Clay-Based Polymer Nanocomposites: Essential Work of Fracture
Abstract
:1. Introduction
2. Clay-Based Polymer Nanocomposites
2.1. Cation Exchange Capacity
2.2. Coupling Agents
2.3. Nanocomposite Structures
2.4. Polymer Intercalation in Solution
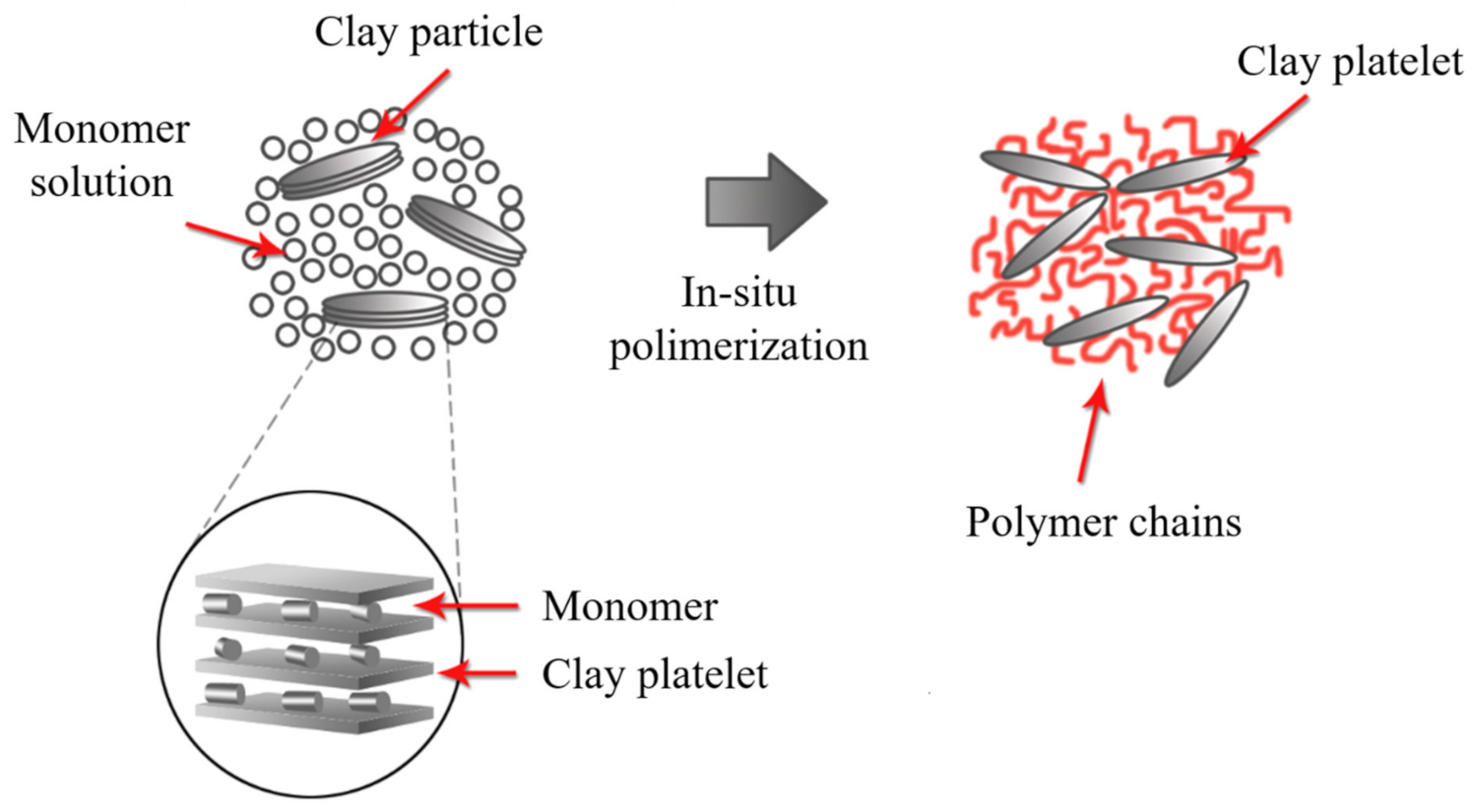
2.5. In Situ Polymerization
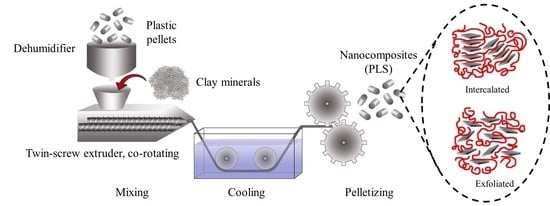
2.6. Melt Blending Process
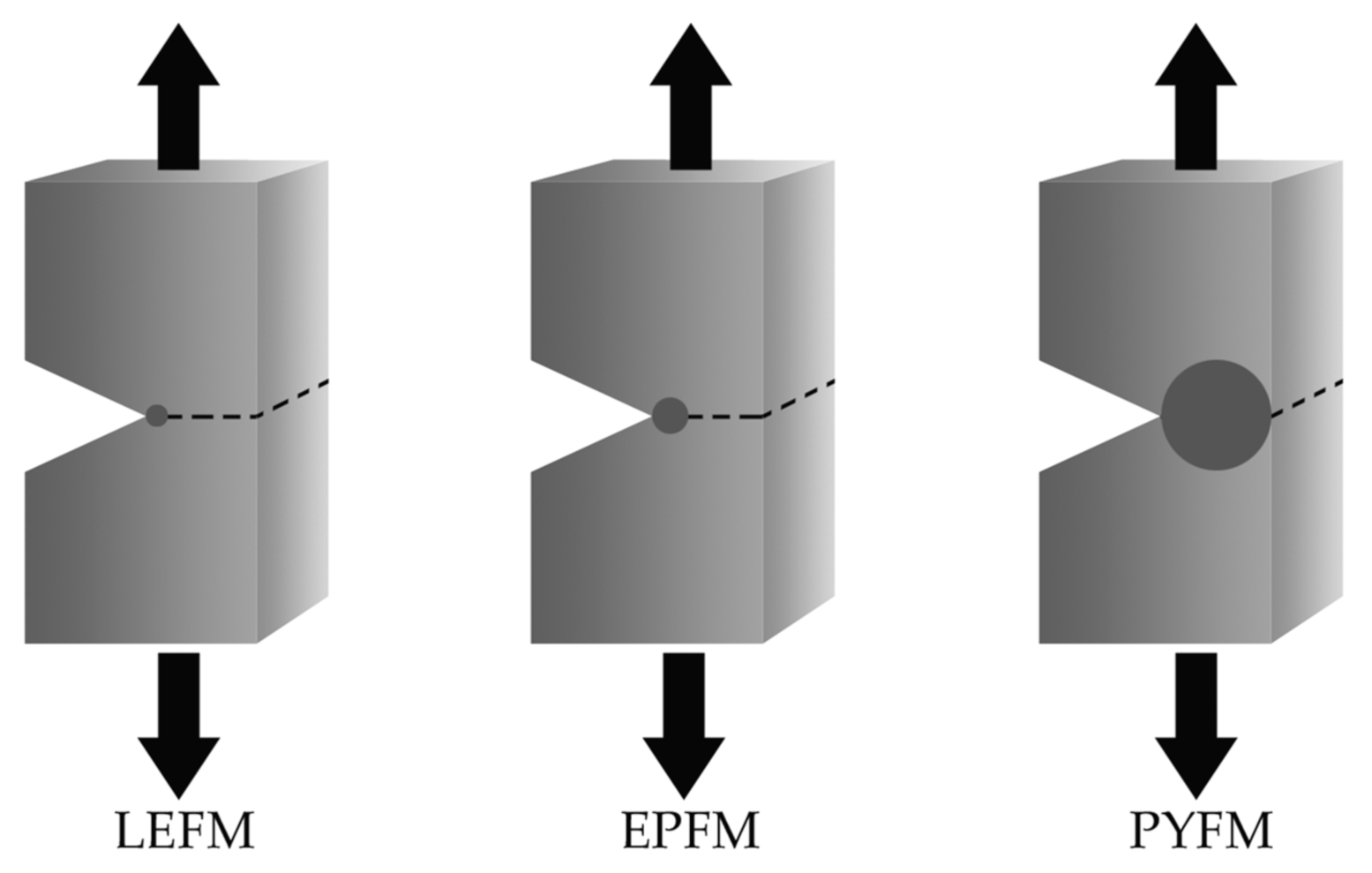
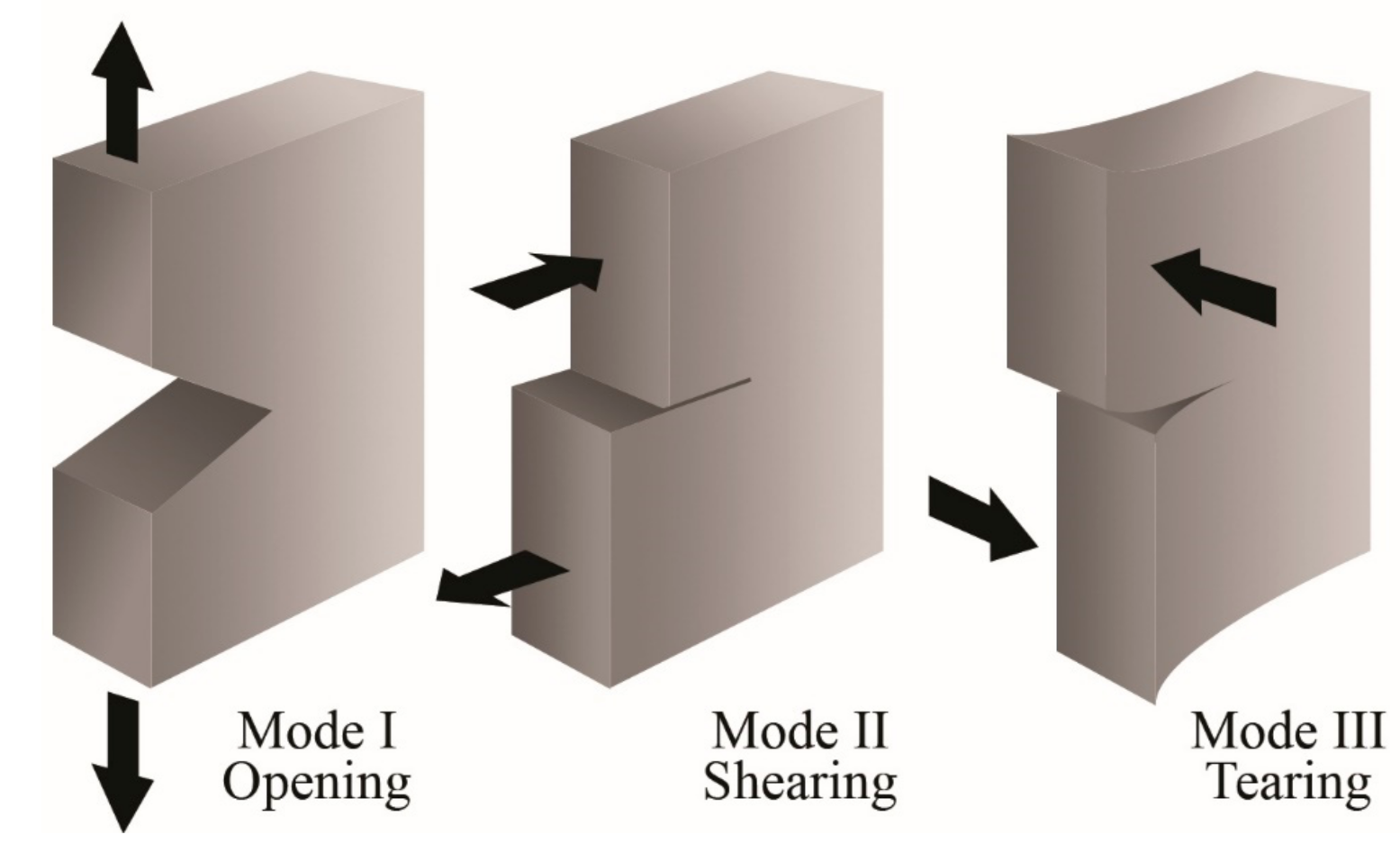
3. Fracture Mechanics
3.1. Essential Work of Fracture
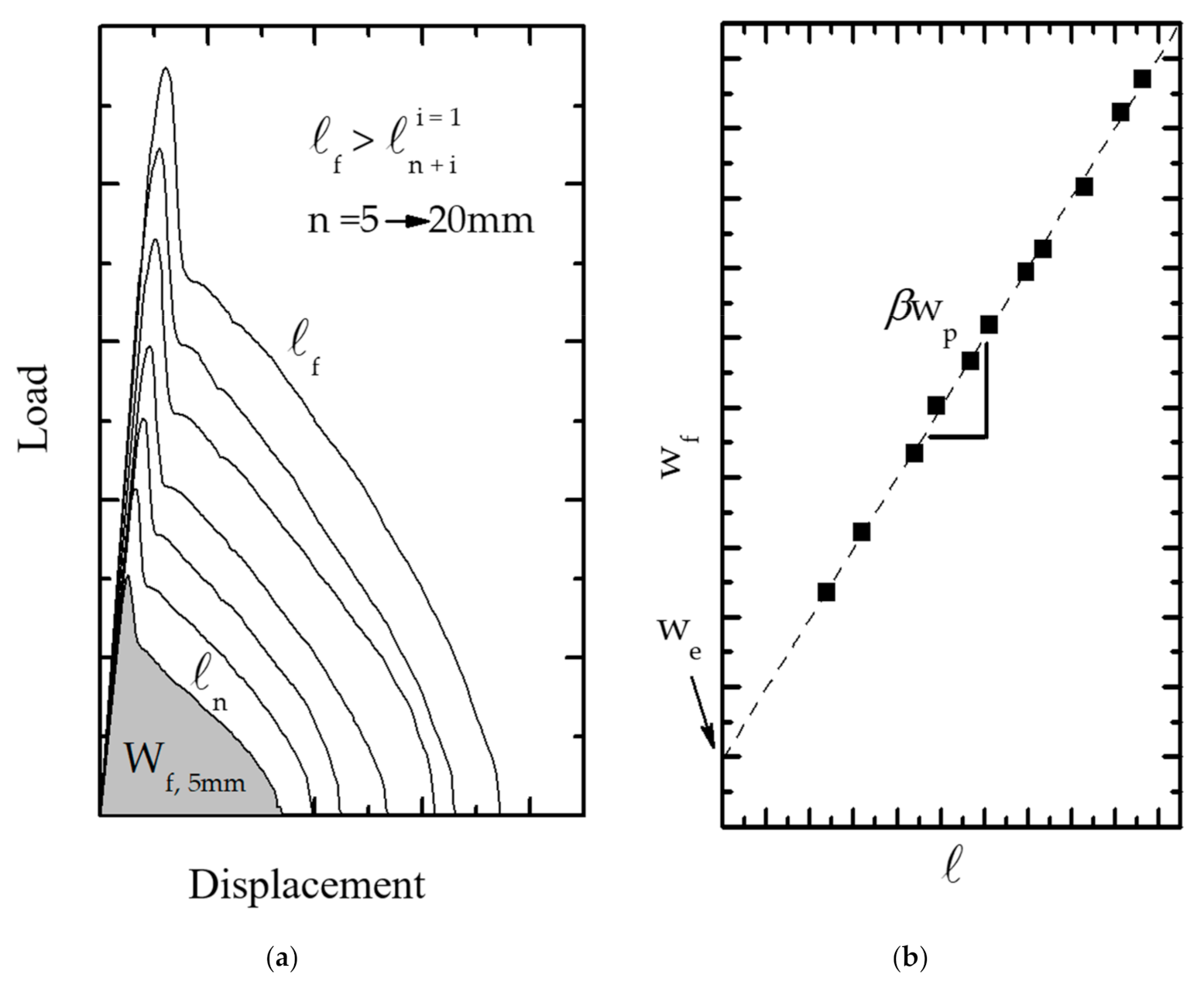
3.1.1. Experimental Considerations for the EWF Method
- Under tensile load, the full ligament yielding should be reached before the crack is initiated
- Equation (3) can be applied if the DDENT specimen is in a plane stress fracture condition, which is verified by applying Hill’s criterion [221].
