Compression Molded Soy Protein Films with Exopolysaccharides Produced by Cider Lactic Acid Bacteria
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Bacterial and Fungal Strains and Their Growth Conditions
2.3. Preparation of Films
2.4. Characterization of Films
2.4.1. Color Measurement
2.4.2. Gloss Measurements
2.4.3. Light Absorption
2.4.4. Differential Scanning Calorimetry (DSC)
2.4.5. Thermo-Gravimetric Analysis (TGA)
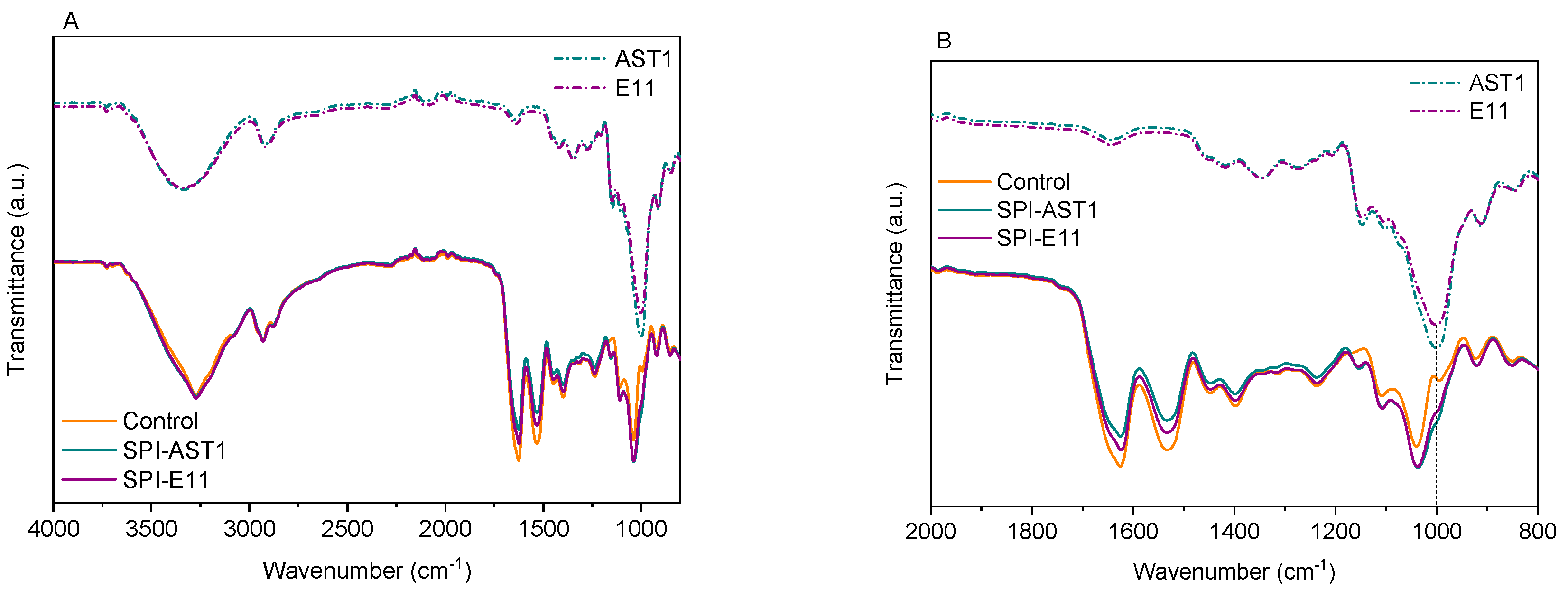
2.4.6. Fourier Transform Infrared (FTIR) Spectroscopy
2.4.7. Moisture Content (MC)
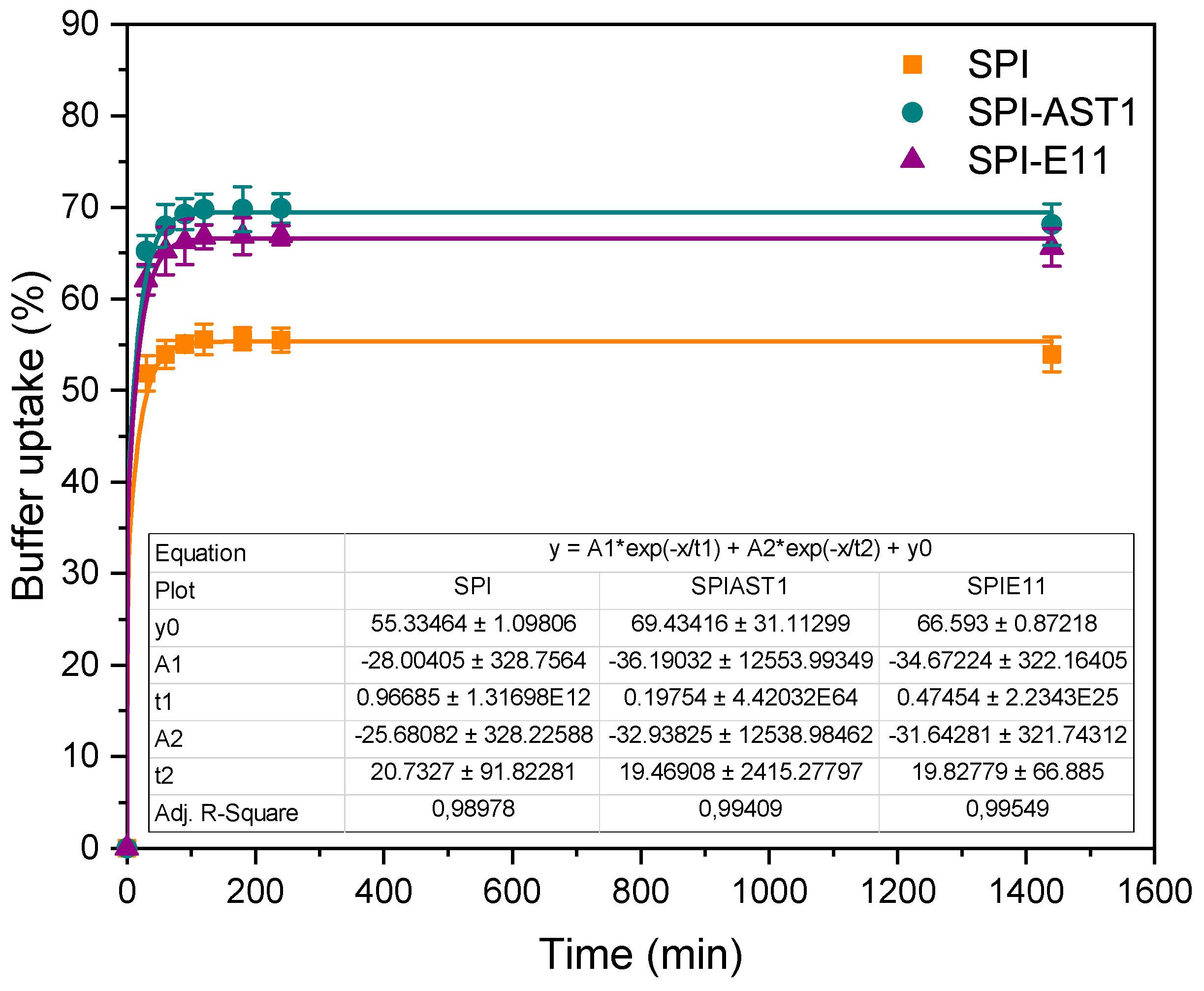
2.4.8. Buffer Uptake (BU)
2.4.9. X-ray Diffraction (XRD)
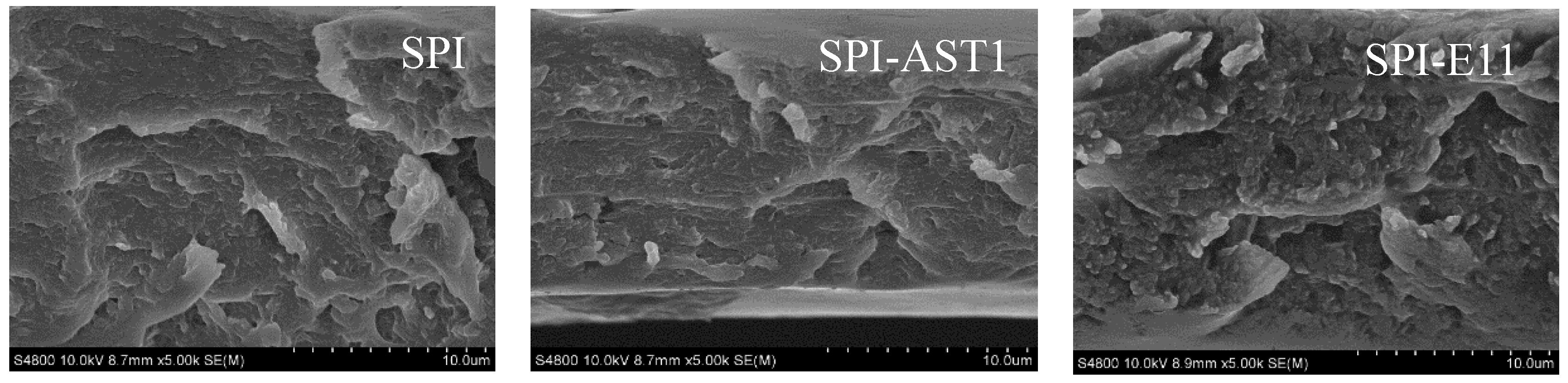
2.4.10. Scanning Electron Microscopy (SEM)
2.4.11. Tensile Test
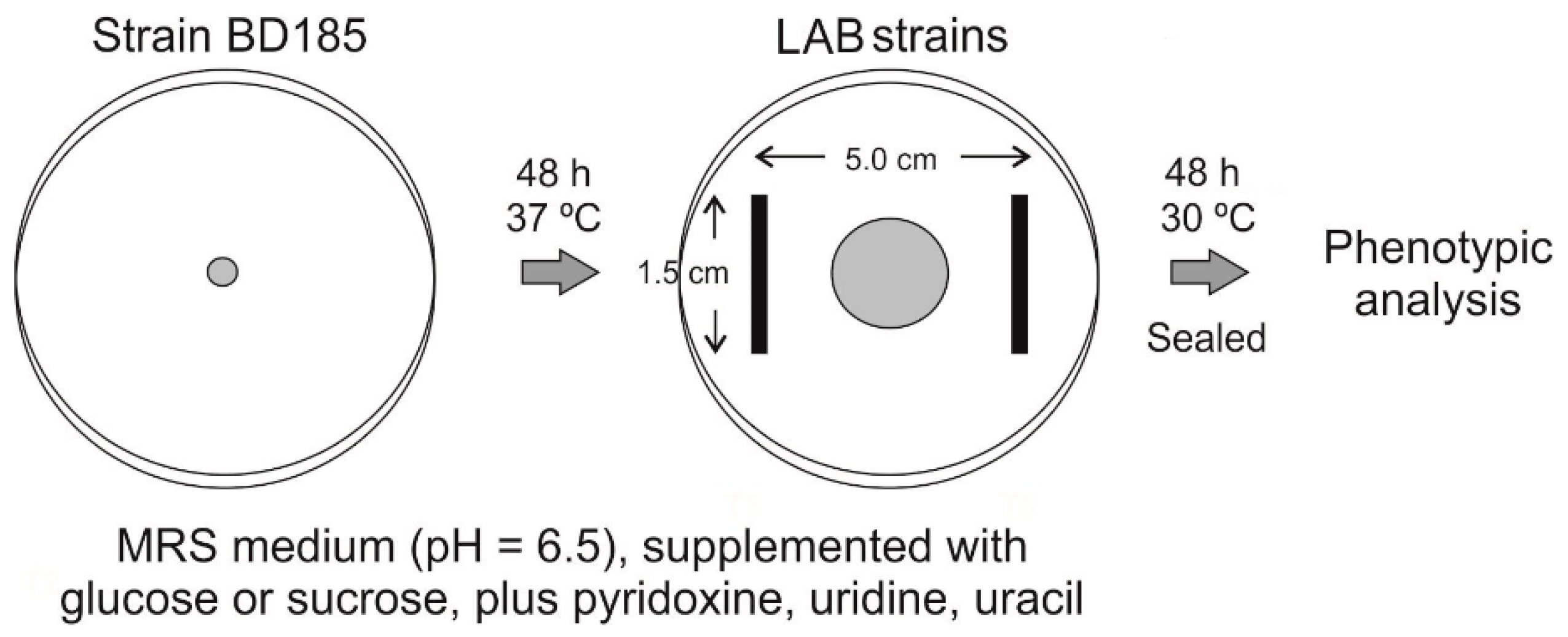
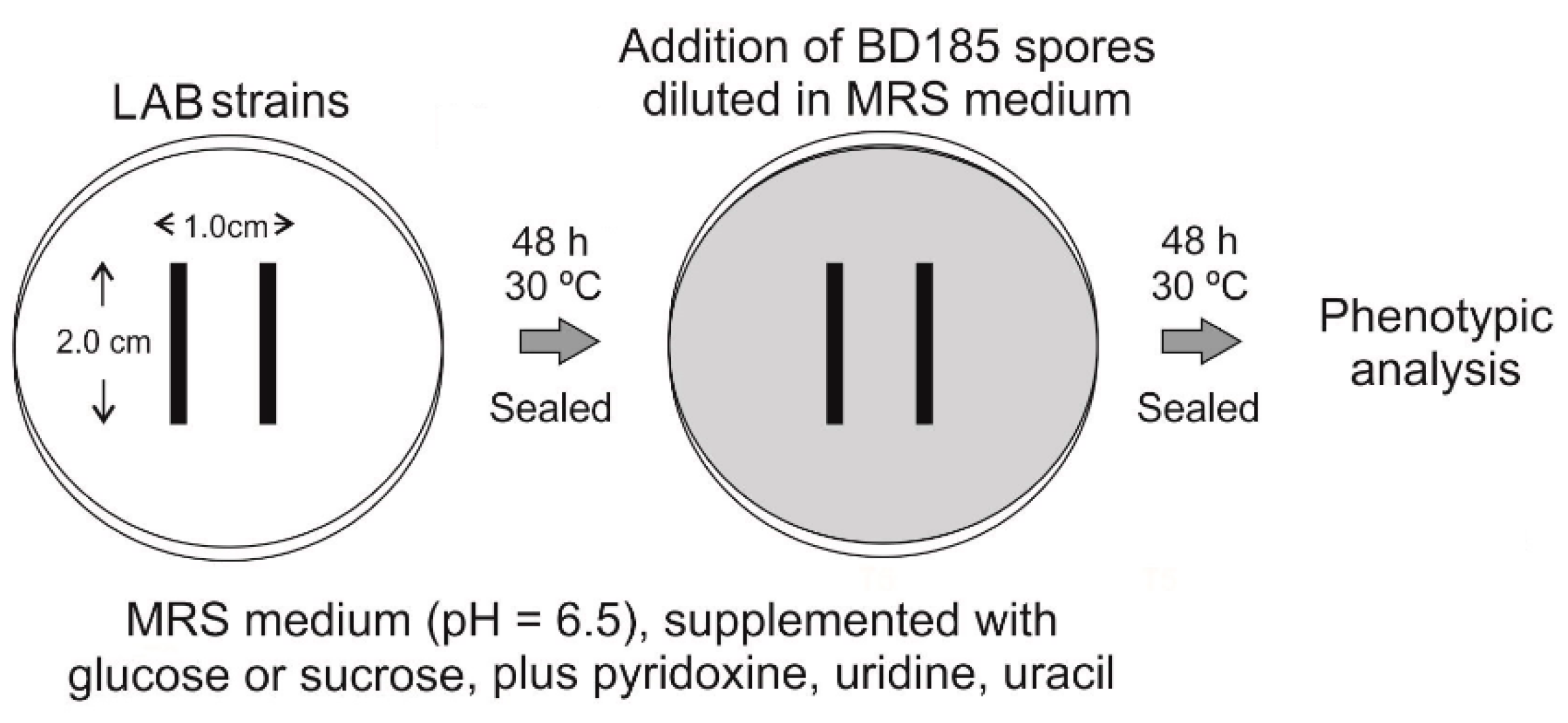
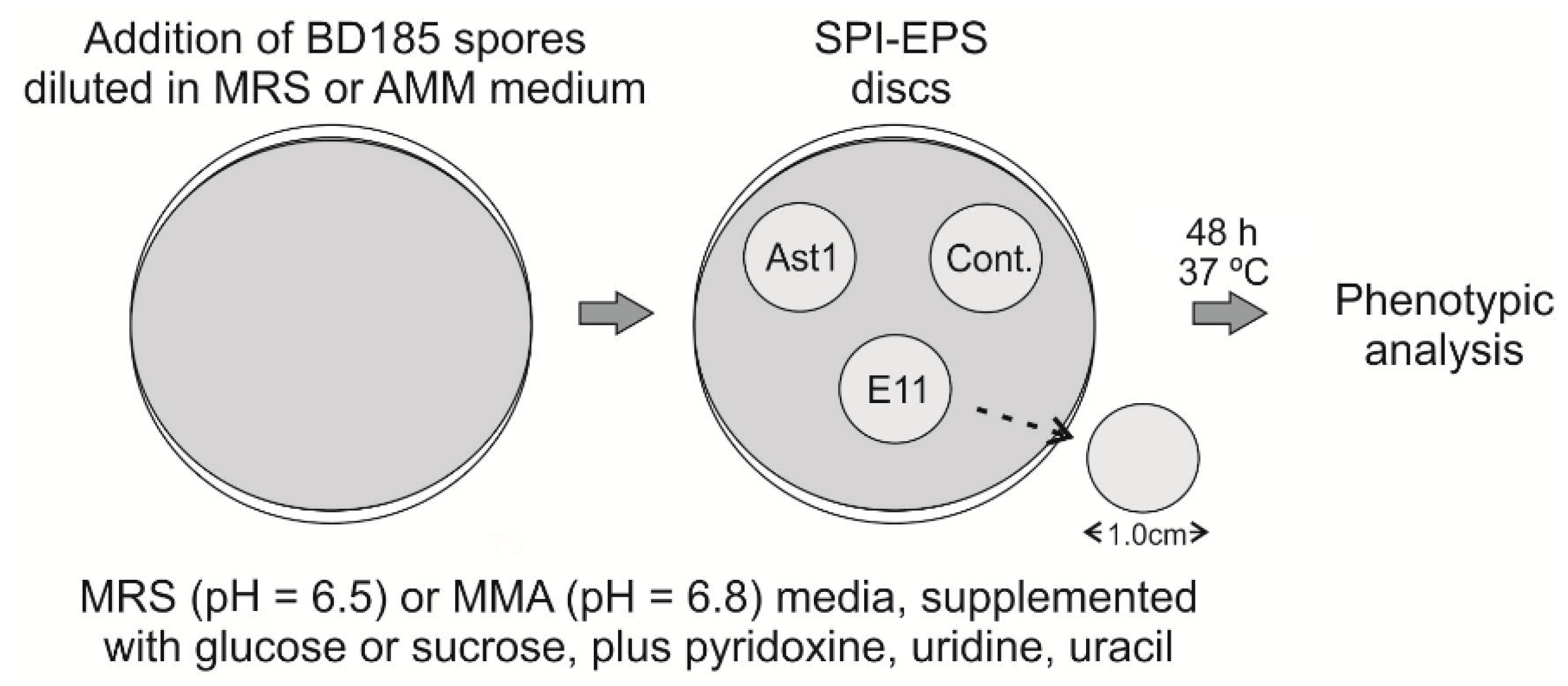
2.4.12. Co-Culturing of Aspergillus nidulans and Bacterial Strains
2.4.13. Statistical Analysis
3. Results and Discussion
3.1. Optical Properties
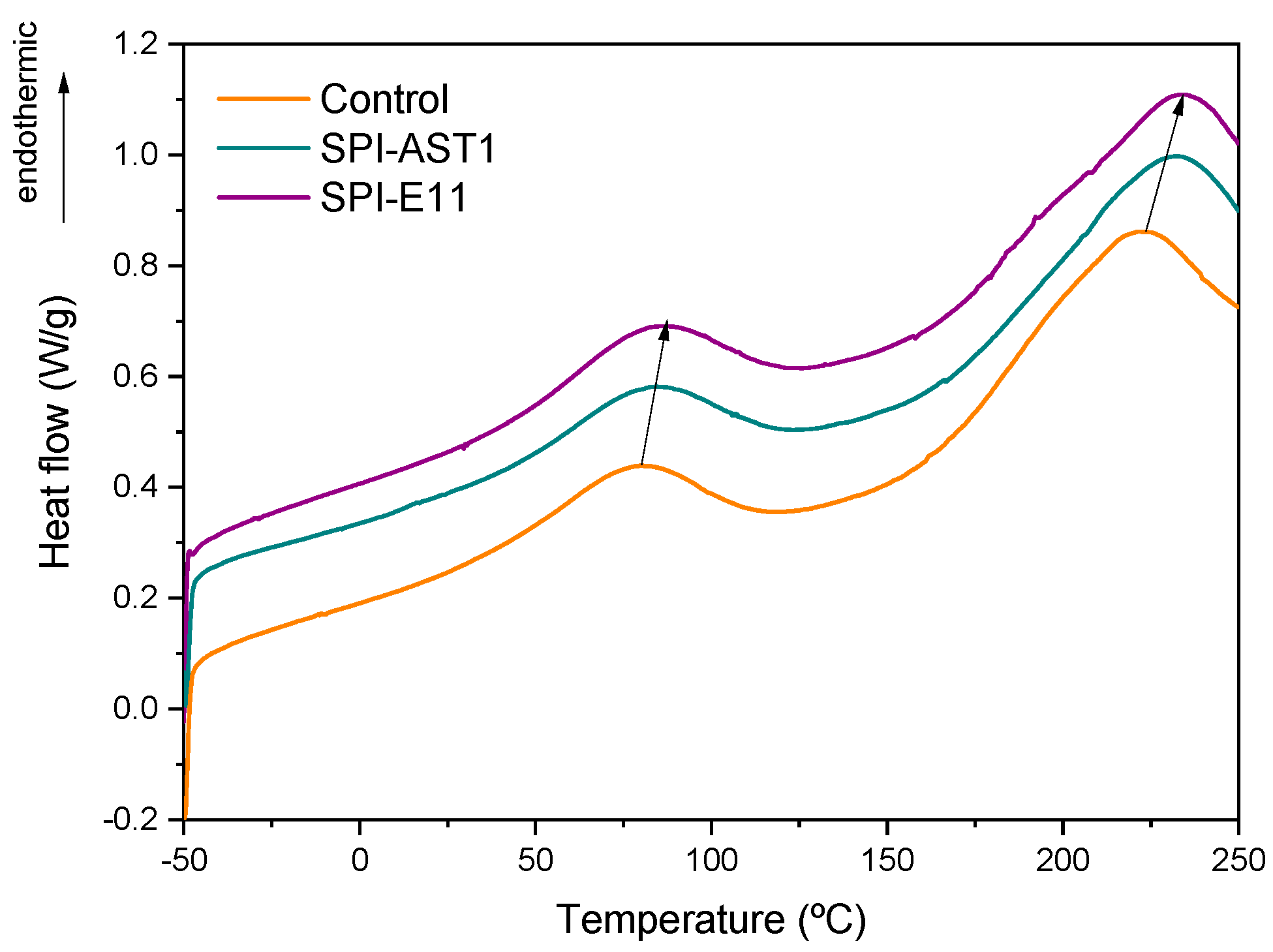
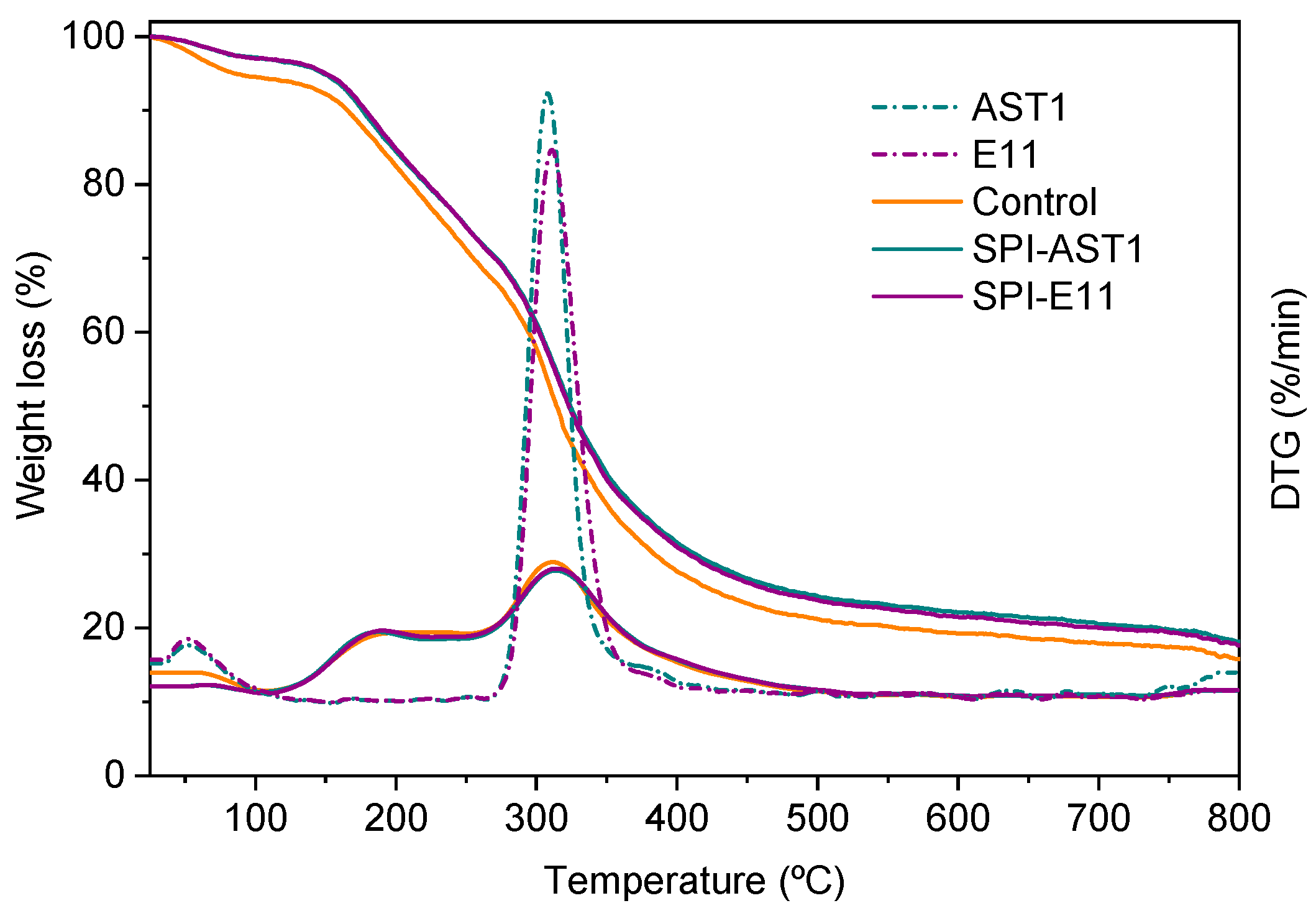
3.2. Thermal Properties of Films
3.3. Physicochemical Properties
3.4. Morphological and Mechanical Properties
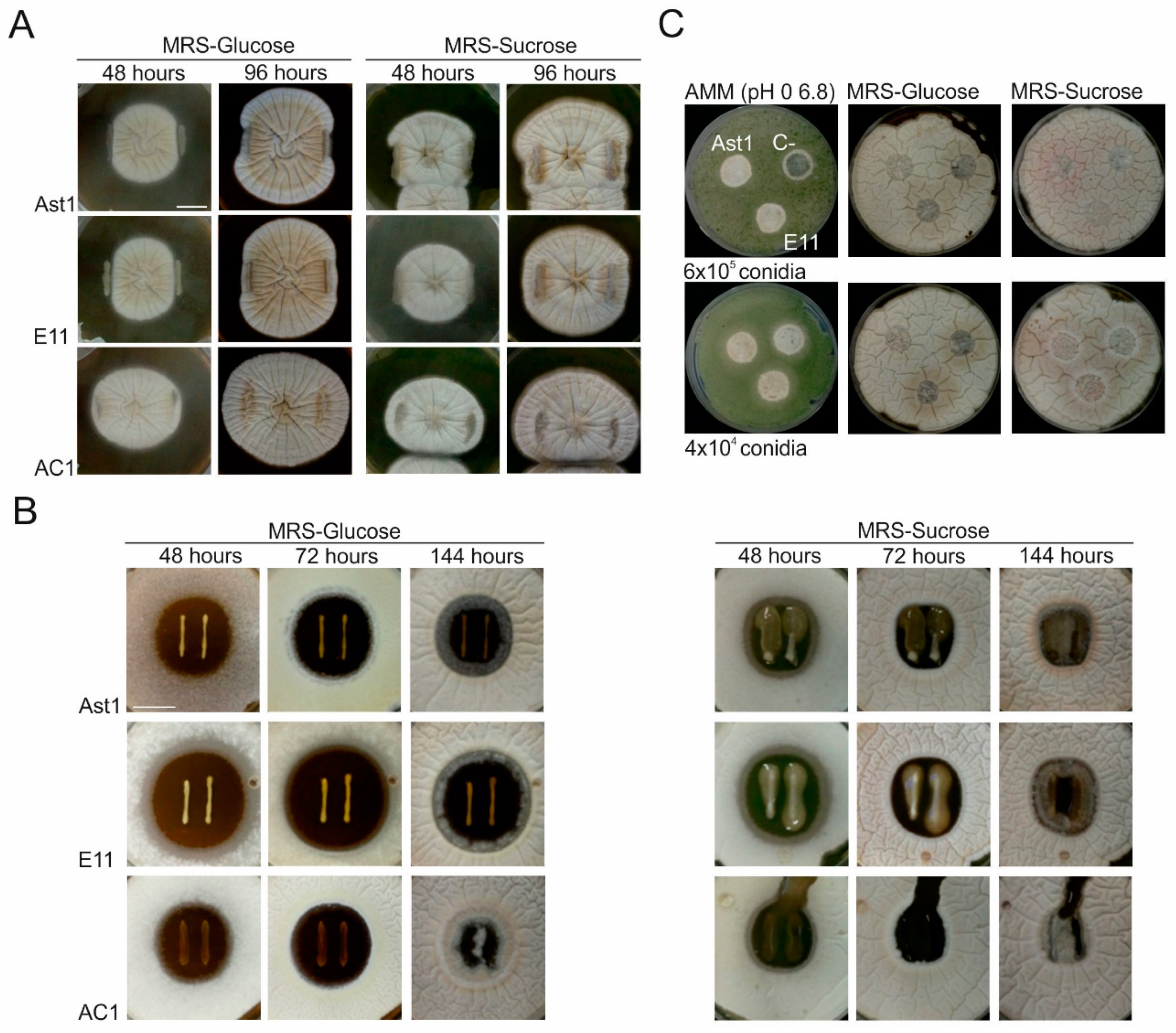
3.5. Germination Delay of the Filamentous Fungus Aspergillus nidulans in Co-Culture with LAB Strains
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Hu, X.; Li, D.; Qiao, Y.; Wang, X.; Zhang, Q.; Zhao, W.; Huang, L. Purification, characterization and anticancer activities of exopolysaccharide produced by Rhodococcus erythropolis HX-2. Int. J. Biol. Macromol. 2020, 145, 646–654. [Google Scholar] [CrossRef]
- Schmid, J. Recent insights in microbial exopolysaccharide biosynthesis and engineering strategies. Curr. Opin. Biotechnol. 2018, 53, 130–136. [Google Scholar] [CrossRef]
