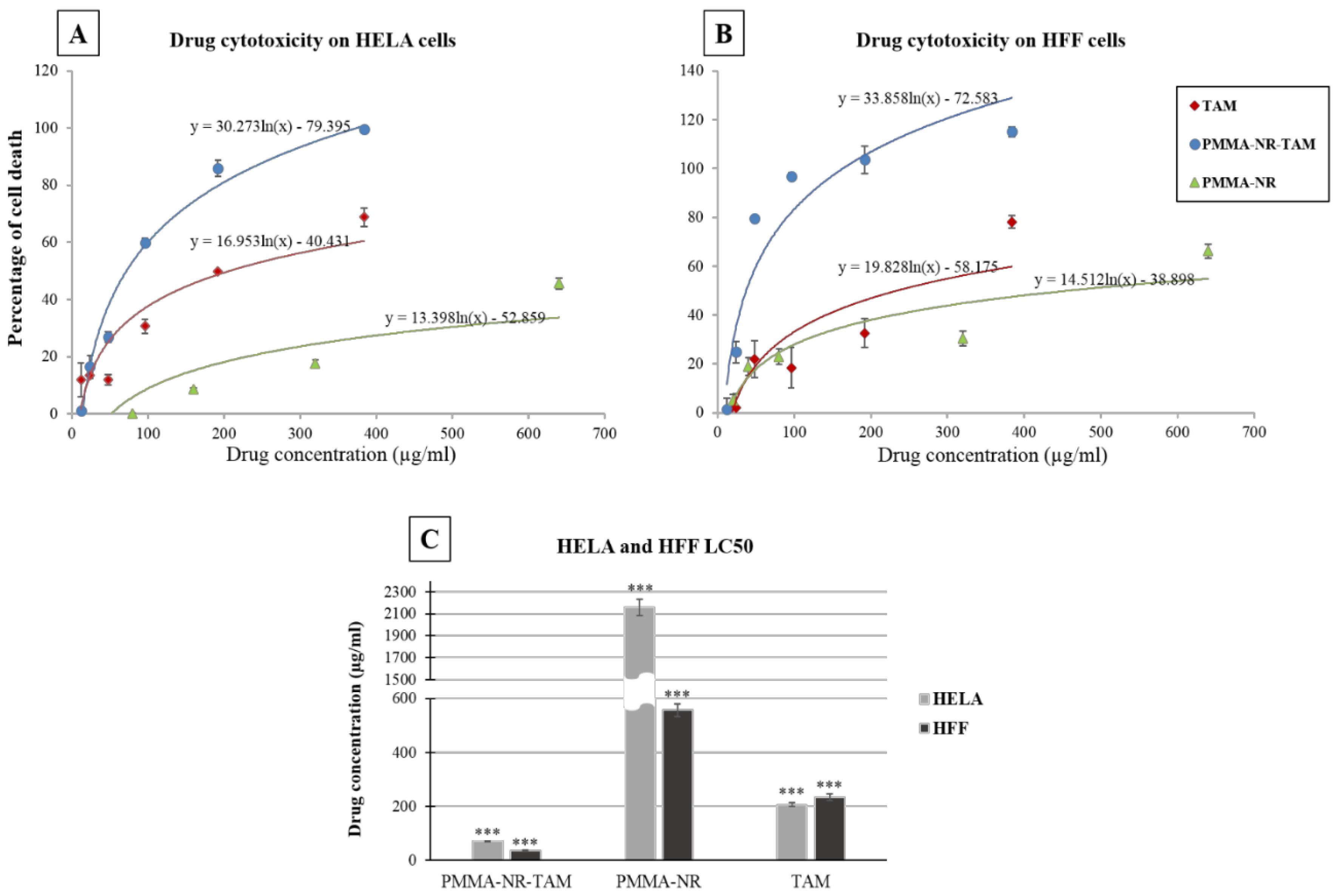
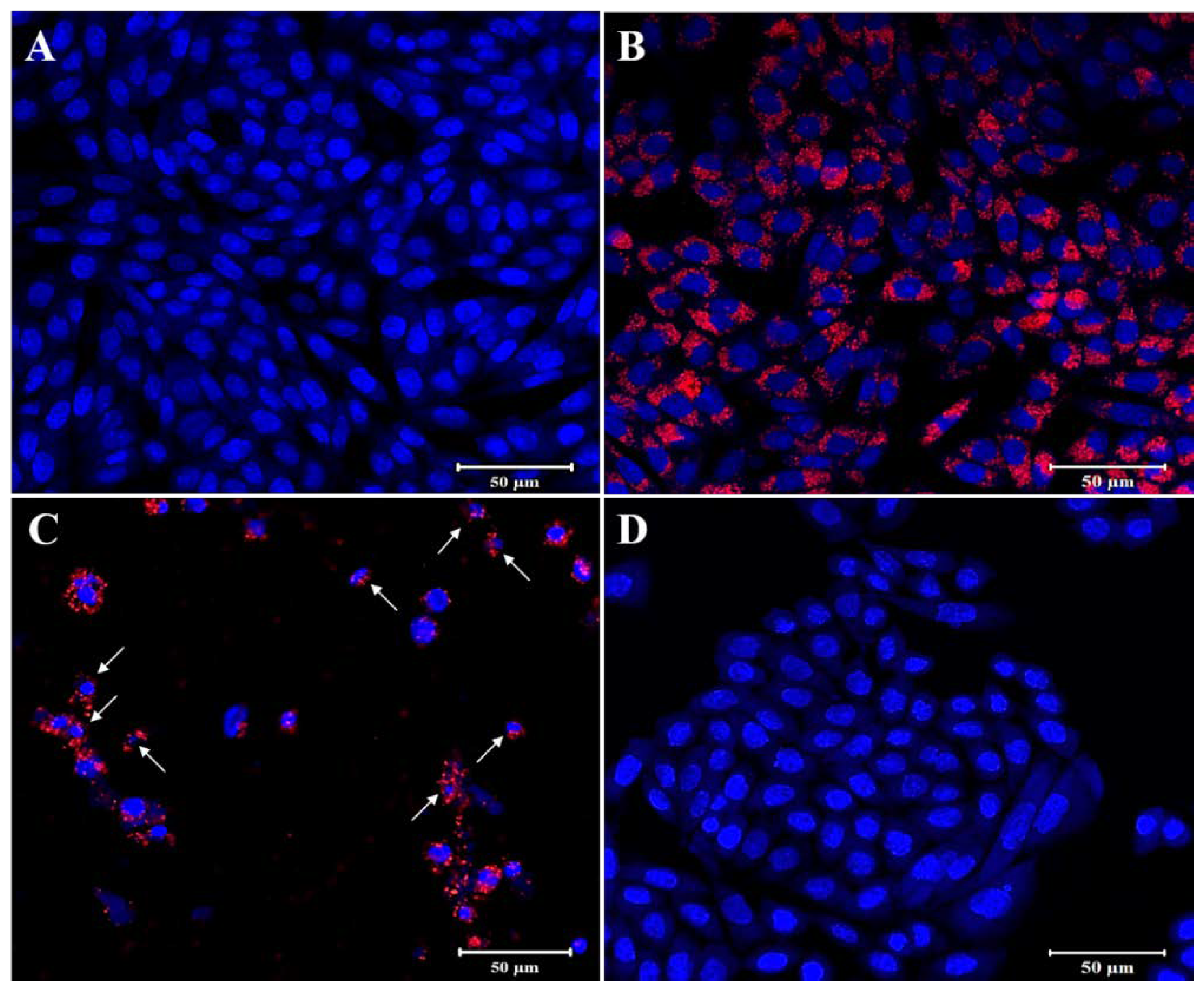
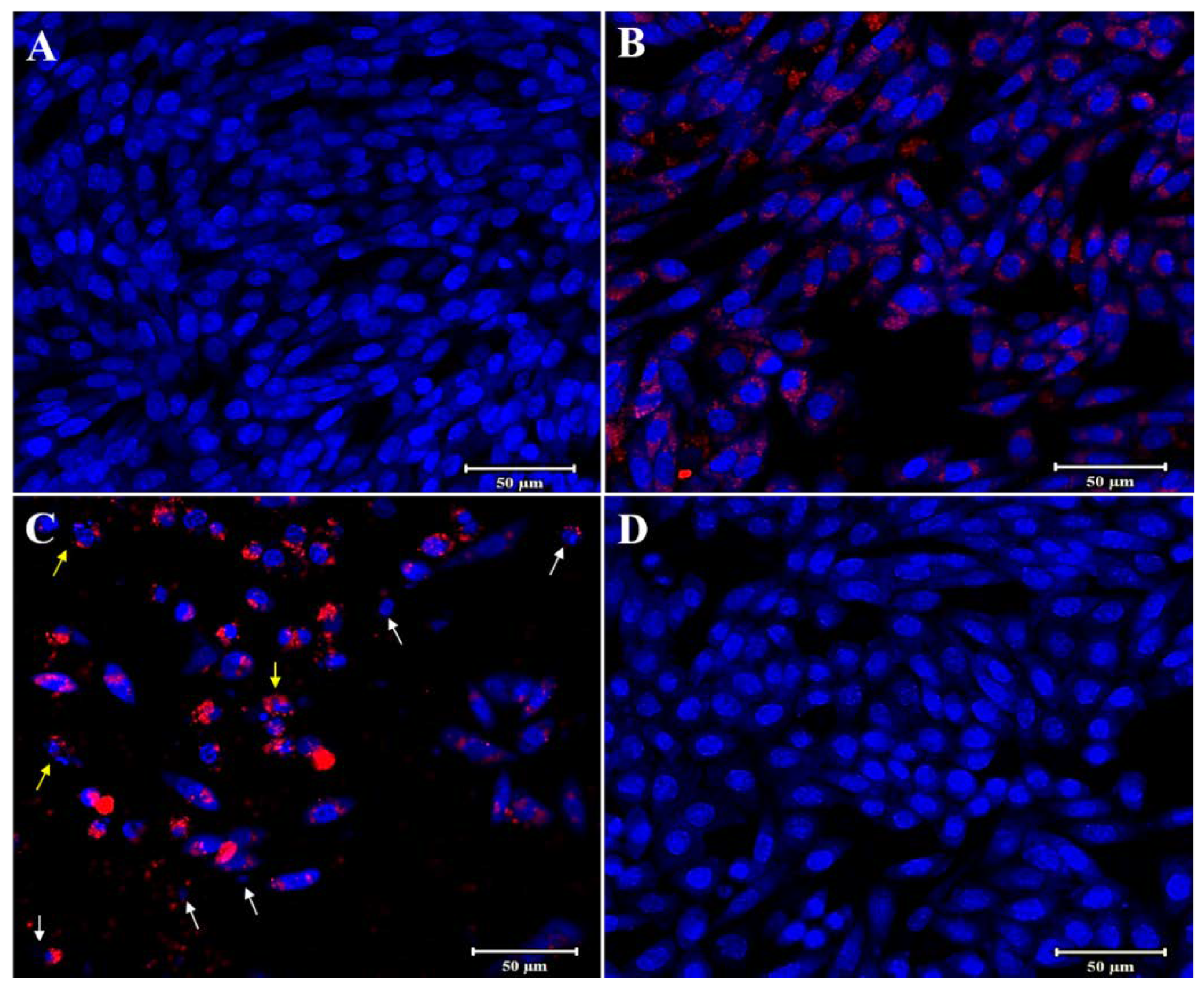
3.3. In Vitro Anti-Cancer Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goetz, M.P.; Knox, S.K.; Suman, V.J.; Rae, J.M.; Safgren, S.L.; Ames, M.M.; Visscher, D.W.; Reynolds, C.; Couch, F.J.; Lingle, W.L.; et al. The impact of cytochrome P450 2D6 metabolism in women receiving adjuvant tamoxifen. Breast Cancer Res. Treat. 2006, 101, 113–121. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, S.R.; Siddhanti, S.; Ciaccia, A.V.; Plouffe, L. A pharmacological review of selective oestrogen receptor modulators. Hum. Reprod. Update 2000, 6, 212–224. [Google Scholar] [CrossRef] [Green Version]
- Sato, M.; Glasebrook, A.L.; Bryant, H.U. Raloxifene: A selective estrogen receptor modulator. J. Bone Miner. Metab. 1994, 12, S9–S20. [Google Scholar] [CrossRef]
- Mürdter, T.E.; Kerb, R.; Turpeinen, M.; Schroth, W.; Ganchev, B.; Böhmer, G.M.; Igel, S.; Schaeffeler, E.; Zanger, U.; Brauch, H.; et al. Genetic polymorphism of cytochrome P450 2D6 determines oestrogen receptor activity of the major infertility drug clomiphene via its active metabolites. Hum. Mol. Genet. 2011, 21, 1145–1154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnson, A.C.; Jürgens, M.D.; Williams, R.; Kümmerer, K.; Kortenkamp, A.; Sumpter, J.P. Do cytotoxic chemotherapy drugs discharged into rivers pose a risk to the environment and human health? An overview and UK case study. J. Hydrol. 2008, 348, 167–175. [Google Scholar] [CrossRef]