- Self-similarity of the experimental load–displacement curves for each l, which supports the development of a fracture geometry common to all DDENT specimens.
- For materials with ductile fracture, the FPZ undergoes a necking process which then breaks into a fracture surface. So, the required conditions involve the specimen thickness (t), the width of DDENT specimen (W), and the plastic zone size (rp):where rp is the radius of the plastic zone, which is defined aswhere E and σy are the elastic modulus and the tensile strength, respectively.
3.1.2. Dimensions of the DDENT Specimen
3.1.3. Use of Video Extensometer
3.1.4. Energy Partitioning
3.1.5. Proposal for the New Shape of the Plastic Zone
3.2. EWF for Clay-Based Polymer Nanocomposites
4. Conclusions
- Although clay minerals are commonly used in the development of clay-based polymer nanocomposites, the montmorillonite clay is widely studied. Therefore, there is a wide field of research to explore with the rest of the phyllosilicates.
- Most clay-based polymer nanocomposites are processed by twin-screw extrusion, leaving aside the use of single-screw extruders. Although the shear stress generated by the twin-screw technology is very efficient in the exfoliation and dispersion of nanoclays (especially polyamides), it promotes the degradation of both the polymer matrix and the organic compounds contained in the modified clays. However, references about processing-induced degradation and its effect on the fracture toughness of polymer nanocomposites were not found.
- The EWF approach allows the evaluation of the fracture behavior of ductile polymers. However, most of the literature focuses on polyolefins and polyamides. One field that is currently being explored is the study of toughness in bio-based polymer nanocomposites. PLA is the most widely used material, but there are many research opportunities in evaluating the fracture behavior of green polymer nanocomposites.
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mittal, V. Polymer layered silicate nanocomposites: A review. Materials 2009, 2, 992–1057. [Google Scholar] [CrossRef] [Green Version]
- Alexandre, M.; Dubois, P. Polymer-layered silicate nanocomposites: Preparation, properties and uses of a new class of materials. Mater. Sci. Eng. R Rep. 2000, 28, 1–63. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Polymer/layered silicate nanocomposites: A review from preparation to processing. Prog. Polym. Sci. 2003, 28, 1539–1641. [Google Scholar] [CrossRef]
- Minerals, S.; Page, I.; The, S.M.; Guggenheim, S.; Martin, R.T.; Alietti, A.; Drits, V.A.; Formoso, M.L.L.; Galán, E.; Köster, H.M.; et al. Clays, nanoclays, and montmorillonite minerals. Dev. Clay Sci. 2017, 148, 255–256. [Google Scholar] [CrossRef]
- Camargo, P.H.C.; Satyanarayana, K.G.; Wypych, F. Nanocomposites: Synthesis, structure, properties and new application opportunities. Mater. Res. 2009, 12, 1–39. [Google Scholar] [CrossRef] [Green Version]
- Giannelis, E.P. Polymer layered silicate nanocomposites. Adv. Mater. 1996, 8, 29–35. [Google Scholar] [CrossRef]
- Hanemann, T.; Szabó, D.V. Polymer-Nanoparticle Composites: From Synthesis to Modern Applications. Materials 2010, 3, 3468–3517. [Google Scholar] [CrossRef]
- Paul, D.R.; Robeson, L.M. Polymer nanotechnology: Nanocomposites. Polymer 2008, 49, 3187–3204. [Google Scholar] [CrossRef] [Green Version]
- Giannelis, E.P.; Krishnamoorti, R.; Manias, E. Polymer-Silicate Nanocomposites: Model Systems for Confined Polymers and Polymer Brushes. In BT—Polymers in Confined Environments; Granick, S., Binder, K., de Gennes, P.-G., Giannelis, E.P., Grest, G.S., Hervet, H., Krishnamoorti, R., Léger, L., Manias, E., Raphaël, E., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 107–147. ISBN 978-3-540-69711-4. [Google Scholar]
- Papp, S.; Szucs, A.; Dékány, I. Colloid synthesis of monodisperse Pd nanoparticles in layered silicates. Solid State Ion. 2001, 141–142, 17169–17211. [Google Scholar] [CrossRef]
- Papp, S.; Szel, J.; Dékány, I. Stabilization of rhodium nanoparticles in an aqueous medium by polymer and layered silicates. Nanotechnology 2003, 5118, 646–656. [Google Scholar]
- Ruiz-Hitzky, E.; Aranda, P.; Darder, M. Hybrid and Biohybrid Materials Based on Layered Clays. In Tailored Organic-Inorganic Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2015; pp. 245–297. ISBN 9781118792223. [Google Scholar]
- Ouchiar, S.; Stoclet, G.; Cabaret, C.; Georges, E.; Smith, A.; Martias, C.; Addad, A.; Gloaguen, V. Comparison of the influence of talc and kaolinite as inorganic fillers on morphology, structure and thermomechanical properties of polylactide based composites. Appl. Clay Sci. 2015, 116–117. [Google Scholar] [CrossRef]
- Sleptsova, S.A.; Okhlopkova, A.A.; Kapitonova, I.V.; Lazareva, N.N.; Makarov, M.M.; Nikiforov, L.A. Spectroscopic study of tribooxidation processes in modified PTFE. J. Frict. Wear 2016, 37. [Google Scholar] [CrossRef]
- Iqbal, S.; Inam, F.; Iqbal, N.; Jamil, T.; Bashir, A.; Shahid, M. Thermogravimetric, differential scanning calorimetric, and experimental thermal transport study of functionalized nanokaolinite-doped elastomeric nanocomposites. J. Therm. Anal. Calorim. 2016, 125. [Google Scholar] [CrossRef]
- Malkappa, K.; Rao, B.N.; Jana, T. Functionalized polybutadiene diol based hydrophobic, water dispersible polyurethane nanocomposites: Role of organo-clay structure. Polymer 2016, 99. [Google Scholar] [CrossRef]
- Neto, J.C.M.; Kimura, S.P.R.; Adeodato, M.G.; Neto, J.E.; Do Nascimento, N.R.; Lona, L.M.F. Intercalation and exfoliation mechanism of kaolinite during the emulsion polymerization. Chem. Eng. Trans. 2017, 57. [Google Scholar] [CrossRef]
- Youssef, A.M.; El-Sayed, S.M. Bionanocomposites materials for food packaging applications: Concepts and future outlook. Carbohydr. Polym. 2018, 193. [Google Scholar] [CrossRef]
- Cabedo, L.; Plackett, D.; Giménez, E.; Lagarón, J.M. Studying the degradation of polyhydroxybutyrate-co-valerate during processing with clay-based nanofillers. J. Appl. Polym. Sci. 2009, 112. [Google Scholar] [CrossRef]
- Letaief, S.; Christian, D. Functionalization of the interlayer surfaces of kaolinite by alkylammonium groups from ionic liquids. Clays Clay Miner. 2009, 57. [Google Scholar] [CrossRef]
- Sun, D.; Li, Y.; Zhang, B.; Pan, X. Preparation and characterization of novel nanocomposites based on polyacrylonitrile/kaolinite. Compos. Sci. Technol. 2010, 70. [Google Scholar] [CrossRef]
- Rhim, J.W.; Park, H.M.; Ha, C.S. Bio-nanocomposites for food packaging applications. Prog. Polym. Sci. 2013, 38. [Google Scholar] [CrossRef]
- Weiss, S.; Hirsemann, D.; Biersack, B.; Ziadeh, M.; Müller, A.H.E.; Breu, J. Hybrid Janus particles based on polymer-modified kaolinite. Polymer 2013, 54. [Google Scholar] [CrossRef]
- Kirillina, I.V.; Nikiforov, L.A.; Okhlopkova, A.A.; Sleptsova, S.A.; Yoon, C.; Cho, J.H. Nanocomposites based on polytetrafluoroethylene and ultrahigh molecular weight polyethylene: A brief review. Bull. Korean Chem. Soc. 2014, 35. [Google Scholar] [CrossRef] [Green Version]
- Cui, Y.; Kumar, S.; Rao Kona, B.; Van Houcke, D. Gas barrier properties of polymer/clay nanocomposites. RSC Adv. 2015, 5. [Google Scholar] [CrossRef]
- Poikelispää, M.; Das, A.; Dierkes, W.; Vuorinen, J. The effect of coupling agents on silicate-based nanofillers/carbon black dual filler systems on the properties of a natural rubber/butadiene rubber compound. J. Elastomers Plast. 2015, 47. [Google Scholar] [CrossRef]
- Dang, W.; Lorenzelli, L.; Vinciguerra, V.; Dahiya, R. Hybrid structure of stretchable interconnect for reliable E-skin application. In Proceedings of the IEEE International Symposium on Industrial Electronics, Edinburgh, UK, 19–21 June 2017. [Google Scholar]
- Dolgopolov, K.N.; Lyubimov, D.N. Evaluation of tribological properties of components of polymer composite selflubricating materials. MATEC Web Conf. 2018, 226, 01005. [Google Scholar] [CrossRef]
- Valentin, T.M.; Dubois, E.M.; Machnicki, C.E.; Bhaskar, D.; Cui, F.R.; Wong, I.Y. 3D printed self-adhesive PEGDA-PAA hydrogels as modular components for soft actuators and microfluidics. Polym. Chem. 2019, 10. [Google Scholar] [CrossRef]
- Kotsilkova, R.; Petkova, V.; Pelovski, Y. Thermal analysis of polymer-silicate nanocomposites. J. Therm. Anal. Calorim. 2001, 64, 591–598. [Google Scholar] [CrossRef]
- Kotsilkova, R. Rheology-structure relationship of polymer/layered silicate hybrids. Mech. Time-Depend. Mater. 2002, 6. [Google Scholar] [CrossRef]
- Kiersnowski, A.; Serwadczak, M.; Kułaga, E.; Futoma-Kołoch, B.; Bugla-Płoskońska, G.; Kwiatkowski, R.; Doroszkiewicz, W.; Pigłowski, J. Delamination of montmorillonite in serum-A new approach to obtaining clay-based biofunctional hybrid materials. Appl. Clay Sci. 2009, 44. [Google Scholar] [CrossRef]
- Szczerba, M.; Środoń, J.; Skiba, M.; Derkowski, A. One-dimensional structure of exfoliated polymer-layered silicate nanocomposites: A polyvinylpyrrolidone (PVP) case study. Appl. Clay Sci. 2010, 47. [Google Scholar] [CrossRef]
- Lan, Y.F.; Lee, R.H.; Lin, J.J. Aqueous dispersion of conjugated polymers by colloidal clays and their film photoluminescence. J. Phys. Chem. B 2010, 114. [Google Scholar] [CrossRef] [PubMed]
- Tamura, K.; Yamada, H. Morphology Generation in Polymer Nanocomposites Using Various Layered Silicates. Optim. Polym. Nanocomposite Prop. 2010, 198. [Google Scholar] [CrossRef]
- Pascua, C.S.; Ohnuma, M.; Matsushita, Y.; Tamura, K.; Yamada, H.; Cuadros, J.; Ye, J. Synthesis of monodisperse Zn-smectite. Appl. Clay Sci. 2010, 48. [Google Scholar] [CrossRef]
- Chang, Y.C.; Chou, C.C.; Lin, J.J. Emulsion intercalation of smectite clays with comb-branched copolymers consisting of multiple quaternary amine salts and a poly(styrene-butadiene- styrene) backbone. Langmuir 2005, 21. [Google Scholar] [CrossRef]
