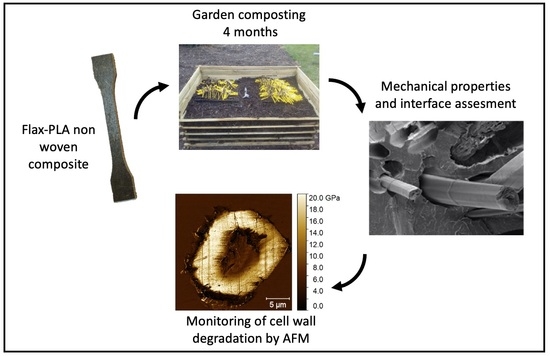
Investigations by AFM of Ageing Mechanisms in PLA-Flax Fibre Composites during Garden Composting
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Manufacturing and Composting Stage
2.3. Composite Tensile Test
2.4. Sample Preparation for AFM Study
2.5. AFM Investigations
2.6. SEM Analysis
3. Results and Discussion
3.1. Evolution of Overall Composite Microstructure with Composting Stage
3.2. Morphology of the Composite Section after Composting
3.3. Assessment of the Flax Fibre Degradation Using AFM
3.4. Degradation of PLA
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bourmaud, A.; Beaugrand, J.; Shah, D.; Placet, V.; Baley, C. Towards the design of high-performance plant fibre composites. Prog. Mater. Sci. 2018, 97, 347–408. [Google Scholar] [CrossRef]
- Pantaloni, D.; Shah, D.; Baley, C.; Bourmaud, A. Monitoring of mechanical performances of flax non-woven biocomposites during a home compost degradation. Polym. Degrad. Stab. 2020, 177, 109166. [Google Scholar] [CrossRef]
- Weng, Y.-X.; Wang, L.; Zhang, M.; Wang, X.-L.; Wang, Y.-Z. Biodegradation behavior of P(3HB,4HB)/PLA blends in real soil environments. Polym. Test. 2013, 32, 60–70. [Google Scholar] [CrossRef]
- Kim, H.-S.; Kim, H.-J.; Lee, J.-W.; Choi, I.-G. Biodegradability of bio-flour filled biodegradable poly(butylene succinate) bio-composites in natural and compost soil. Polym. Degrad. Stab. 2006, 91, 1117–1127. [Google Scholar] [CrossRef]
- Bayerl, T.; Geith, M.; Somashekar, A.A.; Bhattacharyya, D. Influence of fibre architecture on the biodegradability of FLAX/PLA composites. Int. Biodeterior. Biodegrad. 2014, 96, 18–25. [Google Scholar] [CrossRef]
- Alimuzzaman, S.; Gong, R.; Akonda, M. Biodegradability of nonwoven flax fiber reinforced polylactic acid biocomposites. Polym. Compos. 2014, 35, 2094–2102. [Google Scholar] [CrossRef]
- Arnould, O.; Siniscalco, D.; Bourmaud, A.; Le Duigou, A.; Baley, C. Better insight into the nano-mechanical properties of flax fibre cell walls. Ind. Crop. Prod. 2017, 97, 224–228. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.; Greenwood, J. An Adhesion Map for the Contact of Elastic Spheres. J. Colloid Interface Sci. 1997, 192, 326–333. [Google Scholar] [CrossRef]
- Arnould, O.; Arinero, R. Towards a better understanding of wood cell wall characterisation with contact resonance atomic force microscopy. Compos. Part A Appl. Sci. Manuf. 2015, 74, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Melelli, A.; Arnould, O.; Beaugrand, J.; Bourmaud, A. The Middle Lamella of Plant Fibers Used as Composite Reinforcement: Investigation by Atomic Force Microscopy. Molecules 2020, 25, 632. [Google Scholar] [CrossRef] [Green Version]
- Caneva, G.; Nugari, N.; Salvadori, O. La Biologia Vegetale per i Beni Culturali: Biodeterioramento e Conservazione; Nardini: Firence, Italy, 2007. [Google Scholar]
- Grizzi, I.; Garreau, H.; Li, S.; Vert, M. Hydrolytic degradation of devices based on poly(dl-lactic acid) size-dependence. Biomaterials 1995, 16, 305–311. [Google Scholar] [CrossRef]
- Blanchette, R.A. A review of microbial deterioration found in archaeological wood from different environments. Int. Biodeterior. Biodegrad. 2000, 46, 189–204. [Google Scholar] [CrossRef]
- Singh, A.P.; Kim, Y.S.; Singh, T. Chapter 9—Bacterial Degradation of Wood. In Secondary Xylem Biology; Kim, Y.S., Funada, R., Singh, A.P., Eds.; Academic Press: Boston, MA, USA, 2016; pp. 169–190. [Google Scholar] [CrossRef]
- Singh, A.; Hedley, M.; Page, D.; Han, C.; Atisongkroh, K. Microbial Degradation of Cca-Treated Cooling Tower Timbers. IAWA J. 1992, 13, 215–231. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.S.; Singh, A.P. Micromorphological Characteristics of Wood Biodegradation in Wet Environments: A Review. IAWA J. 2000, 21, 135–155. [Google Scholar] [CrossRef]
- Björdal, C.; Nilsson, T.; Bardage, S. Three-dimensional visualisation of bacterial decay in individual tracheids of Pinus sylvestris. Holzforschung 2005, 59, 178–182. [Google Scholar] [CrossRef]
- Basu, S.; Ghose, R. Structural Changes Brought About in the Jute Fiber by Micro-Organisms and their Enzymes. Text. Res. J. 1962, 32, 932–942. [Google Scholar] [CrossRef]