- Brauch, H.; Mürdter, T.E.; Eichelbaum, M.; Schwab, M. Pharmacogenomics of Tamoxifen Therapy. Clin. Chem. 2009, 55, 1770–1782. [Google Scholar] [CrossRef] [Green Version]
- Kisanga, E.R.; Mellgren, G.; Lien, E.A. Excretion of hydroxylated metabolites of tamoxifen in human bile and urine. Anticancer Res. 2005, 25, 4487–4492. [Google Scholar]
- Manna, S.; Holz, M.K. Tamoxifen Action in ER-Negative Breast Cancer. Signal Transduct. Insights 2016, 5, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Majd, M.H.; Asgari, D.; Barar, J.; Valizadeh, H.; Kafil, V.; Abadpour, A.; Moumivand, E.; Mojarrad, J.S.; Rashidic, M.-R.; Coukos, G.; et al. Tamoxifen loaded folic acid armed PEGylated magnetic nanoparticles for targeted imaging and therapy of cancer. Colloids Surf. B Biointerfaces 2013, 106, 117–125. [Google Scholar] [CrossRef]
- Boyd, B.J.; Bergström, C.A.; Vinarov, Z.; Kuentz, M.; Brouwers, J.; Augustijns, P.; Brandl, M.; Bernkop-Schnürch, A.; Shrestha, N.; Preat, V.; et al. Successful oral delivery of poorly water-soluble drugs both depends on the intraluminal behavior of drugs and of appropriate advanced drug delivery systems. Eur. J. Pharm. Sci. 2019, 137, 104967. [Google Scholar] [CrossRef]
- Sahu, A.; Solanki, P.; Mitra, S. Curcuminoid-loaded poly(methyl methacrylate) nanoparticles for cancer therapy. Int. J. Nanomed. 2018, 13, 101–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, L.; Li, Z.; Li, X. Preparation of PMMA nanolatex via microemulsion polymerization initiated by UV direct radiation at low temperature. Ferroelectrics 2019, 549, 111–118. [Google Scholar] [CrossRef]