- Dudkina, M.M.; Tenkovtsev, A.V.; Pospiech, D.; Jehnichen, D.; Häußler, L.; Leuteritz, A. Nanocomposites of NLO chromophore-modified layered silicates and polypropylene. J. Polym. Sci. Part B Polym. Phys. 2005, 43. [Google Scholar] [CrossRef]
- Leroux, F. Organo-modified anionic clays into polymer compared to smectite-type nanofiller: Potential applications of the nanocomposites. J. Nanosci. Nanotechnol. 2006, 6. [Google Scholar] [CrossRef]
- Wang, H.W.; Dong, R.X.; Liu, C.L.; Chang, H.Y. Effect of clay on properties of polyimide-clay nanocomposites. J. Appl. Polym. Sci. 2007, 104. [Google Scholar] [CrossRef]
- Liu, T.; Chen, B.; Evans, J.R.G. Ordered assemblies of clay nano-platelets. Bioinspir. Biomim. 2008, 3. [Google Scholar] [CrossRef]
- Darder, M.; Aranda, P.; Ruiz, A.I.; Fernandes, F.M.; Ruiz-Hitzky, E. Design and preparation of bionanocomposites based on layered solids with functional and structural properties. Mater. Sci. Technol. 2008, 24, 1100–1110. [Google Scholar] [CrossRef]
- Watanabe, H.; Matsushima, H.; Fuji, M.; Takahashi, M. Electrophoretic Deposition of Smectite Particles onto Cupper Plate. Key Eng. Mater. 2009, 412, 195–200. [Google Scholar] [CrossRef]
- Maiti, M.; Bhowmick, A.K. Synthesis and properties of new fluoroelastomer nanocomposites from tailored anionic layered magnesium silicates (hectorite). J. Appl. Polym. Sci. 2009, 111. [Google Scholar] [CrossRef]
- Suh, D.J.; Park, O.O.; Mun, J.; Yoon, C.S. Photorefractive behaviors in a polymer composite including layered silicates. Appl. Clay Sci. 2002, 21. [Google Scholar] [CrossRef]
- Wang, K.; Liang, S.; Du, R.; Zhang, Q.; Fu, Q. The interplay of thermodynamics and shear on the dispersion of polymer nanocomposite. Polymer 2004, 45. [Google Scholar] [CrossRef]
- Shi, X.; Gan, Z. Preparation and characterization of poly(propylene carbonate)/montmorillonite nanocomposites by solution intercalation. Eur. Polym. J. 2007, 43. [Google Scholar] [CrossRef]
- Dornelas, C.B.; Resende, D.K.; Tavares, M.I.B.; Gomes, A.S.; Cabral, L.M. Preparation and reactional evaluation of formation of PVP K-30 - Montmorillonite (natural and organophilic) by X ray diffraction. Polímeros 2008, 18. [Google Scholar] [CrossRef]
- Wang, C.A.; Long, B.; Lin, W.; Huang, Y.; Sun, J. Poly(amic acid)-clay nacrelike composites prepared by electrophoretic deposition. J. Mater. Res. 2008, 23. [Google Scholar] [CrossRef]
- Galimberti, M.; Martino, M.; Guenzi, M.; Leonardi, G.; Citterio, A. Thermal stability of ammonium salts as compatibilizers in polymer/layered silicate nanocomposites. E-Polymers 2009. [Google Scholar] [CrossRef] [Green Version]
- Buruiana, T.; Melinte, V.; Buruiana, E.C.; Mihai, A. Synthesis and characterization of polyurethane cationomer/MMT hybrid composite. Polym. Int. 2009, 58. [Google Scholar] [CrossRef]
- Gao, J.; Guo, N.; Liu, Y.; Li, J.; Hu, H.; Sun, L.; Zhang, X. Effect of compound technology on polyethylene/montmorillonite composites. In Proceedings of the IEEE International Conference on Properties and Applications of Dielectric Materials, Harbin, China, 19–23 July 2009. [Google Scholar]
- Soundararajah, Q.Y.; Karunaratne, B.S.B.; Rajapakse, R.M.G. Mechanical properties of poly(vinyl alcohol) montmorillonite nanocomposites. J. Compos. Mater. 2010, 44. [Google Scholar] [CrossRef]
- Chang, M.K.; Hsieh, H.H.; Li, S.J. A Study of Thermal Stability and Electromaganetic Shielding Behavior of Polyaniline-P-Toluene Sulfonic Acid/Montmorillonite Nanocomposites. Appl. Mech. Mater. 2011, 52–54, 180–185. [Google Scholar] [CrossRef]
- Monsiváis-Barrón, A.J.; Bonilla-Rios, J.; Ramos De Valle, L.F.; Palacios, E. Oxygen permeation properties of HDPE-layered silicate nanocomposites. Polym. Bull. 2013, 70. [Google Scholar] [CrossRef]
- Shiravand, F.; Hutchinson, J.M.; Calventus, Y. Influence of the isothermal cure temperature on the nanostructure and thermal properties of an epoxy layered silicate nanocomposite. Polym. Eng. Sci. 2014, 54. [Google Scholar] [CrossRef]
- Wooster, T.J.; Abrol, S.; MacFarlane, D.R. Cyanate ester polymerization catalysis by layered-silicates. Polymer 2004, 45. [Google Scholar] [CrossRef]
- Jin, X.; Hu, X.; Wang, Q.; Wang, K.; Yao, Q.; Tang, G.; Chu, P.K. Multifunctional cationic polymer decorated and drug intercalated layered silicate (NLS) for early gastric cancer prevention. Biomaterials 2014, 35. [Google Scholar] [CrossRef]
- Riaz, U.; Ashraf, S.M.; Verma, A. Influence of Conducting Polymer as Filler and Matrix on the Spectral, Morphological and Fluorescent Properties of Sonochemically Intercalated poly(o-phenylenediamine)/Montmorillonite Nanocomposites. Recent Pat. Nanotechnol. 2016, 10. [Google Scholar] [CrossRef]
- Ghosh, S.K.; Rahman, W.; Middya, T.R.; Sen, S.; Mandal, D. Improved breakdown strength and electrical energy storage performance of γ-poly(vinylidene fluoride)/unmodified montmorillonite clay nano-dielectrics. Nanotechnology 2016, 27. [Google Scholar] [CrossRef]
- Pu, W.F.; Yang, Y.; Yuan, C.D. Gelation performance of poly(ethylene imine) crosslinking polymer–layered silicate nanocomposite gel system for potential water-shutoff use in high-temperature reservoirs. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Saldābola, R.; Merijs Meri, R.; Zicans, J.; Ivanova, T.; Berzina, R. PC/ABS Nanocomposites with Layered Silicates Obtaining, Structure and Properties. Key Eng. Mater. 2016, 721, 38–42. [Google Scholar] [CrossRef]
- Hernandez-Guerrero, O.; Castillo-Pérez, R.; Hernández-Vargas, M.L.; Campillo-Illanes, B.F. Study of Thermal and Mechanical Properties of Clay/Polymer Nanocomposite Synthesized Via Modified Solution Blending. MRS Adv. 2017, 2, 2757–2762. [Google Scholar] [CrossRef]
- El-Sheikhy, R.; Al-Shamrani, M. Interfacial bond assessment of clay-polyolefin nanocomposites CPNC on view of mechanical and fracture properties. Adv. Powder Technol. 2017, 28. [Google Scholar] [CrossRef]
- İlk, S.; Şener, M.; Vural, M.; Serçe, S. Chitosan/Octadecylamine-Montmorillonite Nanocomposite Containing Nigella arvensis Extract as Improved Antimicrobial Biofilm Against Foodborne Pathogens. Bionanoscience 2018, 8. [Google Scholar] [CrossRef]
- Ashok Gandhi, R.; Jayaseelan, V.; Raghunath, B.K.; Palanikumar, K.; Ramachandran, S. Nano indendation hardness testing of PP-CNT composites. Mater. Today Proc. 2019, 16, 1372–1377. [Google Scholar] [CrossRef]
- Shuai, C.; Li, Y.; Feng, P.; Yang, W.; Zhao, Z.; Liu, W. Montmorillonite reduces crystallinity of poly-l-lactic acid scaffolds to accelerate degradation. Polym. Adv. Technol. 2019, 30. [Google Scholar] [CrossRef]
- Chou, C.C.; Lin, J.J. One-step exfoliation of montmorillonite via phase inversion of amphiphilic copolymer emulsion. Macromolecules 2005, 38. [Google Scholar] [CrossRef]
- Attia, N.F.; Nour, M.; Hassan, M.; Mohamed, G.; Oh, H.; Mahmoud, M. Effect of type of organic modifier on the clay layered-based nanocomposites flammability and toxic gases emission. J. Thermoplast. Compos. Mater. 2020. [Google Scholar] [CrossRef]
- Zubair, M.; Ullah, A. Recent advances in protein derived bionanocomposites for food packaging applications. Crit. Rev. Food Sci. Nutr. 2020, 60. [Google Scholar] [CrossRef]
- Meng, N.; Zhang, M.; Ge, M.Q.; Zhou, N.; Chi, C.; Chu, X.; Sun, B.; Gao, X. Montmorillonite-lecithin-heparin/PDMS films with enhanced mechanical and antithrombogenic properties. Polym. Compos. 2020, 41. [Google Scholar] [CrossRef]
- Sheu, Z.; Cheng, Y.B.; Simon, G.P. Sequential and simultaneous melt intercalation of poly(ethylene oxide) and poly(methyl methacrylate) into layered silicates. Macromolecules 2005, 38. [Google Scholar] [CrossRef]
- Qin, H.; Zhang, S.; Liu, H.; Xie, S.; Yang, M.; Shen, D. Photo-oxidative degradation of polypropylene/montmorillonite nanocomposites. Polymer 2005, 46. [Google Scholar] [CrossRef]
- Rehab, A.; Salahuddin, N. Nanocomposite materials based on polyurethane intercalated into montmorillonite clay. Mater. Sci. Eng. A 2005, 399. [Google Scholar] [CrossRef]
- Hao, X.; Gai, G.; Liu, J.; Yang, Y.; Zhang, Y.; Nan, C.W. Flame retardancy and antidripping effect of OMT/PA nanocomposites. Mater. Chem. Phys. 2006, 96. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, B.; Gong, W.; Kong, L.; Jia, Q. Investigation of the hydrogen-bonding structure and miscibility for PU/EP IPN nanecomposites by PALS. Macromolecules 2006, 39. [Google Scholar] [CrossRef]
- Román, F.; Montserrat, S.; Hutchinson, J.M. On the effect of montmorillonite in the curing reaction of epoxy nanocomposites. J. Therm. Anal. Calorim. 2007, 87, 113–118. [Google Scholar] [CrossRef]
- Vasilev, A.P.; Struchkova, T.S.; Nikiforov, L.A.; Okhlopkova, A.A.; Grakovich, P.N.; Shim, E.L.; Cho, J.H. Mechanical and tribological properties of polytetrafluoroethylene composites with carbon fiber and layered silicate fillers. Molecules 2019, 24, 224. [Google Scholar] [CrossRef] [Green Version]
- Wan, Y.; Fan, Y.; Dan, J.; Hong, C.; Yang, S.; Yu, F. A review of recent advances in two-dimensional natural clay vermiculite-based nanomaterials. Mater. Res. Express 2019, 6. [Google Scholar] [CrossRef]
- Leont’ev, L.B.; Shapkin, N.P.; Leont’ev, A.L. Effect of the Chemical Composition and Structural Characteristics of Vermiculite-Based Tribotechnical Materials on the Operating Ability of the Coatings Formed. J. Mach. Manuf. Reliab. 2020, 49. [Google Scholar] [CrossRef]
- Mittal, V. High CEC generation and surface modification in mica and vermiculite minerals. Philos. Mag. 2013, 93. [Google Scholar] [CrossRef]
- Sleptsova, S.A.; Afanas’eva, E.S.; Grigor’eva, V.P. Structure and tribological behavior of polytetrafluoroethylene modified with layered silicates. J. Frict. Wear 2009, 30. [Google Scholar] [CrossRef]
- Mehrotra, V.; Giannelis, E.P. Metal-insulator molecular multilayers of electroactive polymers: Intercalation of polyaniline in mica-type layered silicates. Solid State Commun. 1991, 77. [Google Scholar] [CrossRef]
