Biocidal Polymers: Synthesis, Characterization and Antimicrobial Activity of Bis-Quaternary Onium Salts of Poly(aspartate-co-succinimide)
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of N,N,N′,N′-Tetramethylethylenediamine Dihydrochloride (TMEDA.2HCl) (II)
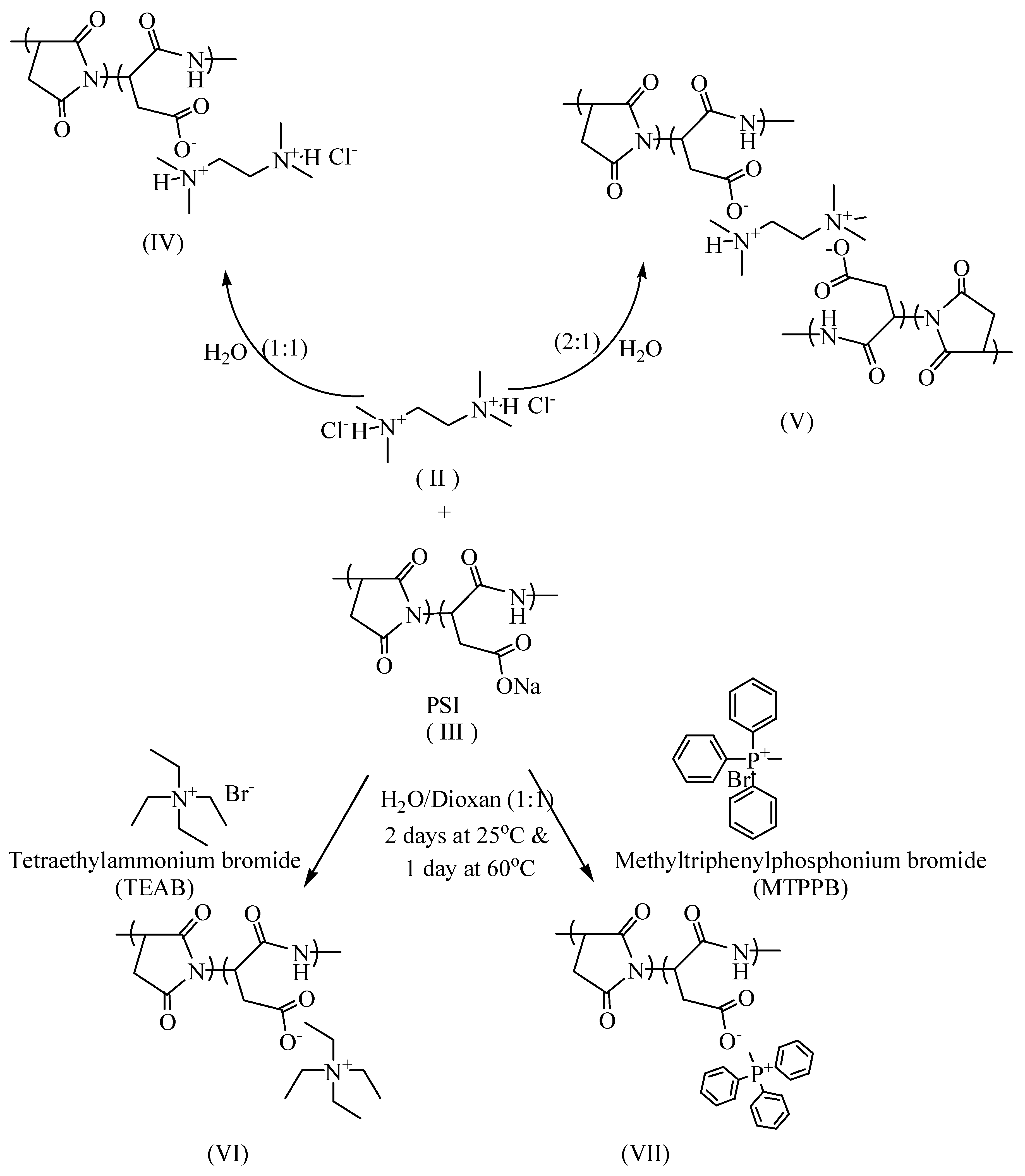
2.3. Synthesis of Antimicrobial Polymers
2.3.1. PSI: TMEDA.2HCl (1:1) (IV)
2.3.2. PSI: TMEDA.2HCl (1:0.5) (V)
2.3.3. Synthesis of Quaternary Onium Salts (VI and VII)
2.4. Characterizations
2.5. Antimicrobial Assessment
2.5.1. Tested Microorganisms
2.5.2. Cut Plug (Agar Diffusion) Procedure for Antimicrobial Activity Test Screening
2.5.3. MIC Evaluation of Effective Antimicrobial Biocidal Polymers
3. Results and Discussion
3.1. Elemental Analysis Halide Ion Estimation
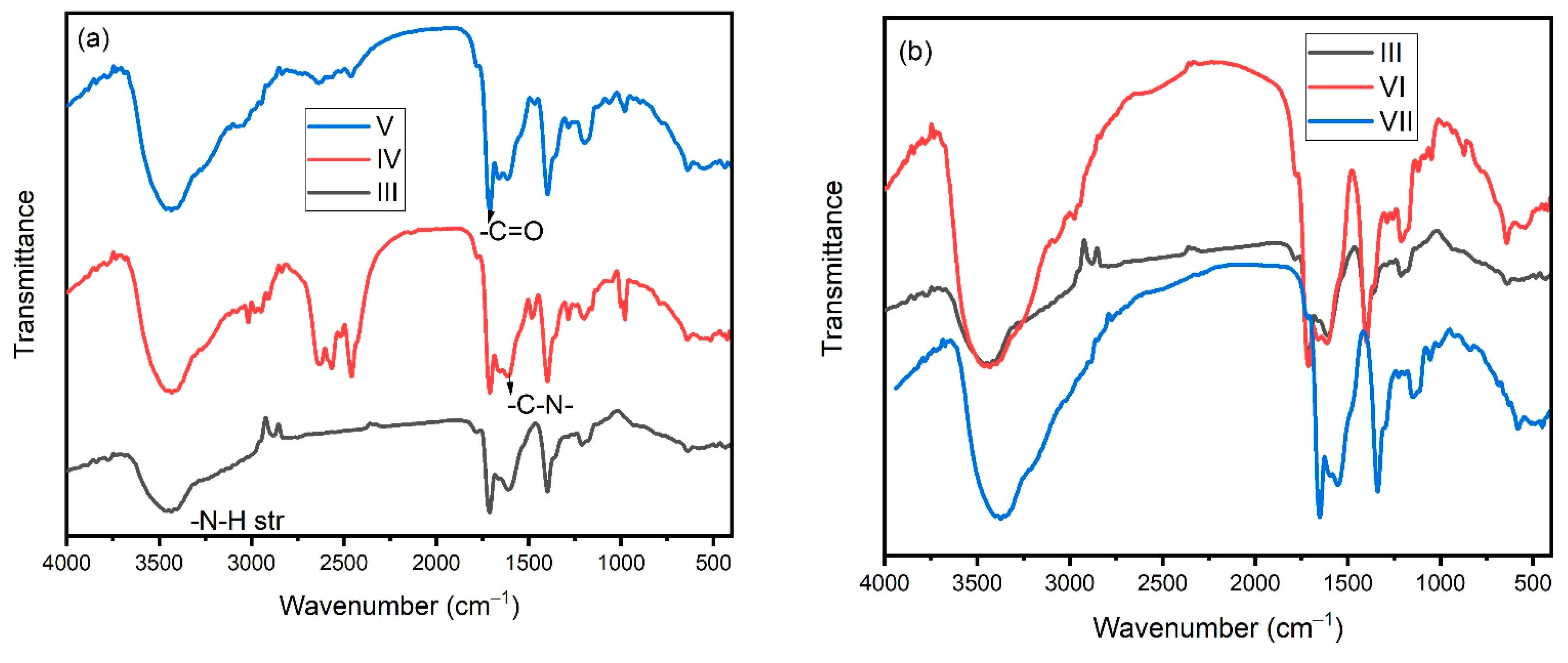
3.2. FTIR Analysis
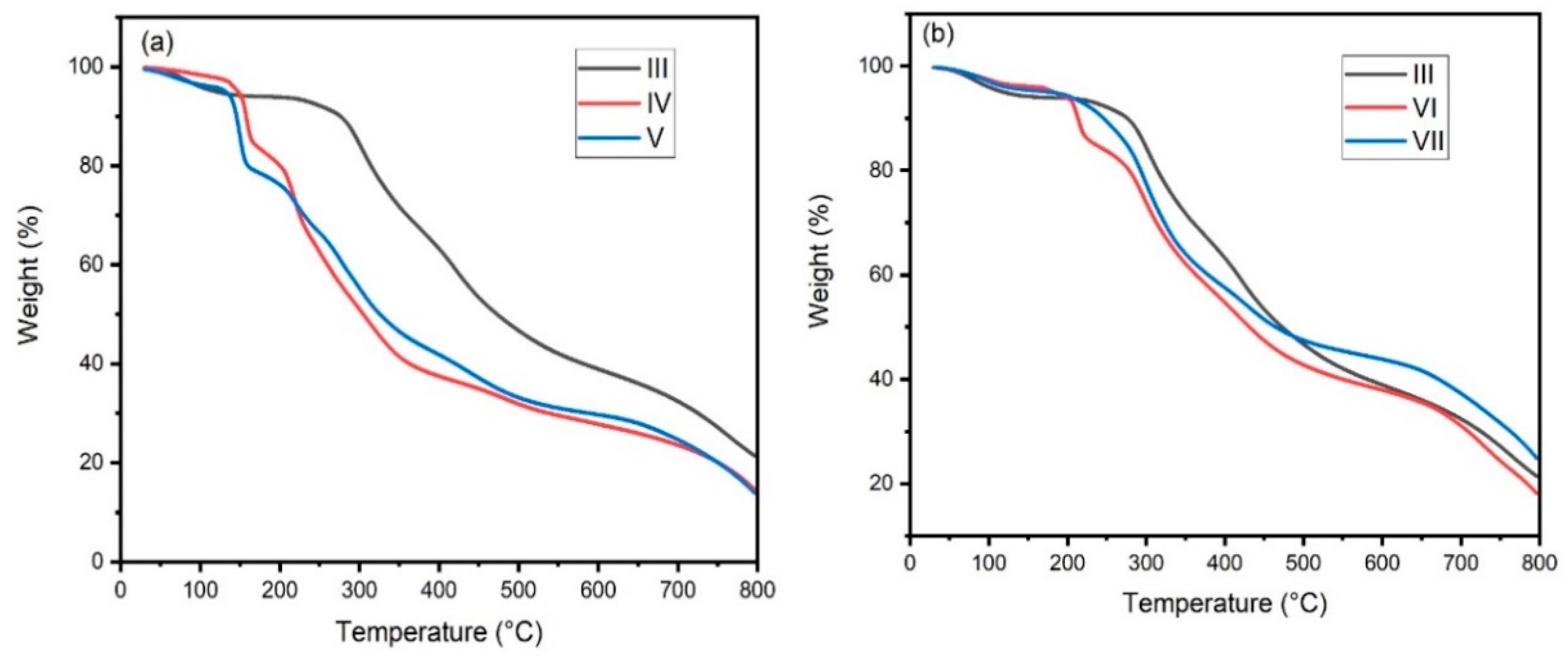
3.3. Thermal Studies
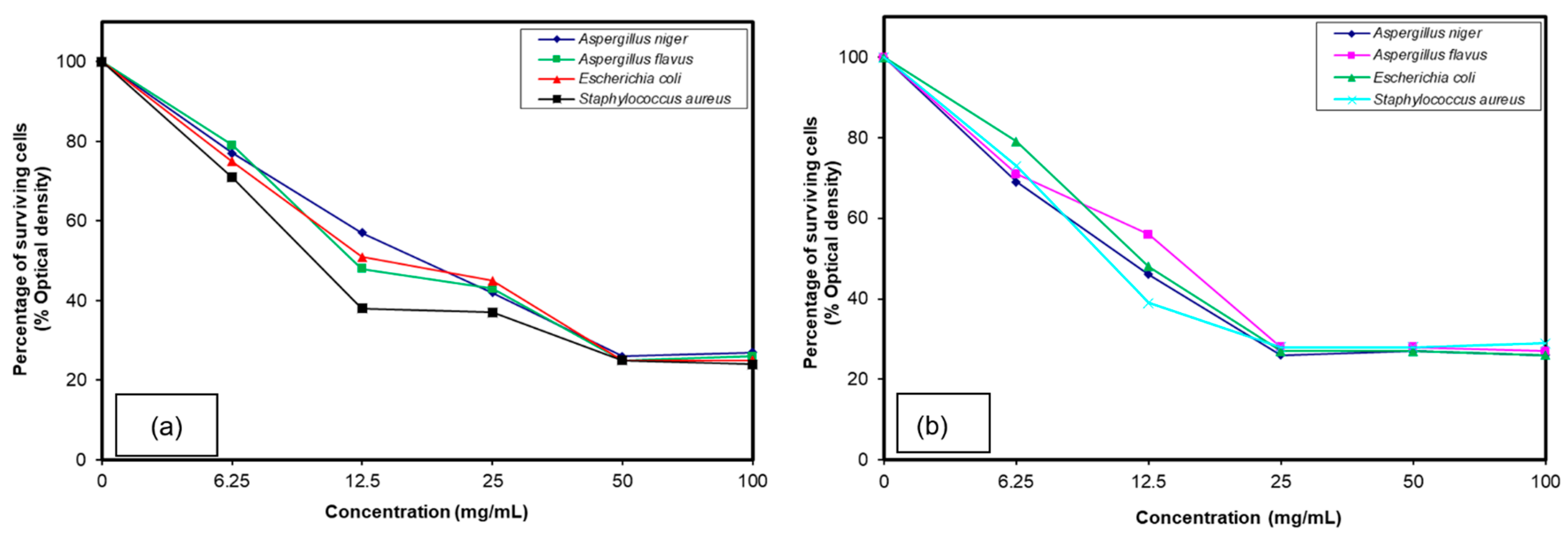
3.4. Antimicrobial Assessments
3.5. Effect of TMEDA Quaternized Copolymer
3.6. Comparison between the Ammonium and Phosphonium Salts
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mahira, S.; Jain, A.; Khan, W.; Domb, A.J. Chapter 1. Antimicrobial materials—An overview. In Antimicrobial Materials for Biomedical Applications; Royal Society of Chemistry: London, UK, 2019; pp. 1–37. [Google Scholar]
- Kenawy, E.-R.; Worley, S.D.; Broughton, R. The chemistry and applications of antimicrobial polymers: A state-of-the-art review. Biomacromolecules 2007, 8, 1359–1384. [Google Scholar] [CrossRef]