- Yuan, L.; Wang, Y.; Pan, M.; Rempel, G.L.; Pan, Q. Synthesis of poly(methyl methacrylate) nanoparticles via differential microemulsion polymerization. Eur. Polym. J. 2013, 49, 41–48. [Google Scholar] [CrossRef]
- Feuser, P.E.; Gaspar, P.C.; Jacques, A.V.; Tedesco, A.C.; Silva, M.C.D.S.; Ricci-Júnior, E.; Sayer, C.; De Araújo, P.H.H. Synthesis of ZnPc loaded poly(methyl methacrylate) nanoparticles via miniemulsion polymerization for photodynamic therapy in leukemic cells. Mater. Sci. Eng. C 2016, 60, 458–466. [Google Scholar] [CrossRef] [PubMed]
- Batista, F.A.; Fontele, S.B.C.; Santos, L.K.B.; Filgueiras, L.A.; Nascimento, S.Q.; Sousa, J.M.D.C.E.; Gonçalves, J.C.R.; Mendes, A.N. Synthesis, characterization of α-terpineol-loaded PMMA nanoparticles as proposed of therapy for melanoma. Mater. Today Commun. 2020, 22, 100762. [Google Scholar] [CrossRef]
- Bang, J.; Rhee, S.E.; Kim, K.; Lee, B.H.; Choe, S. Effect of hydrogen peroxide on the UCST behavior of PMMA in the modified dispersion polymerization. Macromol. Res. 2012, 21, 78–84. [Google Scholar] [CrossRef]
- Peng, B.; Van Der Wee, E.; Velikov, K.P.; Van Blaaderen, A. Synthesis of Monodisperse, Highly Cross-Linked, Fluorescent PMMA Particles by Dispersion Polymerization. Langmuir 2012, 28, 6776–6785. [Google Scholar] [CrossRef]
- Badr, I.H.A.; Lahmar, H.; Kaewsaneha, C.; Saïdi-Besbes, S.; Elaïssari, A. Preparation and Characterization of Poly(methyl methacrylate) Particles by Combined Dispersion and Emulsion Polymerization. Macromol. Res. 2018, 26, 819–824. [Google Scholar] [CrossRef]