- Gaylarde, P.; Gaylarde, C. Deterioration of siliceous stone monuments in Latin America: Microorganisms and mechanisms. Corros. Rev. 2004, 22. [Google Scholar] [CrossRef]
- Heinz, H.; Koerner, H.; Anderson, K.L.; Vaia, R.A.; Farmer, B.L. Force field for mica-type silicates and dynamics of octadecylammonium chains grafted to montmorillonite. Chem. Mater. 2005, 17. [Google Scholar] [CrossRef]
- Zhang, X.; Lin, G.; Abou-Hussein, R.; Hassan, M.K.; Noda, I.; Mark, J.E. Some novel layered-silicate nanocomposites based on a biodegradable hydroxybutyrate copolymer. Eur. Polym. J. 2007, 43. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Su, Q.S.; Yu, L.; Liao, L.B.; Zheng, H.; Huang, H.T.; Zhang, G.G.; Yao, Y.B.; Lau, C.; Chan, H.L.W. Preparation of Low-K Fluorinated Polyimide/Phlogopite Nanocomposites. Adv. Mater. Res. 2008, 47–50, 987–990. [Google Scholar] [CrossRef]
- Bae, S.H.; Yoo, S.I.; Bae, W.K.; Lee, S.; Lee, J.K.; Sohn, B.H. Single-layered films of diblock copolymer micelles containing quantum dots and fluorescent dyes and their fluorescence resonance energy transfer. Chem. Mater. 2008, 20. [Google Scholar] [CrossRef]
- Miwa, Y.; Drews, A.R.; Schlick, S. Unique structure and dynamics of poly(ethylene oxide) in layered silicate nanocomposites: Accelerated segmental mobility revealed by simulating ESR spectra of spin-labels, XRD, FTIR, and DSC. Macromolecules 2008, 41. [Google Scholar] [CrossRef]
- Fujii, K.; Ishihama, Y.; Sakuragi, T.; Ohshima, M.A.; Kurokawa, H.; Miura, H. Heterogeneous catalysts immobilizing α-diimine nickel complexes into fluorotetrasilicic mica interlayers to prepare branched polyethylene from only ethylene. Catal. Commun. 2008, 10. [Google Scholar] [CrossRef]
- Pacheco-Torgal, F.; Castro-Gomes, J.; Jalali, S. Tungsten mine waste geopolymeric binder: Preliminary hydration products investigations. Constr. Build. Mater. 2009, 23. [Google Scholar] [CrossRef] [Green Version]
- Cheng, H.Y.; Jiang, G.J.; Hung, J.Y. Enhanced mechanical and thermal properties of PS/mica and PMMA/mica nanocomposites by emulsion polymerization. Polym. Compos. 2009, 30. [Google Scholar] [CrossRef]
- Tamura, K.; Uno, H.; Yamada, H.; Umeyama, K. Layered silicate-polyamide-6 nanocomposites: Influence of silicate species on morphology and properties. J. Polym. Sci. Part B Polym. Phys. 2009, 47. [Google Scholar] [CrossRef]
- Herzog, E.; Caseri, W.; Suter, U.W. Adsorption of polymers with crown ether substituents on muscovite mica. Colloid Polym. Sci. 1994, 272. [Google Scholar] [CrossRef]
- Möller, M.W.; Handge, U.A.; Kunz, D.A.; Lunkenbein, T.; Altstädt, V.; Breu, J. Tailoring shear-stiff, mica-like nanoplatelets. ICS Nano 2010, 4, 717–724. [Google Scholar] [CrossRef]
- Lin, J.J.; Chan, Y.N.; Lan, Y.F. Hydrophobic modification of layered clays and compatibility for epoxy nanocomposites. Materials 2010, 3, 2588–2605. [Google Scholar] [CrossRef] [Green Version]
- Manias, E.; Heidecker, M.J.; Nakajima, H.; Costache, M.C.; Wilkie, C.A. Poly(ethylene terephthalate) nanocomposites using nanoclays modified with thermally stable surfactants. Therm. Stable Flame Retard. Polym. Nanocompos. 2011, 9780521190, 100. [Google Scholar]
- Schütz, M.R.; Kalo, H.; Lunkenbein, T.; Gröschel, A.H.; Müller, A.H.E.; Wilkie, C.A.; Breu, J. Shear stiff, surface modified, mica-like nanoplatelets: A novel filler for polymer nanocomposites. J. Mater. Chem. 2011, 21. [Google Scholar] [CrossRef]
- Fu, Y.T.; Zartman, G.D.; Yoonessi, M.; Drummy, L.F.; Heinz, H. Bending of layered silicates on the nanometer scale: Mechanism, stored energy, and curvature limits. J. Phys. Chem. C 2011, 115. [Google Scholar] [CrossRef]
- Ahmad Rasyid, M.F.; Hazizan, M.A.; Sharif, J.M. Influence of Organo-Clay on Mechanical and Thermal Properties of O-Muscovite/PP Layered Silicate Nanocomposite. Adv. Mater. Res. 2011, 364, 174–180. [Google Scholar] [CrossRef]
- Xu, X.; Ding, H.; Wang, Y.B.; Liang, Y.; Jiang, W. Preparation and Characterization of Activated Sericite Modified by Fluorosilicate. Adv. Mater. Res. 2012, 427, 70–76. [Google Scholar] [CrossRef]
- Wang, X.Y.; Liu, B.; Tang, Y.F.; Su, H.J.; Han, Y.; Sun, R.C. New progress on rectorite/polymer nanocomposites. Wuji Cailiao Xuebao J. Inorg. Mater. 2012, 27. [Google Scholar] [CrossRef]
- Kudus, M.H.A.; Akil, H.M.; Rasyid, M.F.A. Muscovite-MWCNT hybrid as a potential filler for layered silicate nanocomposite. Mater. Lett. 2012, 79. [Google Scholar] [CrossRef]
- Mittal, V. Surface modification of layered silicates. II. Factors affecting thermal stability. Philos. Mag. 2012, 92. [Google Scholar] [CrossRef]
- Olson, B.G.; Peng, Z.L.; Srithawatpong, R.; McGervey, J.D.; Ishida, H.; Jamieson, A.M.; Manias, E.; Giannelis, E.P. Free volume in layered organosilicate-polystyrene nanocomposites. Mater. Sci. Forum 1997, 255–257. [Google Scholar] [CrossRef]
- Livi, S.; Duchet-Rumeau, J.; Gérard, J.F. Effect of ionic liquid modified synthetic layered silicates on thermal and mechanical properties of high density polyethylene nanocomposites. Macromol. Symp. 2014, 342. [Google Scholar] [CrossRef] [Green Version]
- Omar, M.F.; Abd Wahab, N.S.; Akil, H.M.; Ahmad, Z.A.; Rasyid, M.F.A.; Noriman, N.Z. Effect of Surface Modification on Strain Rate Sensitivity of Polypropylene/Muscovite Layered Silicate Composites. Mater. Sci. Forum 2014, 803, 343–347. [Google Scholar] [CrossRef]
- Omar, M.F.; Jaya, H.; Akil, H.M.; Ahmad, Z.A.; Rasyid, M.F.A.; Noriman, N.Z. Effect of Organic Modification on Dynamic Compression Properties of Polypropylene/Muscovite Layered Silicate Composites. Mater. Sci. Forum 2014, 803, 282–287. [Google Scholar] [CrossRef]
- Ding, J.; Huang, Z.; Luo, H.; Qin, Y.; Shi, M. The role of microcrystalline muscovite to enhance thermal stability of boron-modified phenolic resin, structural and elemental studies in boron-modified phenolic resin/ microcrystalline muscovite composite. Mater. Res. Innov. 2015, 19. [Google Scholar] [CrossRef]
- Kovalevsky, V.; Shchiptsov, V.; Sadovnichy, R. Unique natural carbon deposits of shungite rocks of Zazhogino Ore Field, Republic of Karelia, Russia. In Proceedings of the International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management, SGEM, Albena, 30 June–6 July 2016; Volume 1. [Google Scholar]
- Xia, L.; Wu, H.; Guo, S.; Sun, X.; Liang, W. Enhanced sound insulation and mechanical properties of LDPE/mica composites through multilayered distribution and orientation of the mica. Compos. Part A Appl. Sci. Manuf. 2016, 81. [Google Scholar] [CrossRef]
- Zhang, X.; Yuan, L.; Guan, Q.; Liang, G.; Gu, A. Greatly improving energy storage density and reducing dielectric loss of carbon nanotube/cyanate ester composites through building a unique tri-layered structure with mica paper. J. Mater. Chem. A 2017, 5. [Google Scholar] [CrossRef]
- Michal, O.; Mentlik, V. Influence of Thermal Degradation on the Dielectric Properties of Polymer Composites. In Proceedings of the International Conference on Diagnostics in Electrical Engineering, Diagnostika, Pilsen, Czech Republic, 4–7 September 2018; pp. 1–5. [Google Scholar]
- Bae, H.J.; Goh, Y.; Yim, H.; Yoo, S.Y.; Choi, J.W.; Kwon, D.K. Atomically thin, large area aluminosilicate nanosheets fabricated from layered clay minerals. Mater. Chem. Phys. 2019, 221. [Google Scholar] [CrossRef]
- Lü, R.; Wang, Y.; Wang, J.; Ren, W.; Li, L.; Liu, S.; Chen, Z.; Li, Y.; Wang, H.; Fu, F. Soliton and bound-state soliton mode-locked fiber laser based on a MoS 2 /fluorine mica Langmuir–Blodgett film saturable absorber. Photonics Res. 2019, 7. [Google Scholar] [CrossRef]
- Geke, M.O.; Shelden, R.A.; Caseri, W.R.; Suter, U.W. Ion exchange of cation-terminated poly(ethylene oxide) chains on mica surfaces. J. Colloid Interface Sci. 1997, 189. [Google Scholar] [CrossRef]
- Mohammadi, H.; Moghbeli, M.R. Effect of ethylene-1-butene copolymer on tensile properties and toughness of polypropylene/mica/organoclay hybrid nanocomposites. J. Vinyl Addit. Technol. 2019, 25. [Google Scholar] [CrossRef]
- Fu, Y.; Wang, Y.; Wang, S.; Gao, Z.; Xiong, C. Enhanced breakdown strength and energy storage of PVDF-based dielectric composites by incorporating exfoliated mica nanosheets. Polym. Compos. 2019, 40. [Google Scholar] [CrossRef]
- Mohammadi, H.; Moghbeli, M.R. Polypropylene/organically modified-grafted mica/organoclay hybrid nanocomposites: Preparation, characterization, and mechanical properties. Polym. Compos. 2019, 40. [Google Scholar] [CrossRef]
- Wang, B.; Tang, M.; Wu, Y.; Chen, Y.; Jiang, C.; Zhuo, S.; Zhu, S.; Wang, C. A 2D Layered Natural Ore as a Novel Solid-State Electrolyte. ACS Appl. Energy Mater. 2019, 2. [Google Scholar] [CrossRef]
- Kuznetsov, V.; Ottermann, K.; Helfricht, N.; Kunz, D.; Loch, P.; Kalo, H.; Breu, J.; Papastavrou, G. Surface charge density and diffuse layer properties of highly defined 2:1 layered silicate platelets. Colloid Polym. Sci. 2020, 298. [Google Scholar] [CrossRef]
- Tani, M.; Fukushima, Y. Properties of organic/inorganic hybrid clay-like polymers with epoxy groups. Kobunshi Ronbunshu 2002, 59. [Google Scholar] [CrossRef] [Green Version]
- Imai, Y.; Nishimura, S.; Abe, E.; Tateyama, H.; Abiko, A.; Yamaguchi, A.; Aoyama, T.; Taguchi, H. High-modulus poly(ethylene terephthalate)/expandable fluorine mica nanocomposites with a novel reactive compatibilizer. Chem. Mater. 2002, 14. [Google Scholar] [CrossRef]
- Maiti, P.; Yamada, K.; Okamoto, M.; Ueda, K.; Okamoto, K. New polylactide/layered silicate nanocomposites: Role of organoclays. Chem. Mater. 2002, 14. [Google Scholar] [CrossRef]
- McNally, T.; Murphy, W.R.; Lew, C.Y.; Turner, R.J.; Brennan, G.P. Polyamide-12 layered silicate nanocomposites by melt blending. Polymer 2003, 44. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Yamada, K.; Okamoto, M.; Ogami, A.; Ueda, K. New polylactide/layered silicate nanocomposites. 3. High-performance biodegradable materials. Chem. Mater. 2003, 15. [Google Scholar] [CrossRef]