- Fauci, A.S.; Touchette, N.A.; Folkers, G.K. Emerging infectious diseases: A 10-year perspective from the national institute of allergy and infectious diseases. Emerg. Infect. Dis. 2005, 11, 519–525. [Google Scholar] [CrossRef]
- Gabriel, G.J.; Som, A.; Madkour, A.E.; Eren, T.; Tew, G.N. Infectious disease: Connecting innate immunity to biocidal polymers. Mater. Sci. Eng. R Rep. 2007, 57, 28–64. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Senapati, D.; Khan, S.A.; Singh, A.K.; Hamme, A.; Yust, B.; Sardar, D.; Ray, P.C. Popcorn-shaped magnetic core-plasmonic shell multifunctional nanoparticles for the targeted magnetic separation and enrichment, label-free SERS imaging, and photothermal destruction of multidrug-resistant bacteria. Chem.-A Eur. J. 2013, 19, 2839–2847. [Google Scholar] [CrossRef]
- Kaur, M.; Rai, J.; Randhawa, G. Recent advances in antibacterial drugs. Int. J. Appl. Basic Med. Res. 2013, 3, 3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Hu, C.; Shao, L. The antimicrobial activity of nanoparticles: Present situation and prospects for the future. Int. J. Nanomedicine 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davies, D. Understanding biofilm resistance to antibacterial agents. Nat. Rev. Drug Discov. 2003, 2, 114–122. [Google Scholar] [CrossRef] [PubMed]
- Aziz, A.M. The role of healthcare strategies in controlling antibiotic resistance. Br. J. Nurs. 2013, 22, 1066–1074. [Google Scholar] [CrossRef] [PubMed]
- Exner, M.; Bhattacharya, S.; Christiansen, B.; Gebel, J.; Goroncy-Bermes, P.; Hartemann, P.; Heeg, P.; Ilschner, C.; Kramer, A.; Larson, E.; et al. Antibiotic resistance: What is so special about multidrug-resistant Gram-negative bacteria? GMS Hyg. Infect. Control 2017, 12, Doc05. [Google Scholar] [CrossRef] [PubMed]