- Gad, M.M.; Fouda, S.M.; Al-Harbi, F.A.; Näpänkangas, R.; Raustia, A. PMMA denture base material enhancement: A review of fiber, filler, and nanofiller addition. Int. J. Nanomed. 2017, 12, 3801–3812. [Google Scholar] [CrossRef] [Green Version]
- De Mori, A.; Di Gregorio, E.; Kao, A.P.; Tozzi, G.; Barbu, E.; Sanghani-Kerai, A.; Draheim, R.R.; Roldo, M. Antibacterial PMMA Composite Cements with Tunable Thermal and Mechanical Properties. ACS Omega 2019, 4, 19664–19675. [Google Scholar] [CrossRef] [Green Version]
- Shanmugasundar, S.; Kannan, N.; Sundaravadivel, E.; Zsolt, S.; Mukunthan, K.S.; Manokaran, J.; Narendranath, J.; Kamalakannan, V.P.; Kavitha, P.; Prabhu, V.; et al. Study on the inflammatory response of PMMA/polystyrene/silica nanocomposite membranes for drug delivery and dental applications. PLoS ONE 2019, 14, e0209948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liang, T.; Yan, C.; Zhou, S.; Zhang, Y.; Yang, B. Carbon black reinforced polymethyl methacrylate (PMMA)-based composite particles: Preparation, characterization, and application. J. Geophys. Eng. 2017, 14, 1225–1232. [Google Scholar] [CrossRef]
- Greenspan, P.; Mayer, E.P.; Fowler, S.D. Nile red: A selective fluorescent stain for intracellular lipid droplets. J. Cell Biol. 1985, 100, 965–973. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abasi, C.Y.; Wankasi, D.; Dikio, C. Adsorption Study of Lead(II) Ions on Poly(methyl methacrylate) Waste Material. Asian J. Chem. 2018, 30, 859–867. [Google Scholar] [CrossRef]