- Bokobza, L. Elastomeric composites. I. Silicone composites. J. Appl. Polym. Sci. 2004, 93. [Google Scholar] [CrossRef]
- Zhang, D.; Zhou, C.H.; Lin, C.X.; Tong, D.S.; Yu, W.H. Synthesis of clay minerals. Appl. Clay Sci. 2010, 50. [Google Scholar] [CrossRef]
- Fukushima, K.; Wu, M.H.; Bocchini, S.; Rasyida, A.; Yang, M.C. PBAT based nanocomposites for medical and industrial applications. Mater. Sci. Eng. C 2012, 32. [Google Scholar] [CrossRef]
- Fukushima, K.; Tabuani, D.; Camino, G. Poly(lactic acid)/clay nanocomposites: Effect of nature and content of clay on morphology, thermal and thermo-mechanical properties. Mater. Sci. Eng. C 2012, 32. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Wicklein, B.; Fernandes, F.M.; Castro-Smirnov, F.A.; Martín del Burgo, M.A.; del Real, G.; Aranda, P. Advanced biohybrid materials based on nanoclays for biomedical applications. In Proceedings of the Nanosystems in Engineering and Medicine; Choi, S.H., Choy, J.-H., Lee, U., Varadan, V.K., Eds.; SPIE: Incheon, Korean, 2012; Volume 8548, p. 85480D. [Google Scholar]
- Vahabi, H.; Sonnier, R.; Otazaghine, B.; Le Saout, G.; Lopez-Cuesta, J.M. Nanocomposites of polypropylene/polyamide 6 blends based on three different nanoclays: Thermal stability and flame retardancy. Polim. Polym. 2013, 58. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Aranda, P. Novel architectures in porous materials based on clas. J. Sol-Gel Sci. Technol. 2014, 70, 307–316. [Google Scholar] [CrossRef]
- Ruiz-Hitzky, E.; Darder, M.; Alcântara, A.C.S.; Wicklein, B.; Aranda, P. Recent advances on fibrous clay-based nanocomposites. Adv. Polym. Sci. 2014, 267. [Google Scholar] [CrossRef]
- Frindy, S.; Primo, A.; Qaiss, A.E.K.; Bouhfid, R.; Lahcini, M.; Garcia, H.; Bousmina, M.; El Kadib, A. Insightful understanding of the role of clay topology on the stability of biomimetic hybrid chitosan-clay thin films and CO2-dried porous aerogel microspheres. Carbohydr. Polym. 2016, 146. [Google Scholar] [CrossRef]
- Beyer, G. Nanocomposites: A new class of flame retardants for polymers. Plast. Addit. Compd. 2002, 4, 22–28. [Google Scholar] [CrossRef]
- Kojima, Y.; Usuki, A.; Kawasumi, M.; Okada, A.; Kurauchi, T.; Kamigaito, O. Synthesis of nylon 6–clay hybrid by montmorillonite intercalated with ϵ-caprolactam. J. Polym. Sci. Part A Polym. Chem. 1993, 31, 983–986. [Google Scholar] [CrossRef]
- Jordan, J.W. Organophilic Bentonites. I. Swelling in Organic Liquids. J. Phys. Colloid Chem. 1949, 53, 294–306. [Google Scholar] [CrossRef]
- Weiss, A. Organic Derivatives of Mica-type Layer-Silicates. Angew. Chem. Int. Ed. Engl. 1963, 2, 134–144. [Google Scholar] [CrossRef]
- Lan, T.; Kaviratna, P.D.; Pinnavaia, T.J. Mechanism of Clay Tactoid Exfoliation in Epoxy-Clay Nanocomposites. Chem. Mater. 1995, 7, 2144–2150. [Google Scholar] [CrossRef]
- Vaia, R.A.; Teukolsky, R.K.; Giannelis, E.P. Interlayer Structure and Molecular Environment of Alkylammonium Layered Silicates. Chem. Mater. 1994, 6, 1017–1022. [Google Scholar] [CrossRef]
- Lagaly, G. Interaction of alkylamines with different types of layered compounds. Solid State Ion. 1986, 22, 43–51. [Google Scholar] [CrossRef]
- Zanetti, M.; Lomakin, S.; Camino, G. Polymer layered silicate nanocomposites. Macromol. Mater. Eng. 2000, 279, 1–9. [Google Scholar] [CrossRef]
- Unalan, I.U.; Cerri, G.; Marcuzzo, E.; Cozzolino, C.A.; Farris, S. Nanocomposite films and coatings using inorganic nanobuilding blocks (NBB): Current applications and future opportunities in the food packaging sector. RSC Adv. 2014, 4, 29393–29428. [Google Scholar] [CrossRef] [Green Version]
- Mrah, L.; Meghabar, R. In situ polymerization of styrene–clay nanocomposites and their properties. Polym. Bull. 2020. [Google Scholar] [CrossRef]
- Ekielski, A.; Żelaziński, T.; Durczak, K. The use of wavelet analysis to assess the degree of wear of working elements of food extruders. Eksploat. Niezawodn. Maint. Reliab. 2017, 19, 560–564. [Google Scholar] [CrossRef]
- Mościcki, L.; van Zuilichem, D.J. Extrusion-Cooking and Related Technique. In Extrusion-Cooking Techniques; Wiley Online Books; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011; pp. 1–24. ISBN 9783527634088. [Google Scholar]
- Vaia, R.A.; Giannelis, E.P. Lattice Model of Polymer Melt Intercalation in Organically-Modified Layered Silicates. Macromolecules 1997, 30, 7990–7999. [Google Scholar] [CrossRef]
- Chavarria, F.; Shah, R.K.; Hunter, D.L.; Paul, D.R. Effect of melt processing conditions on the morphology and properties of nylon 6 nanocomposites. Polym. Eng. Sci. 2007, 47, 1847–1864. [Google Scholar] [CrossRef]
- Dennis, H.R.; Hunter, D.L.; Chang, D.; Kim, S.; White, J.L.; Cho, J.W.; Paul, D.R. Effect of melt processing conditions on the extent of exfoliation in organoclay-based nanocomposites. Polymer 2001, 42, 9513–9522. [Google Scholar] [CrossRef]
- Fornes, T.D.; Paul, D.R. Modeling properties of nylon 6/clay nanocomposites using composite theories. Polymer 2003, 44, 4993–5013. [Google Scholar] [CrossRef]
- Lertwimolnun, W.; Vergnes, B. Influence of screw profile and extrusion conditions on the microstructure of polypropylene/organoclay nanocomposites. Polym. Eng. Sci. 2007, 47, 2100–2109. [Google Scholar] [CrossRef]
- Fornes, T.; Yoon, P.; Hunter, D.; Keskkula, H.; Paul, D. Effect of organoclay structure on nylon 6 nanocomposite morphology and properties. Polymer 2002, 43, 5915–5933. [Google Scholar] [CrossRef]
- Fornes, T.D.; Yoon, P.J.; Keskkula, H.; Paul, D.R. Nylon 6 nanocomposites: The effect of matrix molecular weight. Polymer 2001, 42, 9929–9940. [Google Scholar] [CrossRef]
- Cabedo, L.; Villanueva, M.P.; Lagarón, J.M.; Giménez, E. Development and characterization of unmodified kaolinite/EVOH nanocomposites by melt compounding. Appl. Clay Sci. 2017, 135, 300–306. [Google Scholar] [CrossRef] [Green Version]
- Chavarria, F.; Paul, D.R. Comparison of nanocomposites based on nylon 6 and nylon 66. Polymer 2004, 45, 8501–8515. [Google Scholar] [CrossRef]
- Wang, Z.; Pinnavaia, T.J. Hybrid Organic-Inorganic Nanocomposites: Exfoliation of Magadiite Nanolayers in an Elastomeric Epoxy Polymer. Chem. Mater. 1998, 10. [Google Scholar] [CrossRef]
- Ganguly, A.; De Sarkar, M.; Bhowmick, A.K. Thermoplastic elastomeric nanocomposites from poly[styrene-(ethylene-co- butylene)-styrene] triblock copolymer and clay: Preparation and characterization. J. Appl. Polym. Sci. 2006, 100. [Google Scholar] [CrossRef]
- Zhu, L.; Wool, R.P. Nanoclay reinforced bio-based elastomers: Synthesis and characterization. Polymer 2006, 47. [Google Scholar] [CrossRef]
- Százdi, L.; Pozsgay, A.; Pukánszky, B. Factors and processes influencing the reinforcing effect of layered silicates in polymer nanocomposites. Eur. Polym. J. 2007, 43. [Google Scholar] [CrossRef]
- Rehab, A.; Akelah, A.; Agag, T.; Shalaby, N. Polyurethane-nanocomposite materials via in situ polymerization into organoclay interlayers. Polym. Adv. Technol. 2007, 18. [Google Scholar] [CrossRef]
- Chu, D.; Nguyen, Q.; Baird, D.G. Effect of matrix molecular weight on the dispersion of nanoclay in unmodified high density polyethylene. Polym. Compos. 2007, 28. [Google Scholar] [CrossRef] [Green Version]
- Wilkinson, A.N.; Man, Z.; Stanford, J.L.; Matikainen, P.; Clemens, M.L.; Lees, G.C.; Liauw, C.M. Tensile properties of melt intercalated polyamide 6-Montmorillonite nanocomposites. Compos. Sci. Technol. 2007, 67. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, M.; Dana, K.; Ghatak, S.; Banerjee, A. Polypropylene-clay composite prepared from Indian bentonite. Bull. Mater. Sci. 2008, 31. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D.; Pfaendner, R. Polyamide 6 filled with melamine cyanurate and layered silicates: Evaluation of flame retardancy and physical properties. Macromol. Mater. Eng. 2008, 293. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, W.; Han, W.; Guo, W.; Wu, C. Preparation and properties of novel natural rubber/ organo-vermiculite nanocomposites. Polym. Compos. 2009, 30. [Google Scholar] [CrossRef]
- Schmidt, D.F.; Giannelis, E.P. Silicate dispersion and mechanical reinforcement in polysiloxane/layered silicate nanocomposites. Chem. Mater. 2010, 22. [Google Scholar] [CrossRef]
- Lebaron, P.C.; Wang, Z.; Pinnavaia, T.J. Polymer-layered silicate nanocomposites: An overview. Appl. Clay Sci. 1999, 15. [Google Scholar] [CrossRef]
- Zhu, L.; Wool, R.P. Bio-based elastomers from soy oil and nanoclay. In Nanocomposites with Biodegradable Polymers; Oxford University Press: Oxford, UK, 2011; Volume 9780199581, pp. 189–208. [Google Scholar]
- Lipinska, M.; Hutchinson, J.M. Elastomeric epoxy nanocomposites: Nanostructure and properties. Compos. Sci. Technol. 2012, 72. [Google Scholar] [CrossRef]
- Marega, C.; Causin, V.; Saini, R.; Marigo, A.; Meera, A.P.; Thomas, S.; Devi, K.S.U. A direct SAXS approach for the determination of specific surface area of clay in polymer-layered silicate nanocomposites. J. Phys. Chem. B 2012, 116. [Google Scholar] [CrossRef]
- Mallakpour, S.; Dinari, M. The effects of reactive organoclay on the thermal, mechanical, and microstructural properties of polymer/layered silicate nanocomposites based on chiral poly(amide-imide)s. J. Therm. Anal. Calorim. 2013, 114. [Google Scholar] [CrossRef]
- Stojšic, J.; Raos, P.; Kalendova, A. A study of structure and tensile properties of polyamide 12/clay nanocomposites. Polym. Compos. 2016, 37. [Google Scholar] [CrossRef]
- Ghanbari, A.; Heuzey, M.C.; Carreau, P.J.; Ton-That, M.T. Morphology and gas barrier properties of polymer nanocomposites. Polym. Morphol. Princ. Charact. Process. 2016, 394–417. [Google Scholar] [CrossRef]