- Food and Drug Administration. Microorganisms & Microbial-Derived Ingredients Used in Food (Partial List). 2018. Available online: https://www.fda.gov/food/generally-recognized-safe-gras/microorganisms-microbial-derived-ingredients-used-food-partial-list (accessed on 21 August 2020).
- Oleksy-Sobczak, M.; Klewicka, E.; Piekarska-Radzik, L. Exopolysaccharides production by Lactobacillus rhamnosus strains—Optimization of synthesis and extraction conditions. LWT-Food Sci. Technol. 2020, 122, 109055–109062. [Google Scholar] [CrossRef]
- Hu, Y.; Gänzle, M.G. Effect of temperature on production of oligosaccharides and dextran by Weissella cibaria 10 M. Int. J. Food Microbiol. 2018, 280, 27–34. [Google Scholar] [CrossRef]
- Lakshmi Bhavani, A.; Nisha, J. Dextran - the polysaccharide with versatile uses. Int. J. Pharma Bio Sci. 2010, 1, 569–573. [Google Scholar]
- Lynch, K.M.; Coffey, A.; Arendt, E.K. Exopolysaccharide producing lactic acid bacteria: Their techno-functional role and potential application in gluten-free bread products. Food Res. Int. 2018, 110, 52–61. [Google Scholar] [CrossRef]
- Saravanan, C.; Shetty, P.K.H. Isolation and characterization of exopolysaccharide from Leuconostoc lactis KC117496 isolated from idli batter. Int. J. Biol. Macromol. 2016, 90, 100–106. [Google Scholar] [CrossRef]
- Zarour, K.; Llamas, M.G.; Prieto, A.; Ruas-Madiedo, P.; Dueñas, M.T.; de Palencia, P.F.; Aznar, R.; Kihal, M.; Lopez, P. Rheology and bioactivity of high molecular weight dextrans synthesised by lactic acid bacteria. Carbohydr. Polym. 2017, 174, 646–657. [Google Scholar] [CrossRef]
- Mahgoub, A.M.; Mahmoud, M.G.; Selim, M.S.; Awady, M.E.E. Exopolysaccharide from marine Bacillus velezensis MHM3 induces apoptosis of human breast cancer MCF-7 cells through a mitochondrial pathway. Asian Pac. J. Cancer Prev. 2018, 19, 1957–1963. [Google Scholar]
