Preparation and Characterization of PEBAX-5513/AgBF4/BMIMBF4 Membranes for Olefin/Paraffin Separation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
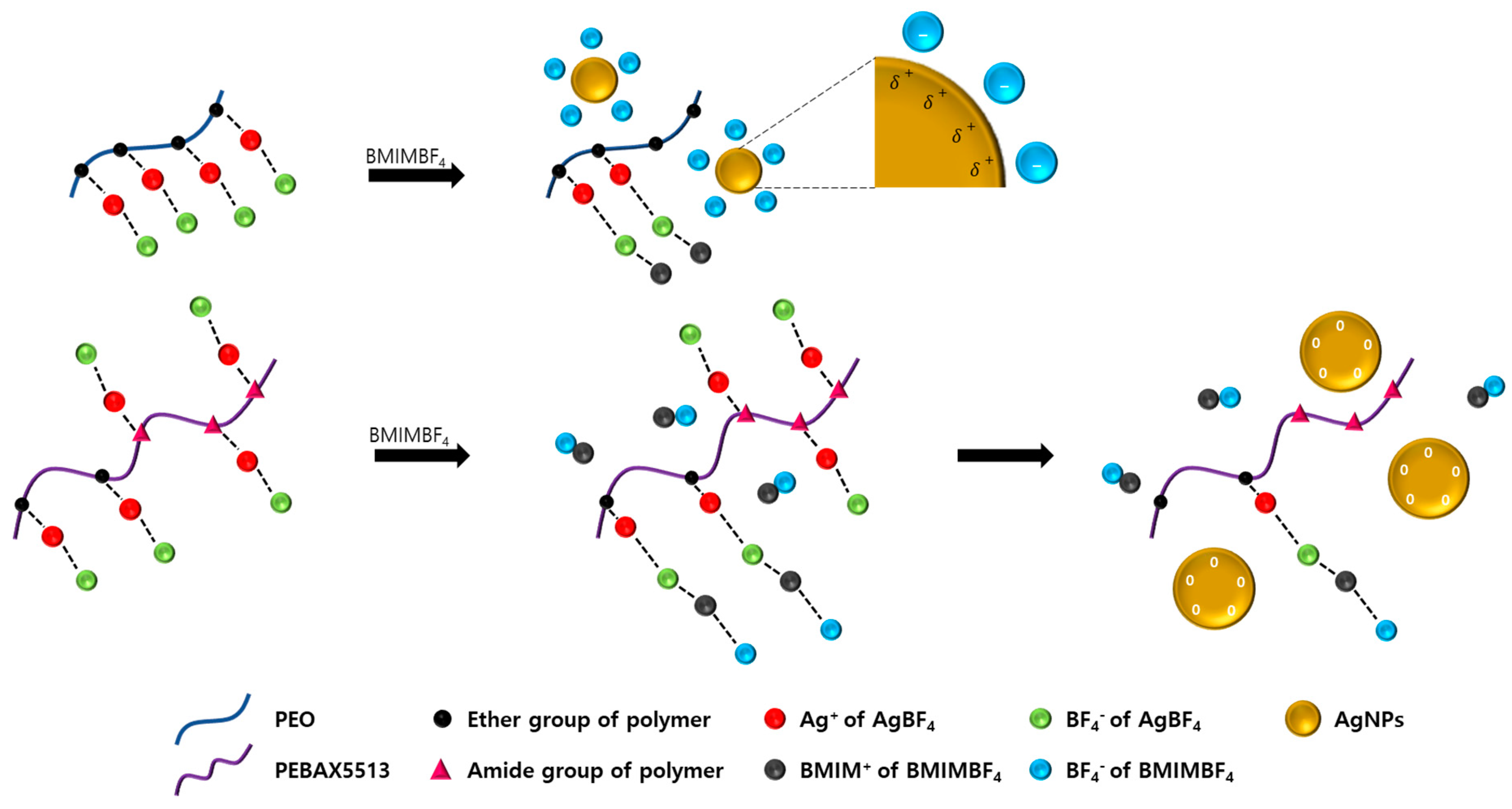
2.2. Preparation of Membrane
2.3. Gas Separation Experiments
2.4. Characterization
3. Results
3.1. Separation Performance
3.2. SEM
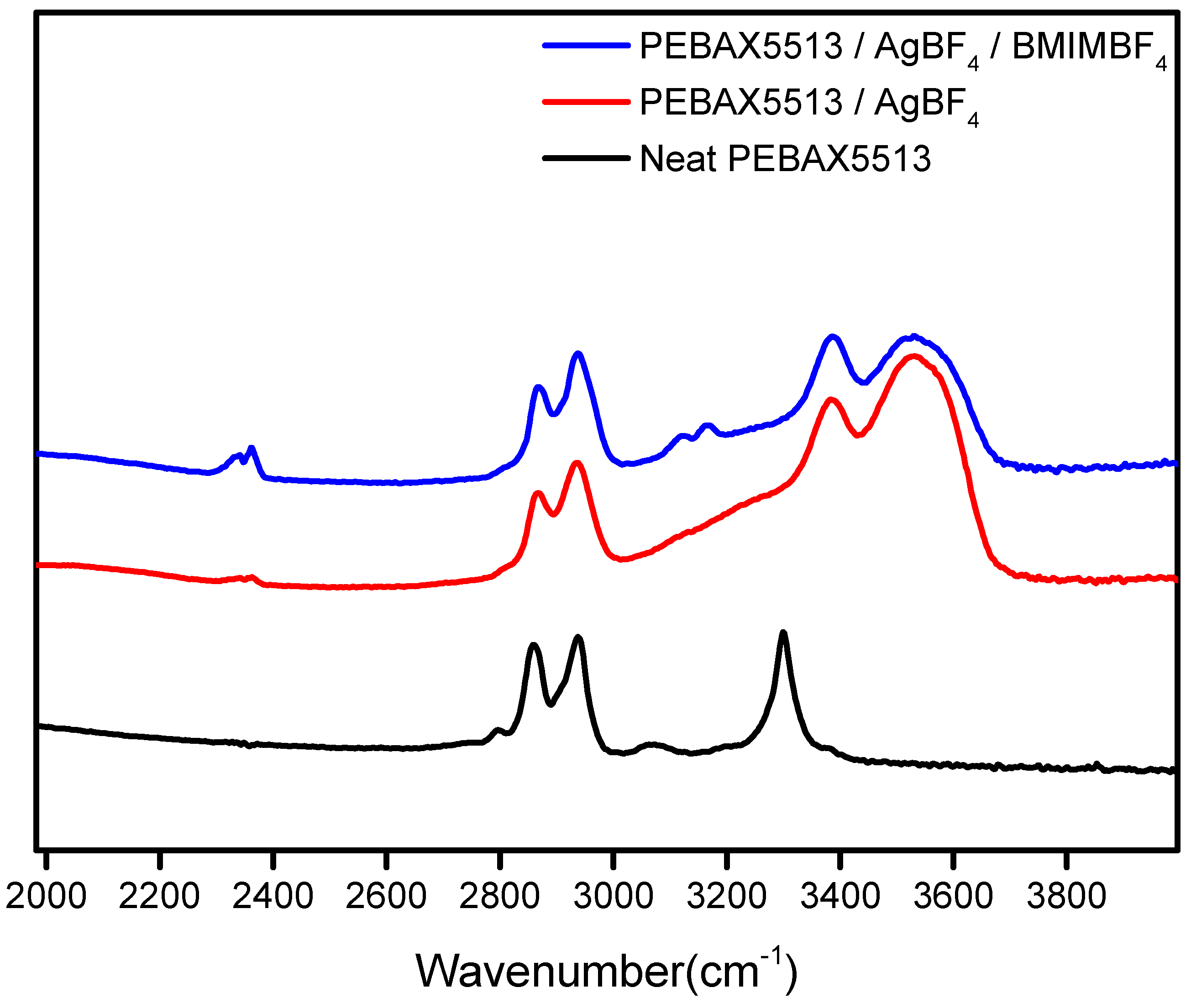
3.3. FT-IR
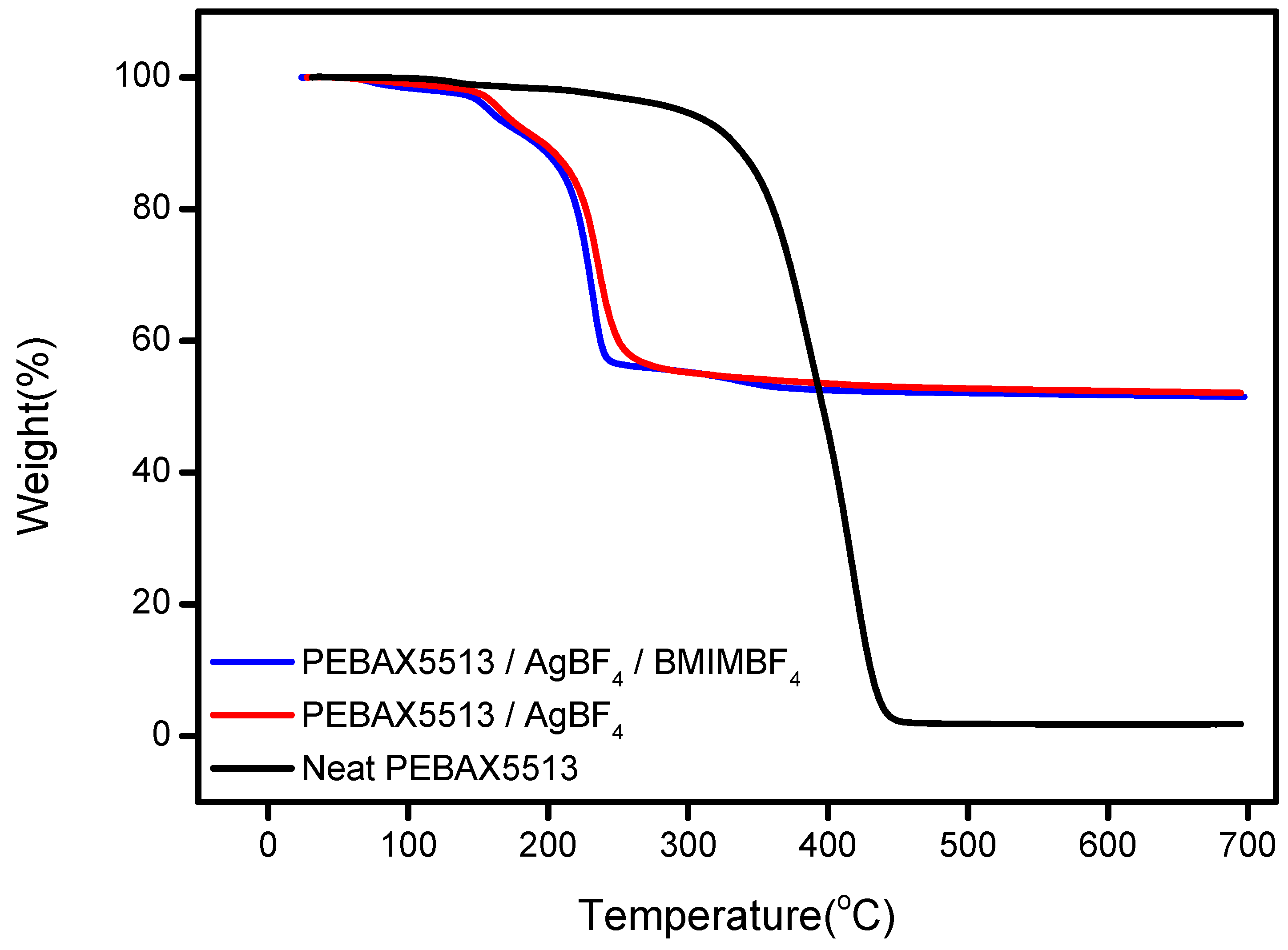
3.4. TGA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sakai, M.; Sasaki, Y.; Tomono, T.; Seshimo, M.; Matsukata, M. Olefin selective Ag-exchanged X-type zeolite membrane for propylene/propane and ethylene/ethane separation. Acs Appl. Mater. Interfaces 2019, 11, 4145–4151. [Google Scholar] [CrossRef]
- Bai, S.; Sridhar, S.; Khan, A. Metal-ion mediated separation of propylene from propane using PPO membranes. J. Membr. Sci. 1998, 147, 131–139. [Google Scholar] [CrossRef]
- Xu, L.; Rungta, M.; Brayden, M.K.; Martinez, M.V.; Stears, B.A.; Barbay, G.A.; Koros, W.J. Olefins-selective asymmetric carbon molecular sieve hollow fiber membranes for hybrid membrane-distillation processes for olefin/paraffin separations. J. Membr. Sci. 2012, 423, 314–323. [Google Scholar] [CrossRef]