- Cassee, F.; Muijser, H.; Duistermaat, E.; Freijer, J.; Geerse, K.; Marijnissen, J.; Arts, J. Particle size-dependent total mass deposition in lungs determines inhalation toxicity of cadmium chloride aerosols in rats. Application of a multiple path dosimetry model. Arch. Toxicol. 2002, 76, 277–286. [Google Scholar] [CrossRef]
- Dong, C.; Ye, Y.; Qian, L.; Zhao, G.; He, B.; Xiao, H. Antibacterial modification of cellulose fibers by grafting β-cyclodextrin and inclusion with ciprofloxacin. Cellulose 2014, 21, 1921–1932. [Google Scholar] [CrossRef]
- Ilker, M.F.; Nüsslein, K.; Tew, G.N.; Coughlin, E.B. Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J. Am. Chem. Soc. 2004, 126, 15870–15875. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, M. Antimicrobial and antiviral hydrogels. Soft Matter 2011, 7, 8725–8736. [Google Scholar] [CrossRef]
- Huang, T.; Qian, Y.; Wei, J.; Zhou, C. Polymeric antimicrobial food packaging and its applications. Polymers 2019, 11, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yavvari, P.S.; Awasthi, A.K.; Sharma, A.; Bajaj, A.; Srivastava, A. Emerging biomedical applications of polyaspartic acid-derived biodegradable polyelectrolytes and polyelectrolyte complexes. J. Mater. Chem. B 2019, 7, 2102–2122. [Google Scholar] [CrossRef] [PubMed]
- Swan, S.K.; Kohlhepp, S.J.; Kohnen, P.W.; Gilbert, D.N.; Bennett, W.M. Long-term protection of polyaspartic acid in experimental gentamicin nephrotoxicity. Antimicrob. Agents Chemother. 1991, 35, 2591–2595. [Google Scholar] [CrossRef] [Green Version]
- Klein, T.; Moritz, R.-J.; Graupner, R. Polyaspartates and polysuccinimide. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2008. [Google Scholar]