- Raposo, M.F.J.; de Morais, R.M.S.C.; de Morais, A.M.M.B. Bioactivity and applications of sulphated polysaccharides from marine microalgae. Mar. Drugs 2013, 11, 233–252. [Google Scholar] [CrossRef] [Green Version]
- Hussain, A.; Zia, K.M.; Tabasum, S.; Noreen, A.; Ali, M.; Iqbal, R.; Zuber, M. Blends and composites of exopolysaccharides; properties and applications: A review. Int. J. Biol. Macromol. 2017, 94, 10–27. [Google Scholar] [CrossRef]
- Etxabide, A.; Garrido, T.; Uranga, J.; Guerrero, P.; de la Caba, K. Extraction and incorporation of bioactives into protein formulations for food and biomedical applications. Int. J. Biol. Macromol. 2018, 120, 2094–2105. [Google Scholar] [CrossRef]
- Garrido, T.; Leceta, I.; de la Caba, K.; Guerrero, P. Chicken feathers as a natural source of sulphur to develop sustainable protein films with enhanced properties. Int. J. Biol. Macromol. 2018, 106, 523–531. [Google Scholar] [CrossRef]
- Wang, S.; Marcone, M.; Barbut, S.; Lim, L.T. The impact of anthocyanin-rich red raspberry extract (ARRE) on the properties of edible soy protein isolate (SPI) films. J. Food Sci. 2012, 77, C497–C505. [Google Scholar] [CrossRef]
- Zou, Y.C.; Wu, C.L.; Ma, C.F.; He, S.; Brennan, C.S.; Yuan, Y. Interactions of grape seed procyanidins with soy protein isolate: Contributing antioxidant and stability properties. LWT-Food Sci. Technol. 2019, 115, 108465–108471. [Google Scholar] [CrossRef]
- Galus, S. Functional properties of soy protein isolate edible films as affected by rapeseed oil concentration. Food Hydrocoll. 2018, 85, 233–241. [Google Scholar] [CrossRef]
- De Man, J.C.; Rogosa, M.; Sharpe, M.E. A medium for the cultivation of lactobacilli. J. Appl. Bacteriol. 1960, 23, 130–135. [Google Scholar] [CrossRef]