- Sanchez, C.M.; Song, T.; Brennecke, J.F.; Freeman, B.D. Hydrogen Stable Supported Ionic Liquid Membranes with Silver Carriers: Propylene and Propane Permeability and Solubility. Ind. Eng. Chem. Res. 2019, 59, 5362–5370. [Google Scholar] [CrossRef]
- Jomekian, A.; Bazooyar, B.; Poormohammadian, S.J.; Darvishi, P. A modified non-equilibrium lattice fluid model based on corrected fractional free volume volume of polymers for gas solubility prediction. Korean J. Chem. Eng. 2019, 36, 1339–1349. [Google Scholar] [CrossRef]
- Hamid, M.R.A.; Jeong, H.K. Recent advances on mixed-matrix membranes for gas separation: Opportunities and engineering challenges. Korean J. Chem. Eng. 2018, 35, 1577–1600. [Google Scholar] [CrossRef]
- Kim, J.; Do, J.Y.; Park, N.; Lee, S.J.; Hong, J.; Kang, M. Photoreduction of CO2 into CH4 using Bi2S3-TiO2 double-layered dense films. Korean J. Chem. Eng. 2018, 35, 1089–1098. [Google Scholar] [CrossRef]
- Raveshiyan, S.; Hosseini, S.S.; Karimi-Sabet, J. Intensification of O2/N2 separation by novel magnetically aligned carbonyl iron powders/polysulfone magnetic mixed matrix membranes. Chem. Eng. Process. 2020, 150, 107866. [Google Scholar] [CrossRef]
- Sanaeepur, H.; Mashhadikhan, S.; Mardassi, G.; Amooghin, A.E.; Bruggen, B.V.; Moghadassi, A. Aminosilane cross-linked poly ether-block-amide PEBAX2533: Characterization and CO2 separation properties. Korean J. Chem. Eng. 2019, 36, 1339–1349. [Google Scholar] [CrossRef]