- Liu, N.; Couto, R.; Seifried, B.; Moquin, P.; Delgado, L.; Temelli, F. Characterization of oat beta-glucan and coenzyme Q10-loaded beta-glucan powders generated by the pressurized gas-expanded liquid (PGX) technology. Food Res. Int. 2018, 106, 354–362. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, B.; Maji, R.; Dey, N.S.; Satapathy, B.S.; Mondal, S. Preparation and characterization of Tamoxifen citrate loaded nanoparticles for breast cancer therapy. Int. J. Nanomed. 2014, 9, 3107–3118. [Google Scholar] [CrossRef] [Green Version]
- Altmeyer, C.; Karam, T.K.; Khalil, N.M.; Mainardes, R.M. Tamoxifen-loaded poly(L-lactide) nanoparticles: Development, characterization and in vitro evaluation of cytotoxicity. Mater. Sci. Eng. C 2016, 60, 135–142. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, R.; Usha, K.; Malik, R.; Sharma, G.; Chitkara, D.; Katare, O.; Raza, K. Biocompatible Phospholipid-Based Mixed Micelles for Tamoxifen Delivery: Promising Evidences from in vitro Anticancer Activity and Dermatokinetic Studies. AAPS PharmSciTech 2016, 18, 2037–2044. [Google Scholar] [CrossRef]
- How, C.W.; Rasedee, A.; Manickam, S.; Rosli, R. Tamoxifen-loaded nanostructured lipid carrier as a drug delivery system: Characterization, stability assessment and cytotoxicity. Colloids Surf. B Biointerfaces 2013, 112, 393–399. [Google Scholar] [CrossRef] [Green Version]




| Time (h) | Drug Release (mg) | Cumulative Drug Release (%) |
|---|---|---|
| 1 | 0.004 | 0.48 |
| 6 | 0.012 | 1.27 |
| 12 | 0.014 | 1.64 |
| 24 | 0.020 | 2.03 |
| 48 | 0.034 | 3.55 |