- Zheng, A.; Xue, Y.; Wei, D.; Li, S.; Xiao, H.; Guan, Y. Synthesis and characterization of antimicrobial polyvinyl pyrrolidone hydrogel as wound dressing. Soft Mater. 2014, 12, 179–187. [Google Scholar] [CrossRef]
- Liu, S.; Ono, R.J.; Wu, H.; Teo, J.Y.; Liang, Z.C.; Xu, K.; Zhang, M.; Zhong, G.; Tan, J.P.K.; Ng, M.; et al. Highly potent antimicrobial polyionenes with rapid killing kinetics, skin biocompatibility and in vivo bactericidal activity. Biomaterials 2017, 127, 36–48. [Google Scholar] [CrossRef]
- Pivcová, H.; Saudek, V.; Drobník, J.; Vlasák, J. Nmr study of poly(aspartic acid). I. α- and β-Peptide bonds in poly(aspartic acid) prepared by thermal polycondensation. Biopolymers 1981, 20, 1605–1614. [Google Scholar] [CrossRef]
- Pivcová, H.; Saudek, V.; Drobnik, H. 13C n.m.r. study of the structure of poly(aspartic acid). Polymer 1982, 23, 1237–1241. [Google Scholar] [CrossRef]
- Valgas, C.; de Souza, S.M.; Smânia, E.F.A.; Smânia, A., Jr. Screening methods to determine antibacterial activity of natural products. Braz. J. Microbiol. 2007, 38, 369–380. [Google Scholar] [CrossRef] [Green Version]
- Shadomy, S.; Epsinel, I.; Cartwright, R. Laboratory studies agents: Susceptibility test and bioassays. In Manual of Clinical Microbiology; Lennette, A., Balows, W., Hausler, H., Shadomy, S., Eds.; Little Brown Co.: Boston, MA, USA, 1985. [Google Scholar]