- Dai, Y.; Li, Q.; Ruan, X.; Hou, Y.; Jiang, X.; Yan, X.; He, G.; Meng, F.; Wang, Z. Fabrication of defect-free Matrimid® asymmetric membranes and the elevated temperature application for N2/SF6 separation. J. Membr. Sci. 2019, 577, 258–265. [Google Scholar] [CrossRef]
- Castro-Dominguez, B.; Leelachaikul, P.; Takagaki, A.; Sugawara, T.; Kikuchi, R.; Oyama, S.T. Supported perfluorotributylamine liquid membrane for H2/O2 separation. J. Membr. Sci. 2013, 448, 262–269. [Google Scholar] [CrossRef]
- Park, H.B.; Kamcev, J.; Robeson, L.M.; Elimelech, M.; Freeman, B.D. Maximizing the right stuff: The trade-off between membrane permeability and selectivity. Science 2017, 356, 6343. [Google Scholar] [CrossRef] [Green Version]
- Hunger, K.; Schmeling, N.; Jeazet, H.B.; Janiak, C.; Staudt, C.; Kleinermanns, K. Investigation of cross-linked and additive containing polymer materials for membranes with improved performance in pervaporation and gas separation. Membranes 2012, 2, 727–763. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beatriz, S.; Joaquin, C.; Ignacio, G.; Miren, E.B.; Oğuz, K.; Jürgen, C.; Freek, K.; Jorge, G. Metal–organic framework based mixed matrix membranes: A solution for highly efficient CO2 capture? Chem. Soc. Rev. 2015, 44, 2421–2454. [Google Scholar]