- Llamas-Arriba, M.G.; Puertas, A.I.; Prieto, A.; López, P.; Cobos, M.; Miranda, J.I.; Marieta, C.; Ruas-Madiedo, P.; Dueñas, M.T. Characterization of dextrans produced by Lactobacillus mali CUPV271 and Leuconostoc carnosum CUPV411. Food Hydrocoll. 2019, 89, 613–622. [Google Scholar] [CrossRef]
- Herrero-Garcia, E.; Perez-de Nanclares-Arregi, E.; Cortese, M.S.; Markina-Iñarrairaegui, A.; Oiartzabal-Arano, E.; Etxebeste, O.; Ugalde, U.; Espeso, E.A. Tip-to-nucleus migration dynamics of the asexual development regulator FlbB in vegetative cells. Mol. Microbiol. 2015, 98, 607–624. [Google Scholar] [CrossRef]
- Otamendi, A.; Perez-de Nanclares-Arregi, E.; Oiartzabal-Arano, E.; Cortese, M.S.; Espeso, E.A.; Etxebeste, O. Developmental regulators FlbE/D orchestrate the polarity site-to-nucleus dynamics of the fungal bZIP transcription factor FlbB. Cell. Mol. Life Sci. 2019, 76, 4369–4390. [Google Scholar] [CrossRef]
- Etxebeste, O.; Ni, M.; Garzia, A.; Kwon, N.J.; Fischer, R.; Yu, J.H.; Espeso, E.A.; Ugalde, U. Basic-zipper-type transcription factor FlbB controls asexual development in Aspergillus nidulans. Eukaryot. Cell 2008, 7, 38–48. [Google Scholar] [CrossRef] [Green Version]
- ASTM D523-14. Standard test method for specular gloss. In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM D638-03. Standard test method for tensile properties of plastics. In Annual Book of ASTM Standards; American Society for Testing and Materials: West Conshohocken, PA, USA, 2003. [Google Scholar]
- Bartkiene, E.; Lele, V.; Ruzauskas, M.; Domig, K.J.; Starkute, V.; Zavistanaviciute, P.; Bartkevics, V.; Pugajeva, I.; Klupsaite, D.; Juodeikiene, G.; et al. Lactic acid bacteria isolation from spontaneous sourdough and their characterization including antimicrobial and antifungal properties evaluation. Microorganisms 2020, 8, 64. [Google Scholar] [CrossRef] [Green Version]