- Xue, Y.; Xiao, H.; Zhang, Y. Antimicrobial polymeric materials with quaternary ammonium and phosphonium salts. Int. J. Mol. Sci. 2015, 16, 3626–3655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Niu, L.; Ma, S.; Li, J.; Tay, F.R.; Chen, J. Quaternary ammonium-based biomedical materials: State-of-the-art, toxicological aspects and antimicrobial resistance. Prog. Polym. Sci. 2017, 71, 53–90. [Google Scholar] [CrossRef] [PubMed]
- Haouet, M.N.; Altissimi, M.S.; Framboas, M.; Galarini, R. Validation of the Volhard method for chloride determination in food. Accredit. Qual. Assur. 2006, 11, 23–28. [Google Scholar] [CrossRef]
- Chen, J.; Xu, L.; Han, J.; Su, M.; Wu, Q. Synthesis of modified polyaspartic acid and evaluation of its scale inhibition and dispersion capacity. Desalination 2015, 358, 42–48. [Google Scholar] [CrossRef]
- Yu, H.; Fu, Y.; Li, G.; Liu, Y. Antimicrobial surfaces of quaternized poly[(2-dimethyl amino)ethyl methacrylate] grafted on wood via ARGET ATRP. Holzforschung 2013, 67, 455–461. [Google Scholar] [CrossRef]
- Wu, S.; Teng, C.; Cai, S.; Jiang, B.; Wang, Y.; Meng, H.; Tao, H. Triphenylphosphine-based functional porous polymer as an efficient heterogeneous catalyst for the synthesis of cyclic carbonates from CO2. Nanoscale Res. Lett. 2017, 12, 609. [Google Scholar] [CrossRef]
- Ikeda, T.; Tazuke, S.; Suzuki, S. Biologically active polycations: Synthesis and antimicrobial activity of poly(trialkyl vinylbenzyl ammonium chloride)s. Makromol. Chem. 1984, 185, 869–876. [Google Scholar] [CrossRef]
- Hancock, R.E.W.; Bellido, F. Antibiotic uptake: Unusual results for unusual molecules. J. Antimicrob. Chemother. 1992, 29, 235–239. [Google Scholar] [CrossRef] [Green Version]