- Roberto, C.M.; Kumar, V.A.; Joaquín, C. Ultrathin permselective membranes: The latent way for efficient gas separation. Rsc Adv. 2020, 10, 12653–12670. [Google Scholar]
- Weston, M.H.; Colón, Y.J.; Bae, Y.; Garibay, S.J.; Snurr, R.Q.; Farha, O.K.; Hupp, J.T.; Nguyen, S.T. High propylene/propane adsorption selectivity in a copper (catecholate)-decorated porous organic polymer. J. Mater. Chem. A 2014, 2, 299–302. [Google Scholar] [CrossRef]
- Ferraz, H.; Duarte, L.; Di Luccio, M.; Alves, T.; Habert, A.; Borges, C. Recent achievements in facilitated transport membranes for separation processes. Braz. J. Chem. Eng. 2007, 24, 101–118. [Google Scholar] [CrossRef] [Green Version]
- Marti, A.M.; Nijem, N.; Chabal, Y.J.; Balkus, K.J., Jr. Selective detection of olefins using a luminescent silver-functionalized metal organic framework, RPM3. Microporous Mesoporous Mater. 2013, 174, 100–107. [Google Scholar] [CrossRef]
- Campos, A.C.C.; dos Reis, R.A.; Ortiz, A.; Gorri, D.; Ortiz, I. A perspective of solutions for membrane instabilities in olefin/paraffin separations: A review. Ind. Eng. Chem. Res. 2018, 57, 10071–10085. [Google Scholar] [CrossRef] [Green Version]