- Magnusson, J.; Schnürer, J. Lactobacillus coryniformis subsp. coryniformis strain Si3 produces a broad-spectrum proteinaceous antifungal compound. Appl. Environ. Microbiol. 2001, 67, 1–5. [Google Scholar] [CrossRef] [Green Version]
- Garrido, T.; Etxabide, A.; Peñalba, M.; de la Caba, K.; Guerrero, P. Preparation and characterization of soy protein thin films: Processing-properties correlation. Mater. Lett. 2013, 105, 110–112. [Google Scholar] [CrossRef]
- Luchese, C.L.; Abdalla, V.F.; Spada, J.C.; Tessaro, I.C. Evaluation of blueberry residue incorporated cassava starch film as pH indicator in different simulants and foodstuffs. Food Hydrocoll. 2018, 82, 209–218. [Google Scholar] [CrossRef]
- Acosta, S.; Jiménez, A.; Cháfer, M.; González-Martínez, C.; Chiralt, A. Physical properties and stability of starch-gelatin based films as affected by the addition of esters of fatty acids. Food Hydrocoll. 2015, 49, 135–143. [Google Scholar] [CrossRef] [Green Version]
- Ren, C.; Xiong, W.; Peng, D.; He, Y.; Zhou, P.; Li, J.; Li, B. Effects of thermal sterilization on soy protein isolate/polyphenol complexes: Aspects of structure, in vitro digestibility and antioxidant activity. Food Res. Int. 2018, 112, 284–290. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, A.; Wang, X.; Xu, N.; Jiang, L. The radiation assisted-Maillard reaction comprehensively improves the freeze-thaw stability of soy protein-stabilized oil-in-water emulsions. Food Hydrocoll. 2020, 103, 105684–105692. [Google Scholar] [CrossRef]
- Sudha; Gupta, C.; Aggarwal, S. Dyeing wet blue goat nappa skin with a microbial colorant obtained from Penicillium minioluteum. J. Clean. Prod. 2016, 127, 585–590. [Google Scholar] [CrossRef]
- Zhou, B.; Gao, M.; Feng, X.; Huang, L.; Huang, Q.; Kootala, S.; Larsson, T.E.; Zheng, L.; Bowden, T. Carbazate modified dextrans as scavengers for carbonylated proteins. Carbohydr. Polym. 2020, 232, 115802–115809. [Google Scholar] [CrossRef]