- Kanazawa, A.; Ikeda, T.; Endo, T. Polymeric phosphonium salts as a novel class of cationic biocides. V. Synthesis and antibacterial activity of polyesters releasing phosphonium biocides. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 3003–3011. [Google Scholar] [CrossRef]
- Kawabata, N.; Nishiguchi, M. Antibacterial activity of soluble pyridinium-type polymers. Appl. Environ. Microbiol. 1988, 54, 2532–2535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kenawy, E.-R.; Abdel-Hay, F.I.; El-Magd, A.A.; Mahmoud, Y. Biologically active polymers: VII. Synthesis and antimicrobial activity of some crosslinked copolymers with quaternary ammonium and phosphonium groups. React. Funct. Polym. 2006, 66, 419–429. [Google Scholar] [CrossRef]


| Sample Code | Carbon (%) | Hydrogen (%) | Nitrogen (%) | Halide (%) | ||||
|---|---|---|---|---|---|---|---|---|
| Calc. | Found | Calc. | Found | Calc. | Found | Calc. | Found | |
| III | 44.05 | 36.76 | 3.23 | 2.92 | 12.84 | 9.92 | - | - |
| IV | 42.85 | 38.85 | 6.25 | 7.20 | 16.66 | 10.93 | 9.72 | 7.33 |
| V | 46.87 | 39.44 | 5.46 | 5.22 | 16.40 | 10.91 | - | 0.33 |
| VI | 56.14 | 37.53 | 7.89 | 4.17 | 12.28 | 8.69 | - | - |
| VII | 66.25 | 37.44 | 5.11 | 3.77 | 5.72 | 8.84 | - | - |
| Polymer Code | Ton (°C) | T50 (°C) | Residue (at 600 °C) |
|---|---|---|---|
| III | 283 | 473 | 39 |
| VI | 206 | 431 | 38 |
| VII | 254 | 465 | 44 |
| IV | 150 | 305 | 27.9 |
| V | 140 | 326 | 29.7 |
| Tested Microorganism | Diameter (mm) of the Inhibition Zone * | ||||
|---|---|---|---|---|---|
| Control | Biocidal Polymers | ||||
| III | VI | VII | IV | V | |
| Candida albicans | 10 ± 1.0 | 12 ± 1.2 | 14 ± 1.3 | 13 ± 1.2 | 13 ± 1.1 |
| Cryptococcus neoformans | 9± 0.8 | 13 ± 1.1 | 13 ± 1.2 | 14 ± 1.3 | 12 ± 1.2 |