- Salgado-Gordon, H.; Valbuena-Moreno, G. Technical and economic evaluation of the separation of light olefins (ethylene and propylene) by using p-complexation with silver salts. Ciencia, Tecnología y Futuro 2011, 4, 73–87. [Google Scholar]
- Liu, Z.; Zhang, L.; Li, L.; Zhang, S. Separation of olefin/paraffin by electrodialysis. Sep. Purif. Technol. 2019, 218, 20–24. [Google Scholar] [CrossRef]
- Merkel, T.C.; Blanc, R.; Ciobanu, I.; Firat, B.; Suwarlim, A.; Zeid, J. Silver salt facilitated transport membranes for olefin/paraffin separations: Carrier instability and a novel regeneration method. J. Membr. Sci. 2013, 447, 177–189. [Google Scholar] [CrossRef]
- Jeong, S.; Kang, S.W. Effect of Ag2O nanoparticles on long-term stable polymer/AgBF4/Al(NO3)3 complex membranes for olefin/paraffin separation. Chem. Eng. J. 2017, 327, 500–504. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, J.H.; Won, J.; Char, K.; Kang, Y.S. Enhancement of facilitated olefin transport by amino acid in silver–polymer complex membranes. Chem. Commun. 2003, 6, 768–769. [Google Scholar] [CrossRef]
- Dastafkan, K.; Khajeh, M.; Ghaffari-Moghaddam, M.; Bohlooli, M. Silver nanoparticles for separation and preconcentration processes. Trends Anal. Chem. 2015, 64, 118–126. [Google Scholar] [CrossRef]