- Mo, X.; Sun, X. Plasticization of soy protein polymer by polyol-based plasticizers. J. Am. Oil Chem. Soc. 2002, 79, 197–202. [Google Scholar] [CrossRef]
- Garrido, T.; Leceta, I.; Cabezudo, S.; Guerrero, P.; de la Caba, K. Tailoring soy protein film properties by selecting casting or compression as processing methods. Eur. Polym. J. 2016, 85, 499–507. [Google Scholar] [CrossRef]
- Garrido, T.; Uranga, J.; Guerrero, P.; de la Caba, K. The potential of vegetal and animal proteins to develop more sustainable food packaging. In Polymers for Food Applications; Gutierrez, T.J., Ed.; Springer: Cham, Switzerland, 2018; pp. 25–59. [Google Scholar]
- Kang, H.; Wang, Z.; Zhang, W.; Li, J.; Zhang, S. Physico-chemical properties improvement of soy protein isolate films through caffeic acid incorporation and tri-functional aziridine hybridization. Food Hydrocoll. 2016, 61, 923–932. [Google Scholar] [CrossRef]
- Zheng, T.; Yu, X.; Pilla, S. Mechanical and moisture sensitivity of fully bio-based dialdehyde carboxymethyl cellulose cross-linked soy protein isolate films. Carbohydr. Polym. 2017, 157, 1333–1340. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Yu, M.; Wang, L. Preparation and characterization of antioxidant soy protein isolate films incorporating licorice residue extract. Food Hydrocoll. 2018, 75, 13–21. [Google Scholar] [CrossRef]
- Uranga, J.; Etxabide, A.; Cabezudo, S.; de la Caba, K.; Guerrero, P. Valorization of marine-derived biowaste to develop chitin/fish gelatin products as bioactive carriers and moisture scavengers. Sci. Total Environ. 2020, 706, 135747–135756. [Google Scholar] [CrossRef]
- Carpiné, D.; Dagostin, J.L.A.; Bertan, L.C.; Mafra, M.R. Development and characterization of soy protein isolate emulsion-based edible films with added coconut oil for olive oil packaging: Barrier, mechanical, and thermal properties. Food Bioprocess Technol. 2015, 8, 1811–1823. [Google Scholar] [CrossRef]