- Gorshkova, T.; Mikshina, P.; Petrova, A.; Chernova, T.; Mokshina, N.; Gorshkov, O. Plants at Bodybuilding: Development of Plant “Muscles”. In Plant Biomechanics from Structure to Function at Multiple Scales; Geitmann, A., Gril, J., Eds.; Springer: Cham, Switzerland, 2018; pp. 141–163. [Google Scholar] [CrossRef]
- Gorshkova, T.; Brutch, N.; Chabbert, B.; Deyholos, M.; Hayashi, T.; Lev-Yadun, S.; Mellerowicz, E.J.; Morvan, C.; Neutelings, G.; Pilate, G. Plant Fiber Formation: State of the Art, Recent and Expected Progress, and Open Questions. Crit. Rev. Plant Sci. 2012, 31, 201–228. [Google Scholar] [CrossRef]
- Xu, H.; Yang, X.; Xie, L.; Hakkarainen, M. Conformational Footprint in Hydrolysis-Induced Nanofibrillation and Crystallization of Poly(lactic acid). Biomacromolecules 2016, 17, 985–995. [Google Scholar] [CrossRef]
- Narladkar, A.; Balnois, E.; Grohens, Y. An AFM Study of Poly(L-lactic acid) and Poly(D-lactic acid) Macromolecules and Their Stereocomplexes at the Solid-Air Interface. Macromol. Symp. 2006, 241, 34–44. [Google Scholar] [CrossRef]
- Pantani, R.; Sorrentino, A. Influence of crystallinity on the biodegradation rate of injection-moulded poly(lactic acid) samples in controlled composting conditions. Polym. Degrad. Stab. 2013, 98, 1089–1096. [Google Scholar] [CrossRef]
- Soccalingame, L. Étude des Scénarios de fin de vie des Biocomposites: Vieillissement et Retransformation de Biocomposites PP/Farine De bois et PLA/Fibres de lin. Ph.D. Thesis, Université Montpellier II-Sciences et Techniques du Languedoc, Montpellier, France, 2014. [Google Scholar]
- Sawpan, M.A.; Islam, M.R.; Beg, M.D.H.; Pickering, K. Effect of Accelerated Weathering on Physico-Mechanical Properties of Polylactide Bio-Composites. J. Polym. Environ. 2019, 27, 942–955. [Google Scholar] [CrossRef]
- Müller, R.-J. Biodegradability of Polymers: Regulations and Methods for Testing. In Biopolymers Online; American Cancer Society: Atlanta, GA, USA, 2005. [Google Scholar] [CrossRef]
- Qi, X.; Ren, Y.; Wang, X. New advances in the biodegradation of Poly(lactic) acid. Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Laycock, B.; Nikolić, M.; Colwell, J.M.; Gauthier, E.; Halley, P.; Bottle, S.; George, G. Lifetime prediction of biodegradable polymers. Prog. Polym. Sci. 2017, 71, 144–189. [Google Scholar] [CrossRef] [Green Version]
- Butbunchu, N.; Pathom-Aree, W. Actinobacteria as Promising Candidate for Polylactic Acid Type Bioplastic Degradation. Front. Microbiol. 2019, 10, 2834. [Google Scholar] [CrossRef] [PubMed]
- Suyama, T.; Tokiwa, Y.; Ouichanpagdee, P.; Kanagawa, T.; Kamagata, Y. Phylogenetic Affiliation of Soil Bacteria That Degrade Aliphatic Polyesters Available Commercially as Biodegradable Plastics. Appl. Environ. Microbiol. 1998, 64, 5008–5011. [Google Scholar] [CrossRef] [Green Version]
- Saadi, Z.; Rasmont, A.; Cesar, G.; Bewa, H.; Benguigui, L. Fungal Degradation of Poly(l-lactide) in Soil and in Compost. J. Polym. Environ. 2011, 20, 273–282. [Google Scholar] [CrossRef]
- Repeèkienë, J.; Lugauskas, A.; Jankauskiene, Z. Diversity of fungal species on laid and stand-retted flax. Bot. Lith. 2007, 13, 51–59. [Google Scholar]
- Djemiel, C.; Goulas, E.; Badalato, N.; Chabbert, B.; Hawkins, S.; Grec, S. Targeted Metagenomics of Retting in Flax: The Beginning of the Quest to Harness the Secret Powers of the Microbiota. Front. Genet. 2020, 11, 581664. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melelli, A.; Pantaloni, D.; Balnois, E.; Arnould, O.; Jamme, F.; Baley, C.; Beaugrand, J.; Shah, D.U.; Bourmaud, A. Investigations by AFM of Ageing Mechanisms in PLA-Flax Fibre Composites during Garden Composting. Polymers 2021, 13, 2225. https://doi.org/10.3390/polym13142225
Melelli A, Pantaloni D, Balnois E, Arnould O, Jamme F, Baley C, Beaugrand J, Shah DU, Bourmaud A. Investigations by AFM of Ageing Mechanisms in PLA-Flax Fibre Composites during Garden Composting. Polymers. 2021; 13(14):2225. https://doi.org/10.3390/polym13142225
Chicago/Turabian StyleMelelli, Alessia, Delphin Pantaloni, Eric Balnois, Olivier Arnould, Frédéric Jamme, Christophe Baley, Johnny Beaugrand, Darshil U. Shah, and Alain Bourmaud. 2021. "Investigations by AFM of Ageing Mechanisms in PLA-Flax Fibre Composites during Garden Composting" Polymers 13, no. 14: 2225. https://doi.org/10.3390/polym13142225