- Han, K.I.; Kang, S.W.; Kim, J.; Kang, Y.S. Effect of ionic liquids on dissociation of copper flake into copper nanoparticles and its application to facilitated olefin transport membranes. J. Membr. Sci. 2011, 374, 43–48. [Google Scholar] [CrossRef]
- Ozawa, K.; Munakata, S.; Edamoto, K.; Mase, K. Electron Donor Molecule on the Oxide Surface: Influence of Surface Termination of ZnO on Adsorption of Tetrathiafulvalene. J. Phys. Chem. C 2011, 115, 21843–21851. [Google Scholar] [CrossRef]
- Lee, J.H.; Sudhager, P.; Cho, Y.; Kang, S.W. Effect of temperature on separation performance in ionic liquid/Ag nanocomposite membranes for olefin/paraffin mixtures. J. Ind. Eng. Chem. 2019, 74, 103–107. [Google Scholar] [CrossRef]
- Jeon, H.; Cho, Y.; Kang, S.W. Structural Effect of Ionic Liquid on Long-Term Stability in Poly (ethylene oxide)/Ag Ions/Ag Nanoparticles Composite for Olefin Separation. Macromol. Res. 2019, 28, 445–449. [Google Scholar] [CrossRef]
- Jeon, H.; Kang, S.W. Hybrid effect of Ag ions and polarized Ag nanoparticles in poly (ethylene oxide)/AgBF4/ionic liquid composites for long-term stable membranes. Polym. Compos. 2019, 40, 2745–2750. [Google Scholar] [CrossRef]