- Rahman, M.R.; Hamdan, S.B.; Hossen, M.F. The effect of clay dispersion on polypropylene nanocomposites: Physico-mechanical, thermal, morphological, and optical properties. In Silica and Clay Dispersed Polymer Nanocomposites; Elsevier: Cambridge, UK, 2018; pp. 201–257. [Google Scholar]
- Msekh, M.A.; Cuong, N.H.; Zi, G.; Areias, P.; Zhuang, X.; Rabczuk, T. Fracture properties prediction of clay/epoxy nanocomposites with interphase zones using a phase field model. Eng. Fract. Mech. 2018, 188. [Google Scholar] [CrossRef]
- Rauschendorfer, J.E.; Thien, K.M.; Denz, M.; Köster, S.; Ehlers, F.; Vana, P. Tuning the Mechanical Properties of Poly(Methyl Acrylate) via Surface-Functionalized Montmorillonite Nanosheets. Macromol. Mater. Eng. 2021, 306. [Google Scholar] [CrossRef]
- Chang, M.-K.; Wei, H.-L.; Wu, K.-S. The Strength and Thermal Stability of Low-Density Polyethylene Grafted Maleic Anhydride/Montmorillonite Nanocomposites. Adv. Sci. Lett. 2012, 13, 240–244. [Google Scholar] [CrossRef]
- Kuchta, F.D.; Lemstra, P.J.; Keller, A.; Batenburg, L.F.; Fischer, H.R. Polymer crystallization studied in confined dimensions using nanocomposites from polymers and layered minerals. Mater. Res. Soc. Symp. Proc. 2000, 628. [Google Scholar] [CrossRef]
- Filippone, G.; Dintcheva, N.T.; Acierno, D.; La Mantia, F.P. The role of organoclay in promoting co-continuous morphology in high-density poly(ethylene)/poly(amide) 6 blends. Polymer 2008, 49, 1312–1322. [Google Scholar] [CrossRef]
- Tillekeratne, M.; Jollands, M.; Cser, F.; Bhattacharya, S.N. Role of mixing parameters in the preparation of poly(ethylene vinyl acetate) nanocomposites by melt blending. J. Appl. Polym. Sci. 2006, 100, 2652–2658. [Google Scholar] [CrossRef]
- Liao, B.; Song, M.; Liang, H.; Pang, Y. Polymer-layered silicate nanocomposites. 1. A study of poly(ethylene oxide)/Na+-montmorillonite nanocomposites as polyelectrolytes and polyethylene-block-poly(ethylene glycol) copolymer/Na+-montmorillonite nanocomposites as fillers for reinforcement of po. Polymer 2001, 42. [Google Scholar] [CrossRef]
- Lim, S.K.; Lim, S.T.; Kim, H.B.; Chin, I.; Choi, H.J. Preparation and Physical Characterization of Polyepichlorohydrin Elastomer/Clay Nanocomposites. J. Macromol. Sci. Phys. 2003, 42. [Google Scholar] [CrossRef]
- Li, X.; Mishra, J.K.; Seul, S.D.; Kim, I.; Ha, C.S. Microstructure and properties of poly (butylene terephthalate) based nanocomposites. Compos. Interfaces 2004, 11, 335–346. [Google Scholar] [CrossRef]
- Ahmadi, S.J.; Huang, Y.D.; Li, W. Synthetic routes, properties and future applications of polymer-layered silicate nanocomposites. J. Mater. Sci. 2004, 39. [Google Scholar] [CrossRef]
- Song, L.; Hu, Y.; Tang, Y.; Zhang, R.; Chen, Z.; Fan, W. Study on the properties of flame retardant polyurethane/organoclay nanocomposite. Polym. Degrad. Stab. 2005, 87. [Google Scholar] [CrossRef]
- Li, J.; Zhao, L.; Guo, S. Ultrasonic preparation of polymer/layered silicate nanocomposites during extrusion. Polym. Bull. 2005, 55. [Google Scholar] [CrossRef]
- Chang, M.-K. Mechanical properties and thermal stability of low-density polyethylene grafted maleic anhydride/montmorillonite nanocomposites. J. Ind. Eng. Chem. 2015, 27, 96–101. [Google Scholar] [CrossRef]
- Sinha Ray, S.; Okamoto, M. Biodegradable polylactide and its nanocomposites: Opening a new dimension for plastics and composites. Macromol. Rapid Commun. 2003, 24. [Google Scholar] [CrossRef]
- Vlasveld, D.P.N.; Daud, W.; Bersee, H.E.N.; Picken, S.J. Continuous fibre composites with a nanocomposite matrix: Improvement of flexural and compressive strength at elevated temperatures. Compos. Part A Appl. Sci. Manuf. 2007, 38. [Google Scholar] [CrossRef]
- Akbari, B.; Bagheri, R. Deformation mechanism of epoxy/clay nanocomposite. Eur. Polym. J. 2007, 43. [Google Scholar] [CrossRef]
- Yamagata, S.; Iida, J.; Watari, F. FRP Esthetic Orthodontic Wire and Development of Matrix Strengthening with Poly(methyl methacrylate)/Montmorillonite Nanocomposite. In Handbook of Polymernanocomposites. Processing, Performance and Application; Springer: Berlin/Heidelberg, Germany, 2014; pp. 319–328. ISBN 9783642386497. [Google Scholar]
- Tasan, C.C.; Kaynak, C. Mechanical performance of resol type phenolic resin/layered silicate nanocomposites. Polym. Compos. 2009, 30. [Google Scholar] [CrossRef]
- Inglis, C.E. Stress in a plate due to the presence of cracks and sharp corners. Trans. R. Inst. Nav. Archit. 1913, 60, 219–241. [Google Scholar]
- Griffith, A.A. The Phenomena of Rupture and Flow in Solids. Philos. Trans. R. Soc. London Ser. A 1921, 221, 163–198. [Google Scholar]
- Irwin, G.R. Analysis of stresses and strains near the end of a crack traversing a plate. J. Appl. Mech. 1957, 24, 361–364. [Google Scholar] [CrossRef]
- Westergaard, H.M. Bearing Pressures and Cracks. J. Appl. Mech. 1939, 6, 49–53. [Google Scholar] [CrossRef]
- Wells, A.A. Application of fracture mechanics at and beyond general yielding. Br. Weld. J. 1961, 11, 563–570. [Google Scholar]
- Rice, J.R. A path independent integral and the approximate analysis of strain concentrations by notches and cracks. J. Appl. Mech. 1968, 35, 379–389. [Google Scholar] [CrossRef] [Green Version]
- Broberg, K.B. On stable crack growth. J. Mech. Phys. Solids 1975, 23, 443–445. [Google Scholar] [CrossRef]
- Broberg, K.B. Crack-Growth Criteria and Non-linear Fracture Mechanics. J. Mech. Phys. Solids 1971, 19, 407. [Google Scholar] [CrossRef]
- Cotterell, B.; Reddel, J.K. The Essential Work of Plane Stress Ductile Fracture. Int. J. Fract. 1977, 13, 267–277. [Google Scholar]
- Mai, Y.W.; Cotterell, B. The Essential Work of fracture for tearing of ductile metals. Int. J. Fract. 1984, 24, 229–236. [Google Scholar] [CrossRef]
- Paton, C.A.; Hashemi, S. Plane-stress essential work of ductile fracture for polycarbonate. J. Mater. Sci. 1992, 27, 2279–2290. [Google Scholar] [CrossRef]
- Wu, J.; Mai, Y.W. Cotterell Fracture toughness and fracture mechanisms of PBT/PC/IM blend (Part I: Fracture properties). J. Mater. Sci. 1993, 28, 3373–3384. [Google Scholar] [CrossRef]
- Sanchez, J.J.; Santana, O.O.; Gordillo, A.; Maspoch, M.L.; Martinez, A.B. Essential work of fracture of injection moulded samples of PET and PET/PC blends. Fract. Polym. Compos. Adhes. 2003, 32, 77–88. [Google Scholar]
- Pardoen, T.; Marchal, Y.; Delannay, F. Essential work of fracture compared to fracture mechanics—towards a thickness independent plane stress toughness. Eng. Fract. Mech. 2002, 69, 617–631. [Google Scholar] [CrossRef]
- Rae, P.J.; Brown, E.N.; Orler, E.B. The mechanical properties of poly(ether-ether-ketone) (PEEK) with emphasis on the large compressive strain response. Polymer 2007, 48, 598–615. [Google Scholar] [CrossRef]
- Arkhireyeva, A.; Hashemi, S. Fracture behaviour of polyethylene naphthalate (PEN). Polymer 2002, 43, 289–300. [Google Scholar] [CrossRef]
- Knockaert, R.; Doghri, I.; Marchal, Y.; Pardoen, T.; Delannay, F. Experimental and numerical investigation of fracture in double-edge notched steel plates. Int. J. Fract. 1996, 81, 383–399. [Google Scholar] [CrossRef]
- Cotterell, B.; Pardoen, T.; Atkins, A.G. Measuring toughness and the cohesive stress-displacement relationship by the essential work of fracture concept. Eng. Fract. Mech. 2005, 72, 827–848. [Google Scholar] [CrossRef]
- Chen, Y.H.; Mai, Y.W.; Tong, P.; Zhang, L.C. Numerical Simulation of the Essential Fracture Work Method. In Fracture of Polymers, Composites and Adhesion; Williams, J.G., Pavan, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2000; Volume 27, p. 175. [Google Scholar]
- Chen, H.B.; Wu, J.S. Understanding the underlying physics of the essential work of fracture on the molecular level. Macromolecules 2007, 40, 4322–4326. [Google Scholar] [CrossRef]
- Kwon, H.J.; Jar, P.Y.B. Fracture toughness of polymers in shear mode. Polymer 2005, 46, 12480–12492. [Google Scholar] [CrossRef]
- Kwon, H.J.; Jar, P.Y.B. Toughness of high-density polyethylene in shear fracture. Int. J. Fract. 2007, 145, 123–133. [Google Scholar] [CrossRef]
- Kim, H.S.; Karger-Kocsis, J. Tearing resistance of some co-polyester sheets. Acta Mater. 2004, 52, 3123–3133. [Google Scholar] [CrossRef]
- Wong, J.S.S.; Ferrer-Balas, D.; Li, R.K.Y.; Mai, Y.W.; Maspoch, M.L.; Sue, H.J. On tearing of ductile polymer films using the essential work of fracture (EWF) method. Acta Mater. 2003, 51, 4929–4938. [Google Scholar] [CrossRef]
- Schuller, T.; Lauke, B. Finite-element simulation of interfacial crack propagation: Methods and tools for the complete failure process under large scale yielding. Eng. Fract. Mech. 2006, 73, 2252–2263. [Google Scholar] [CrossRef]
- Haughie, D.W.; Buckley, C.P.; Wu, J.J. The integrity of welded interfaces in ultra-high molecular weight polyethylene: Part 2 - Interface toughness. Biomaterials 2006, 27, 3875–3881. [Google Scholar] [CrossRef]
- Gray, A. Testing protocol for Essential Work of Fracture. ESIS-TC4 1993. [Google Scholar]
- Hill, R. On discontinuous plastic states, with special reference to localized necking in thin sheets. Journa Mech. Phys. Solids 1952, 1, 19–30. [Google Scholar] [CrossRef]
- Clutton, E.Q. ESIS TC4 experience with the essential work of fracture method. In European Structural Integrity Society; Williams, J.G., Pavan, A., Eds.; Elsevier Science, Ltd.: Oxford, LB, USA, 2000; Volume 27, pp. 187–199. [Google Scholar]
- Maspoch, M.L.; Gamez-Perez, J.; Gordillo, A.; Sánchez-Soto, M.; Velasco, J.I. Characterisation of injected EPBC plaques using the essential work of fracture (EWF) method. Polymer 2002, 43, 4177–4183. [Google Scholar] [CrossRef]
- Maspoch, M.L.; Santana, O.O.; Cailloux, J.; Franco-Urquiza, E.; Rodriguez, C.; Belzunce, J.; Martínez, A.B. Ductile-brittle transition behaviour of PLA/o-MMT films during the physical aging process. Express Polym. Lett. 2015, 9, 185–195. [Google Scholar] [CrossRef]