| 72 | 0.137 | 13.98 |
| 120 | 0.204 | 21.26 |
| 240 | 0.234 | 26.01 |
| 300 | 0.263 | 29.94 |
| Drug | HFF | HELA | ||||
|---|---|---|---|---|---|---|
| LC50 (µg/mL) | SD | p Value | LC50 (µg/mL) | SD | p Value | |
| PMMA-NR-Si-TAM | 37.36 | 2.72 | 3.33 × 10−6 | 71.83 | 0.88 | 1.09 × 10−6 |
| PMMA-NR | 556.89 | 24.32 | 3.33 × 10−6 | 2158.55 | 73.24 | 1.09 × 10−6 |
| TAM | 234.08 | 11.90 | 9.72 × 10−6 | 207.31 | 7.89 | 7.4 × 10−6 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alomari, M.; Balasamy, R.J.; Almohazey, D.; Ravinayagam, V.; Al Hamad, M.; Ababneh, D.; Bahmdan, H.; Alomari, A.-H.; Mokadem, Z.; Elaissari, A. Nile Red-Poly(Methyl Methacrylate)/Silica Nanocomposite Particles Increase the Sensitivity of Cervical Cancer Cells to Tamoxifen. Polymers 2020, 12, 1516. https://doi.org/10.3390/polym12071516
Alomari M, Balasamy RJ, Almohazey D, Ravinayagam V, Al Hamad M, Ababneh D, Bahmdan H, Alomari A-H, Mokadem Z, Elaissari A. Nile Red-Poly(Methyl Methacrylate)/Silica Nanocomposite Particles Increase the Sensitivity of Cervical Cancer Cells to Tamoxifen. Polymers. 2020; 12(7):1516. https://doi.org/10.3390/polym12071516
Chicago/Turabian StyleAlomari, Munther, Rabindran Jermy Balasamy, Dana Almohazey, Vijaya Ravinayagam, Mohammad Al Hamad, Deena Ababneh, Hiba Bahmdan, Abdul-Hakeem Alomari, Zakaria Mokadem, and Abdelhamid Elaissari. 2020. "Nile Red-Poly(Methyl Methacrylate)/Silica Nanocomposite Particles Increase the Sensitivity of Cervical Cancer Cells to Tamoxifen" Polymers 12, no. 7: 1516. https://doi.org/10.3390/polym12071516