- Jung, K.W.; Kang, S.W. Effect of functional group ratio in PEBAX copolymer on propylene/propane separation for facilitated olefin transport membranes. Sci. Rep. 2019, 9, 11454. [Google Scholar] [CrossRef] [PubMed]


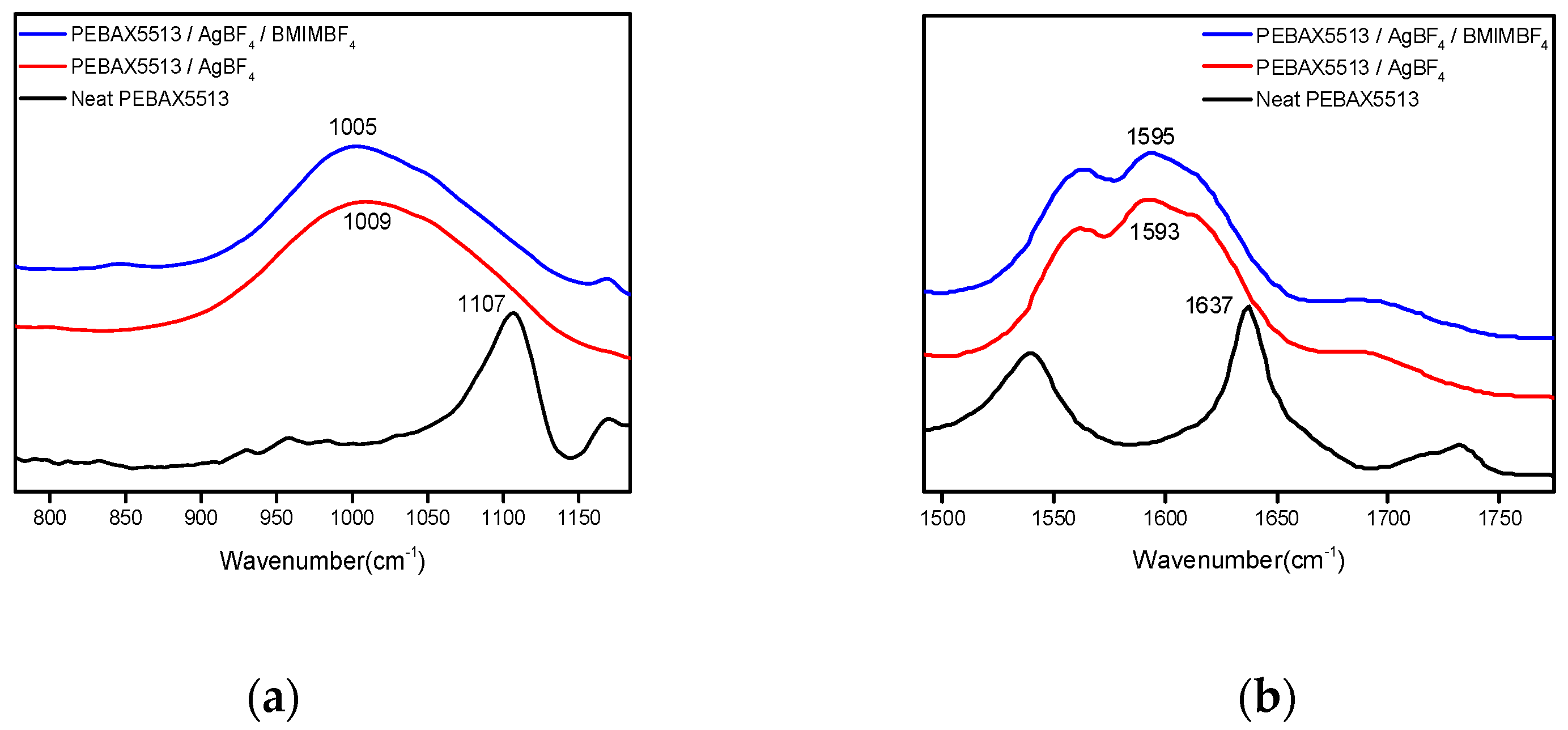
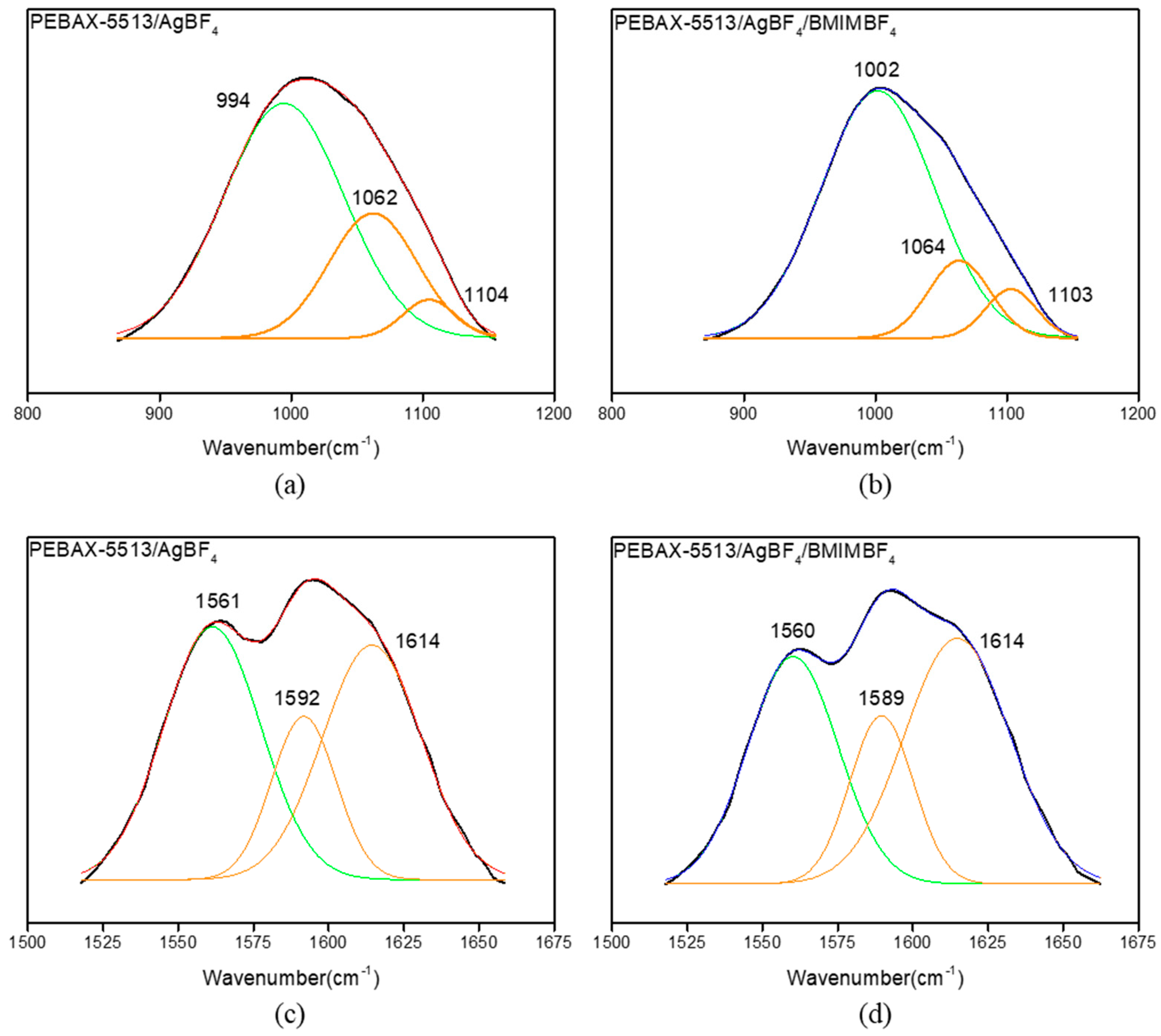
| Wavenumber (cm−1) | PEBAX-5513/AgBF4 (%) | PEBAX-5513/AgBF4/BMIMBF4 (%) |
|---|---|---|
| 994–1002 | 68.3 | 79.5 |
| 1062–1064 | 31.7 | 20.5 |
| 1103–1104 |
| Wavenumber (cm−1) | PEBAX-5513/AgBF4 (%) | PEBAX-5513/AgBF4/BMIMBF4 (%) |
|---|---|---|
| 1589–1592 | 29.2 | 31.2 |
| 1614 | 70.8 | 68.8 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.Y.; Cho, Y.; Kang, S.W. Preparation and Characterization of PEBAX-5513/AgBF4/BMIMBF4 Membranes for Olefin/Paraffin Separation. Polymers 2020, 12, 1550. https://doi.org/10.3390/polym12071550
Kim SY, Cho Y, Kang SW. Preparation and Characterization of PEBAX-5513/AgBF4/BMIMBF4 Membranes for Olefin/Paraffin Separation. Polymers. 2020; 12(7):1550. https://doi.org/10.3390/polym12071550
Chicago/Turabian StyleKim, So Young, Younghyun Cho, and Sang Wook Kang. 2020. "Preparation and Characterization of PEBAX-5513/AgBF4/BMIMBF4 Membranes for Olefin/Paraffin Separation" Polymers 12, no. 7: 1550. https://doi.org/10.3390/polym12071550