- Martínez, A.B.; Segovia, A.; Gamez-Perez, J.; Maspoch, M.L. Essential work of fracture (EWF) analysis of the tearing of a ductile polymer film. Eng. Fract. Mech. 2010, in press. [Google Scholar] [CrossRef]
- Pettarin, V.; Frontini, P.M.; Elicabe, G.E. Optimal ligament lengths in impact fracture toughness estimation by the essential work of fracture method. Polym. Test. 2005, 24, 189–196. [Google Scholar] [CrossRef]
- Tjong, S.C.; Bao, S.P. Impact fracture toughness of polyamide-6/montmorillonite nanocomposites toughened with a maleated styrene/ethylene butylene/styrene elastomer. J. Polym. Sci. Part B Polym. Phys. 2005, 43. [Google Scholar] [CrossRef]
- Ozkoc, G.; Bayram, G.; Bayramli, E. Impact essential work of fracture toughness of ABS/polyamide-6 blends compatibilized with olefin based copolymers. J. Mater. Sci. 2008, 43, 2642–2652. [Google Scholar] [CrossRef]
- Bao, S.P.; Tjong, S.C. Fracture characterization of high density polyethylene/organoclay nanocomposites toughened with SEBS-g-MA. Key Eng. Mater. 2006, 312, 187–192. [Google Scholar] [CrossRef]
- Fayolle, B.; Tcharkhtchi, A.; Verdu, J. Temperature and molecular weight dependence of fracture behaviour of polypropylene films. Polym. Test. 2004, 23, 939–947. [Google Scholar] [CrossRef]
- Wainstein, J.; Fasce, L.A.; Cassanelli, A.; Frontini, P.M. High rate toughness of ductile polymers. Eng. Fract. Mech. 2007, 74, 2070–2083. [Google Scholar] [CrossRef]
- Saminathan, K.; Selvakumar, P.; Bhatnagar, N. Fracture studies of polypropylene/nanoclay composite. Part I: Effect of loading rates on essential work of fracture. Polym. Test. 2008, 27, 296–307. [Google Scholar] [CrossRef]
- Saminathan, K.; Selvakumar, P.; Bhatnagar, N. Fracture studies of polypropylene/nanoclay composite. Part II: Failure mechanism under fracture loads. Polym. Test. 2008, 27, 453–458. [Google Scholar] [CrossRef]
- Santana, O.O.; Maspoch, M.L.; Martinez, A.B. Plane strain essential work of fracture in SENB geometry at low and high strain rates of PC/ABS blends. Polym. Bull. 1997, 39, 511–518. [Google Scholar] [CrossRef]
- Maspoch, M.L.; Gamez-Perez, J.; Karger-Kocsis, J. Effects of thickness, deformation rate and energy partitioning on the work of fracture parameters of uPVC films. Polym. Bull. 2003, 50, 279–286. [Google Scholar] [CrossRef]
- Arkhireyeva, A.; Hashemi, S. Effect of temperature on work of fracture parameters in poly(ether-ether ketone) (PEEK) film. Eng. Fract. Mech. 2004, 71, 789–804. [Google Scholar] [CrossRef]
- Arkhireyeva, A.; Hashemi, S. Combined effect of temperature and thickness on work of fracture parameters of unplasticized PVC film. Polym. Eng. Sci. 2002, 42, 504–518. [Google Scholar] [CrossRef]
- Korsunsky, A.M.; Kim, K. Determination of essential work of necking and tearing from a single tensile test. Int. J. Fract. 2005, 132, L37–L44. [Google Scholar] [CrossRef]
- Korsunsky, A.M.; Nguyen, G.D.; Houlsby, G.T. Analysis of essential work of rupture using non-local damage-plasticity modelling. Int. J. Fract. 2005, 135, L19–L26. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Ferrer-Balas, D. On the plane-strain essential work of fracture of polymer sheets. Polym. Bull. 2001, 46, 507–512. [Google Scholar] [CrossRef]
- Kwon, H.J.; Jar, P.Y.B. New energy partitioning approach to the measurement of plane-strain fracture toughness of high-density polyethylene based on the concept of essential work of fracture. Eng. Fract. Mech. 2007, 74, 2471–2480. [Google Scholar] [CrossRef]
- Kwon, H.J.; Jar, R.Y.B. Application of essential work of fracture concept to toughness characterization of high-density polyethylene. Polym. Eng. Sci. 2007, 47, 1327–1337. [Google Scholar] [CrossRef]
- Pegoretti, A.; Ricco, T. On the essential work of fracture of neat and rubber toughened polyamide-66. Eng. Fract. Mech. 2006, 73, 2486–2502. [Google Scholar] [CrossRef]
- Martinez, A.B.; Segovia, A.; Gamez-Perez, J.; Maspoch, M.L. Influence of femtolaser notch sharpening technique in the determination of essential work of fracture (EWF) parameters. Eng. Fract. Mech. 2009, 76, 1247–1254. [Google Scholar] [CrossRef]
- Maspoch, M.L.; Ferrer, D.; Gordillo, A.; Santana, O.O. Effect of the Specimen Dimensions and the test Speed on the Fracture Toughness of iPP by the Essential Work of Fracture (EWF) Method. J. Appl. Polym. Sci. 1999, 73, 177–187. [Google Scholar] [CrossRef]
- Gamez-Perez, J.; Santana, O.; Martinez, A.B.; Maspoch, M.L. Use of extensometers on essential work of fracture (EWF) tests. Polym. Test. 2008, 27, 491–497. [Google Scholar] [CrossRef]
- Fung, K.L.; Li, R.K.Y. A study on the fracture characteristics of rubber toughened poly(ethylene terephthalate) blends. Polym. Test. 2005, 24, 863–872. [Google Scholar] [CrossRef]
- Mai, Y.-W.; Cotterell, B.; Horlyck, R.; Vigna, G. The essential Work of Plane Stress Ductile Fracture of Linear Polyethylenes. Polym. Eng. Sci. 1987, 27, 804. [Google Scholar] [CrossRef]
- Karger-Kocsis, J.; Czigány, T.; Moskala, E.J. Thickness Dependence of work of fracture Parameters of an Amorphous Copolyester. Polymer 1997, 38, 4587. [Google Scholar] [CrossRef]
- Ferrer-Balas, D.; Maspoch, M.L.; Martínez, A.B.; Santana, O.O. On the essential work of fracture method: Energy partitioning of the fracture process in iPP films. Polym. Bull. 1999, 42, 101–108. [Google Scholar] [CrossRef]
- Ferrer-Balas, D.; Maspoch, M.L.; Martinez, A.B.; Santana, O.O. Influence of annealing on the microstructural, tensile and fracture properties of polypropylene films. Polymer 2001, 42, 1697–1705. [Google Scholar] [CrossRef]
- Casellas, J.J.; Frontini, P.M.; Carella, J.M. Fracture characterization of low-density polyethylenes by the essential work of fracture: Changes induced by thermal treatments and testing temperature. J. Appl. Polym. Sci. 1999, 74, 781–796. [Google Scholar] [CrossRef]
- Ho, C.H.; Vu-Khanh, T. Physical aging and time-temperature behavior concerning fracture performance of polycarbonate. Theor. Appl. Fract. Mech. 2004, 41, 103–114. [Google Scholar] [CrossRef]
- Kayano, Y.; Keskkula, H.; Paul, D.R. Fracture behaviour of some rubber-toughened nylon 6 blends. Polymer 1998, 39, 2835–2845. [Google Scholar] [CrossRef]
- Van der Wal, A.; Mulder, J.J.; Thijs, H.A.; Gaymans, R.J. Fracture of polypropylene: 1. The effect of molecular weight and temperature at low and high test speed. Polymer 1998, 39, 5467–5475. [Google Scholar] [CrossRef]
- Yamakawa, R.S.; Razzinoa, C.A.; Correab, C.A.; Hage, E., Jr. Influence of notching and molding conditions on determination of EWF parameters in polyamide 6. Polym. Test. 2004, 23, 195–202. [Google Scholar] [CrossRef]
- Baldi, F.; Bignotti, F.; Fina, A.; Tabuani, D.; Riccò, T. Mechanical characterization of polyhedral oligomeric silsesquioxane/ polypropylene blends. J. Appl. Polym. Sci. 2007, 105. [Google Scholar] [CrossRef]
- Arencon, D.; Velasco, J.I.; Realinho, V.; Sanchez-Soto, M.; Gordillo, A. Fracture toughness of glass microsphere-filled polypropylene and polypropylene/poly (ethylene terephthalate-co-isophthalate) blend-matrix composites. J. Mater. Sci. 2007, 42, 19–29. [Google Scholar] [CrossRef]
- Nekhlaoui, S.; Essabir, H.; Bensalah, M.O.; Fassi-Fehri, O.; Qaiss, A.; Bouhfid, R. Fracture study of the composite using essential work of fracture method: PP-SEBS-g-MA/E1 clay. Mater. Des. 2014, 53. [Google Scholar] [CrossRef]
- Maspoch, M.L.; Franco-Urquiza, E.; Gamez-Perez, J.; Santana, O.O.; Sánchez-Soto, M. Fracture behaviour of poly[ethylene-(vinyl alcohol)]/organo-clay composites. Polym. Int. 2009, 58. [Google Scholar] [CrossRef]
- Ganß, M.; Staudinger, U.; Satapathy, B.K.; Leuteritz, A.; Weidisch, R. Mechanism of strengthening and toughening of a nanostructured styrene-butadiene based block copolymer by oligostyrene-modified montmorillonites. Polymer 2021, 213, 123328. [Google Scholar] [CrossRef]
- Bao, S.P.; Tjong, S.C. Impact essential work of fracture of polypropylene / montmorillonite nanocomposites toughened with SEBS-g-MA elastomer. Compos. Part A Appl. Sci. Manuf. 2007, 38, 378–387. [Google Scholar] [CrossRef]
- Bureau, M.N.; Perrin-sarazin, F. Essential work of fracture and failure mechanisms of polypropylene–clay nanocomposites. Eng. Fract. Mech. 2006, 73, 2360–2374. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.W.; Hassan, A.; Rahmat, A.R.; Wahit, M.U. Mechanical behaviour and fracture toughness evaluation of rubber toughened polypropylene nanocomposites. Plast. Rubber Compos. 2006, 35, 37–46. [Google Scholar] [CrossRef]
- Bureau, M.N.; Perrin-Sarazin, F.; Ton-That, M.T. Polyolefin nanocomposites: Essential work of fracture analysis. Polym. Eng. Sci. 2004, 44, 1142–1151. [Google Scholar] [CrossRef]
- Tjong, S.C.; Ruan, Y.H. Fracture behavior of thermoplastic polyolefin/clay nanocomposites. J. Appl. Polym. Sci. 2008, 110. [Google Scholar] [CrossRef]
- Pedrazzoli, D.; Tuba, F.; Khumalo, V.M.; Pegoretti, A.; Karger-Kocsis, J. Mechanical and rheological response of polypropylene/boehmite nanocomposites. J. Reinf. Plast. Compos. 2014, 33. [Google Scholar] [CrossRef] [Green Version]
- Turcsán, T.; Mészáros, L.; Khumalo, V.M.; Thomann, R.; Karger-Kocsis, J. Fracture behavior of boehmite-filled polypropylene block copolymer nanocomposites as assessed by the essential work of fracture concept. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef] [Green Version]
- Karger-Kocsis, J.; Khumalo, V.M.; Bárány, T.; Mészáros, L.; Pegoretti, A. On the toughness of thermoplastic polymer nanocomposites as assessed by the essential work of fracture (EWF) approach. Compos. Interfaces 2013, 20, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Xie, B.H.; Yang, W.; Yang, M.B. Grafted polyolefin-coated synthetic mica-filled polypropylene-co-ethylene composites: A study on the interfacial morphology and properties. J. Macromol. Sci. Part B Phys. 2010, 49. [Google Scholar] [CrossRef]