- Qu, P.; Huang, H.; Wu, G.; Sun, E.; Chang, Z. Effects of hydrolysis degree of soy protein isolate on the structure and performance of hydrolyzed soy protein isolate/urea/formaldehyde copolymer resin. J. Appl. Polym. Sci. 2015, 132, 41469–41476. [Google Scholar] [CrossRef]
- Desam, G.P.; Li, J.; Chen, G.; Campanella, O.; Narsimhan, G. Prediction of swelling behavior of crosslinked maize starch suspensions. Carbohydr. Polym. 2018, 199, 331–340. [Google Scholar] [CrossRef]
- Ye, Q.; Han, Y.; Zhang, J.; Zhang, W.; Xia, C.; Li, J. Bio-based films with improved water resistance derived from soy protein isolate and stearic acid via bioconjugation. J. Clean. Prod. 2019, 214, 125–131. [Google Scholar] [CrossRef]
- Garrido, T.; Etxabide, A.; Leceta, I.; Cabezudo, S.; de la Caba, K.; Guerrero, P. Valorization of soya by-products for sustainable packaging. J. Clean. Prod. 2014, 64, 228–233. [Google Scholar] [CrossRef]
- Guerrero, P.; Retegi, A.; Gabilondo, N.; de la Caba, K. Mechanical and thermal properties of soy protein films processed by casting and compression. J. Food Eng. 2010, 100, 145–151. [Google Scholar] [CrossRef]
- Sadiq, F.A.; Yan, B.; Tian, F.; Zhao, J.; Zhang, H.; Chen, W. Lactic acid bacteria as antifungal and anti-mycotoxigenic agents: A comprehensive review. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1403–1436. [Google Scholar] [CrossRef] [Green Version]
- Etxebeste, O.; Espeso, E.A. Aspergillus nidulans in the post-genomic era: A top-model filamentous fungus for the study of signaling and homeostasis mechanisms. Int. Microbiol. 2020, 23, 5–22. [Google Scholar] [CrossRef]
- Nützmann, H.W.; Scazzocchio, C.; Osbourn, A. Metabolic gene clusters in eukaryotes. Annu. Rev. Genet. 2018, 52, 159–183. [Google Scholar] [CrossRef]