| Aspergillus niger | 9 ± 0.7 | 16 ± 1.4 | 21 ± 2.0 | 17 ± 1.5 | 21 ± 2.0 |
| Aspergillus flavus | 11 ± 1.0 | 18 ± 1.7 | 20 ± 1.9 | 19 ± 1.8 | 20 ± 1.8 |
| Escherichia coli | 10 ± 0.8 | 16 ± 1.5 | 19 ± 1.5 | 16 ± 1.5 | 21 ± 1.7 |
| Staphylococcus aureus | 8 ± 0.8 | 17 ± 1.6 | 18 ± 1.6 | 18 ± 1.7 | 17 ± 1.5 |
| Pseudomonas aeruginosa | 9 ± 0.6 | 12 ± 1.2 | 14 ± 1.3 | 13 ± 1.2 | 11 ± 1.0 |
| Salmonella typhi | 8 ± 0.7 | 14 ± 1.3 | 13 ± 1.2 | 11 ± 1.0 | 12 ± 1.0 |
| Tested Microorganism | MIC (mg/mL) of Biocidal Polymers | |||
|---|---|---|---|---|
| VI | VII | IV | V | |
| Aspergillus niger | 50 | 25 | 50 | 25 |
| Aspergillus flavus | ||||
| Escherichia coli | ||||
| Staphylococcus aureus | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
El-Newehy, M.H.; A., M.M.; Aldalbahi, A.K.; Thamer, B.M.; Mahmoud, Y.A.-G.; El-Hamshary, H. Biocidal Polymers: Synthesis, Characterization and Antimicrobial Activity of Bis-Quaternary Onium Salts of Poly(aspartate-co-succinimide). Polymers 2021, 13, 23. https://doi.org/10.3390/polym13010023
El-Newehy MH, A. MM, Aldalbahi AK, Thamer BM, Mahmoud YA-G, El-Hamshary H. Biocidal Polymers: Synthesis, Characterization and Antimicrobial Activity of Bis-Quaternary Onium Salts of Poly(aspartate-co-succinimide). Polymers. 2021; 13(1):23. https://doi.org/10.3390/polym13010023
Chicago/Turabian StyleEl-Newehy, Mohamed H., Meera Moydeen A., Ali K. Aldalbahi, Badr M. Thamer, Yehia A.-G. Mahmoud, and Hany El-Hamshary. 2021. "Biocidal Polymers: Synthesis, Characterization and Antimicrobial Activity of Bis-Quaternary Onium Salts of Poly(aspartate-co-succinimide)" Polymers 13, no. 1: 23. https://doi.org/10.3390/polym13010023