- Schroeckh, V.; Scherlach, K.; Nützmann, H.W.; Shelest, E.; Schmidt-Heck, W.; Schuemann, J.; Martin, K.; Hertweck, C.; Brakhage, A.A. Intimate bacterial-fungal interaction triggers biosynthesis of archetypal polyketides in Aspergillus nidulans. Proc. Natl. Acad. Sci. USA 2009, 106, 14558–14563. [Google Scholar] [CrossRef] [Green Version]


| Film | L* | a* | b* | ΔE* | Gloss (GU) |
|---|---|---|---|---|---|
| Control SPI-AST1 | 94.56 ± 0.34 a 95.31 ± 0.45 b | −0.84 ± 0.04 b −0.61 ± 0.03 a | 8.66 ± 0.31 b 6.78 ± 0.31 a | --- 2.08 ± 0.29 a | 23.4 ± 2.7 a 18.1 ± 2.2 b |
| SPI-E11 | 94.17 ± 0.20 a | −0.91 ± 0.04 c | 11.93 ± 0.58 c | 3.29 ± 0.59 b | 20.9 ± 2.5 ab |
| Film | E (MPa) | TS (MPa) | EB (%) |
|---|---|---|---|
| Control SPI-AST1 | 213.56 ± 16.68 a 278.59 ± 18.79 b | 12.47 ± 0.46 ab 13.03 ± 0.41 a | 48.17 ± 4.01 a 40.50 ± 5.41 b |
| SPI-E11 | 342.51 ± 15.87 c | 11.54 ± 0.88 b | 25.04 ± 3.41 c |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uranga, J.; Llamas, M.G.; Agirrezabala, Z.; Dueñas, M.T.; Etxebeste, O.; Guerrero, P.; de la Caba, K. Compression Molded Soy Protein Films with Exopolysaccharides Produced by Cider Lactic Acid Bacteria. Polymers 2020, 12, 2106. https://doi.org/10.3390/polym12092106
Uranga J, Llamas MG, Agirrezabala Z, Dueñas MT, Etxebeste O, Guerrero P, de la Caba K. Compression Molded Soy Protein Films with Exopolysaccharides Produced by Cider Lactic Acid Bacteria. Polymers. 2020; 12(9):2106. https://doi.org/10.3390/polym12092106
Chicago/Turabian StyleUranga, Jone, Mª Goretti Llamas, Ziortza Agirrezabala, María Teresa Dueñas, Oier Etxebeste, Pedro Guerrero, and Koro de la Caba. 2020. "Compression Molded Soy Protein Films with Exopolysaccharides Produced by Cider Lactic Acid Bacteria" Polymers 12, no. 9: 2106. https://doi.org/10.3390/polym12092106