- Fu, Z.; Dai, W.; Yu, H.; Zou, X.; Chen, B. Effect of composition on fracture behavior of polypropylene-wollastonite- polyolefin elastomer system. J. Mater. Sci. 2011, 46. [Google Scholar] [CrossRef]
- Mouzakis, D.E.; Papanicolaou, G.C.; Giannadakis, K.; Zuburtikudis, I. On the toughness response of iPP and sPP/MWNT nanocomposites. Strain 2013, 49. [Google Scholar] [CrossRef]
- Das, D.; Satapathy, B.K. Designing tough and fracture resistant polypropylene/multi wall carbon nanotubes nanocomposites by controlling stereo-complexity and dispersion morphology. Mater. Des. 2014, 54. [Google Scholar] [CrossRef]
- Khodabandelou, M.; Razavi Aghjeh, M.K.; Mazidi, M.M. Fracture toughness and failure mechanisms in un-vulcanized and dynamically vulcanized PP/EPDM/MWCNT blend-nanocomposites. RSC Adv. 2015, 5. [Google Scholar] [CrossRef]
- Khodabandelou, M.; Razavi Aghjeh, M.K.; Khonakdar, H.A.; Mehrabi Mazidi, M. Effect of localization of carbon nanotubes on fracture behavior of un-vulcanized and dynamically vulcanized PP/EPDM/MWCNT blend-nanocomposites. Compos. Sci. Technol. 2017, 149. [Google Scholar] [CrossRef]
- Satapathy, B.K.; Ganß, M.; Weidisch, R.; Po, P.; Jehnichen, D.; Keller, T.; Jandt, K.D. Ductile-to-Semiductile Transition in PP-MWNT Nanocomposites. Macromol. Rapid Commun. 2007, 28, 834–841. [Google Scholar] [CrossRef]
- Haghnegahdar, M.; Naderi, G.; Ghoreishy, M.H.R. Fracture toughness and deformation mechanism of un-vulcanized and dynamically vulcanized polypropylene/ethylene propylene diene monomer/graphene nanocomposites. Compos. Sci. Technol. 2017, 141. [Google Scholar] [CrossRef]
- Zhang, H.; Zhang, Z.; Park, H.W.; Zhu, X. Influence of surface-modified TiO2 nanoparticles on fracture behavior of injection molded polypropylene. Front. Mater. Sci. China 2008, 2. [Google Scholar] [CrossRef]
- Hajibabazadeh, S.; Razavi Aghjeh, M.K.; Palahang, M. Study on the fracture toughness and deformation micro-mechanisms of PP/EPDM/SiO2 ternary blend-nanocomposites. J. Compos. Mater. 2020, 54. [Google Scholar] [CrossRef]
- Tjong, S.C.; Bao, S.P. Fracture toughness of high density polyethylene/SEBS-g-MA/montmorillonite nanocomposites. Compos. Sci. Technol. 2007, 67. [Google Scholar] [CrossRef]
- Franco-Urquiza, E.; Perez, J.G.; Sánchez-Soto, M.; Santana, O.O.; Maspoch, M.L. The effect of organo-modifier on the structure and properties of poly[ethylene-(vinyl alcohol)]/organo-modified montmorillonite composites. Polym. Int. 2010, 59. [Google Scholar] [CrossRef]
- Na, S.; Spatari, S.; Hsuan, Y.G. Fracture characterization of recycled high density polyethylene/nanoclay composites using the essential work of fracture concept. Polym. Eng. Sci. 2016, 56. [Google Scholar] [CrossRef]
- Pedrazzoli, D.; Ceccato, R.; Karger-Kocsis, J.; Pegoretti, A. Viscoelastic behaviour and fracture toughness of linear-low-density polyethylene reinforced with synthetic boehmite alumina nanoparticles. Express Polym. Lett. 2013, 7. [Google Scholar] [CrossRef]
- Costa, F.R.; Satapathy, B.K.; Wagenknecht, U. POLYMER Morphology and fracture behaviour of polyethylene/Mg–Al layered double hydroxide ( LDH) nanocomposites. Eur. Polym. J. 2006, 42, 2140–2152. [Google Scholar] [CrossRef]
- Liao, C.Z.; Bao, S.P.; Tjong, S.C. Microstructure and fracture behavior of maleated high-density polyethylene/ silicon carbide nanocomposites toughened with poly(styrene-ethylene-butylene- styrene) triblock copolymer. Adv. Polym. Technol. 2011, 30. [Google Scholar] [CrossRef]
- Dorigato, A.; Pegoretti, A. Fracture behaviour of linear low density polyethylene-fumed silica nanocomposites. Eng. Fract. Mech. 2012, 79. [Google Scholar] [CrossRef]
- Pedrazzoli, D.; Pegoretti, A.; Thomann, R.; Kristõf, J.; Karger-Kocsis, J. Toughening linear low-density polyethylene with halloysite nanotubes. Polym. Compos. 2015, 36. [Google Scholar] [CrossRef]
- Mohsenzadeh, M.S.; Mazinani, M.; Zebarjad, S.M. Evaluation of fracture behavior of polyethylene/CaCO3 nanocomposite using essential work of fracture (EWF) approach. Nanocomposites 2015, 1. [Google Scholar] [CrossRef] [Green Version]
- Shir Mohammadi, M.; Hammerquist, C.; Simonsen, J.; Nairn, J.A. The fracture toughness of polymer cellulose nanocomposites using the essential work of fracture method. J. Mater. Sci. 2016, 51. [Google Scholar] [CrossRef]
- Tjong, S.C.; Bao, S.P.; Liang, G.D. Polypropylene/montmorillonite nanocomposites toughened with SEBS-g-MA: Structure-property relationship. J. Polym. Sci. Part B Polym. Phys. 2005, 43. [Google Scholar] [CrossRef]
- Heidari, F.; Aghalari, M.; Chalabi Tehran, A.; Shelesh-Nezhad, K. Study on the fluidity, mechanical and fracture behavior of ABS/TPU/CNT nanocomposites. J. Thermoplast. Compos. Mater. 2020. [Google Scholar] [CrossRef]
- Liao, C.Z.; Tjong, S.C. Effects of carbon nanofibers on the fracture, mechanical, and thermal properties of PP/SEBS-g-MA blends. Polym. Eng. Sci. 2011, 51. [Google Scholar] [CrossRef]
- Baldi, F.; Bignotti, F.; Tieghi, G.; Riccò, T. Rubber toughening of polyamide 6/organoclay nanocomposites obtained by melt blending. J. Appl. Polym. Sci. 2006, 99. [Google Scholar] [CrossRef]
- Xu, W.; Lv, R.; Na, B.; Tian, N.; Li, Z.; Fu, Q. Micro-FTIR study of molecular orientation at crack tip in nylon 6/clay nanocomposite: Insight into fracture mechanism. J. Phys. Chem. B 2009, 113. [Google Scholar] [CrossRef]
- Dayma, N.; Jaggi, H.S.; Satapathy, B.K. Post-yield fracture behaviour of PA-6/LDPE-g-MA/nanoclay ternary nanocomposites: Semiductile-to-ductile transition. J. Polym. Res. 2012, 19. [Google Scholar] [CrossRef]
- Dayma, N.; Jaggi, H.S.; Satapathy, B.K. Post-yield crack toughness behavior of polyamide-6/polypropylene grafted maleic anhydride/nanoclay ternary nanocomposites. Mater. Des. 2013, 49. [Google Scholar] [CrossRef]
- González, I.; Zabaleta, A.; Eguiazábal, J.I. Toughening and brittle-tough transition in polyamide 12-organoclay/maleated styrene-ethylene-co-butylene-styrene nanocomposites. Polym. Eng. Sci. 2013, 53. [Google Scholar] [CrossRef]
- Dayma, N.; Kumar, S.; Das, D.; Satapathy, B.K. Melt-mixed PA-6/LDPE-g-MA/nanoclay ternary nanocomposite: Micro-mechanisms from post-yield fracture kinetics and strain field analysis. Mater. Chem. Phys. 2013, 142. [Google Scholar] [CrossRef]
- Nakhaei, M.R.; Naderi, G.; Reza Ghoreishy, M.H. Experimental investigation of mechanical properties, fracture mechanism and crack propagation of PA6/NBR/Clay nanocomposites. Iran. J. Polym. Sci. Technol. 2020, 33. [Google Scholar] [CrossRef]
- Fu, L.D.; Chen, S.; Wang, Y.J.; Wang, X.D.; Wang, X. Fracture toughness of polyamide 6/maleated ethylene-propylene-diene terpolymer rubber/nano calcium carbonate ternary composites according to essential work of fracture analysis. J. Appl. Polym. Sci. 2011, 120. [Google Scholar] [CrossRef]
- Prashantha, K.; Schmitt, H.; Lacrampe, M.F.; Krawczak, P. Mechanical behaviour and essential work of fracture of halloysite nanotubes filled polyamide 6 nanocomposites. Compos. Sci. Technol. 2011, 71. [Google Scholar] [CrossRef]
- Liao, C.Z.; Tjong, S.C. Fracture Toughness of Polyamide 6/ Maleated Styrene-Ethylene-Butylene-Styrene/Silicon Carbide Nanocomposites. Adv. Mater. Res. 2011, 275, 229–233. [Google Scholar] [CrossRef]
- Liao, C.Z.; Tjong, S.C. Mechanical and thermal behaviour of polyamide 6/silicon carbide nanocomposites toughened with maleated styrene-ethylene-butylene-styrene elastomer. Fatigue Fract. Eng. Mater. Struct. 2012, 35, 56–63. [Google Scholar] [CrossRef]
- Satapathy, B.K.; Weidisch, R.; Po, P.; Janke, A. SCIENCE AND Tough-to-brittle transition in multiwalled carbon nanotube (MWNT)/polycarbonate nanocomposites. Compos. Sci. Technol. 2007, 67, 867–879. [Google Scholar] [CrossRef]
- Janke, A. Crack Toughness Behaviour of Multiwalled Carbon Nanotube ( MWNT)/Polycarbonate Nanocomposites. Macromol. Rapid Commun. 2005, 1246–1252. [Google Scholar] [CrossRef]
- Rodríguez, C.; Arencón, D.; Belzunce, J.; Maspoch, M.L. Small punch test on the analysis of fracture behaviour of PLA-nanocomposite films. Polym. Test. 2014, 33, 21–29. [Google Scholar] [CrossRef]
- Tuba, F.; Khumalo, V.M.; Karger-Kocsis, J. Essential work of fracture of poly(Ïμ-caprolactone)/boehmite alumina nanocomposites: Effect of surface coating. J. Appl. Polym. Sci. 2013, 129. [Google Scholar] [CrossRef] [Green Version]
- Arroyo, O.H.; Huneault, M.A.; Favis, B.D.; Bureau, M.N. Processing and properties of PLA/Thermoplastic starch/Montmorillonite nanocomposites. Polym. Compos. 2010, 31. [Google Scholar] [CrossRef]
- Ahmad, S.H.; Rasid, R.; Surip, S.N.; Anuar, H.; Czigany, T.; Razak, S.B.A. Mechanical and fracture toughness behavior of TPNR nanocomposites. J. Compos. Mater. 2007, 41. [Google Scholar] [CrossRef]
- Penaloza, D.P. Review on the preparation and properties of clay-based nanocomposites with covalently-bound polymer architecture. Philipp. J. Sci. 2019, 148, 813–824. [Google Scholar]
- Peres, F.M.; Schon, C.G. Application of the essential work of fracture method in ranking the performance in service of high-density polyethylene resins employed in pressure pipes. J. Mater. Sci. 2008, 43, 1844–1850. [Google Scholar] [CrossRef]
- Rodriguez-Gonzalez, F.; Ramsay, B.; Favis, B. High performance LDPE/thermoplastic starch blends: A sustainable alternative to pure polyethylene. Polymer 2003, 44, 1517–1526. [Google Scholar] [CrossRef]

 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
magnesium/aluminum. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
magnesium/aluminum. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
magnesium/aluminum. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
magnesium/aluminum. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
 oxygen,
oxygen,  silicon,
silicon,  hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
 oxygen,
oxygen,  silicon,
silicon,  hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.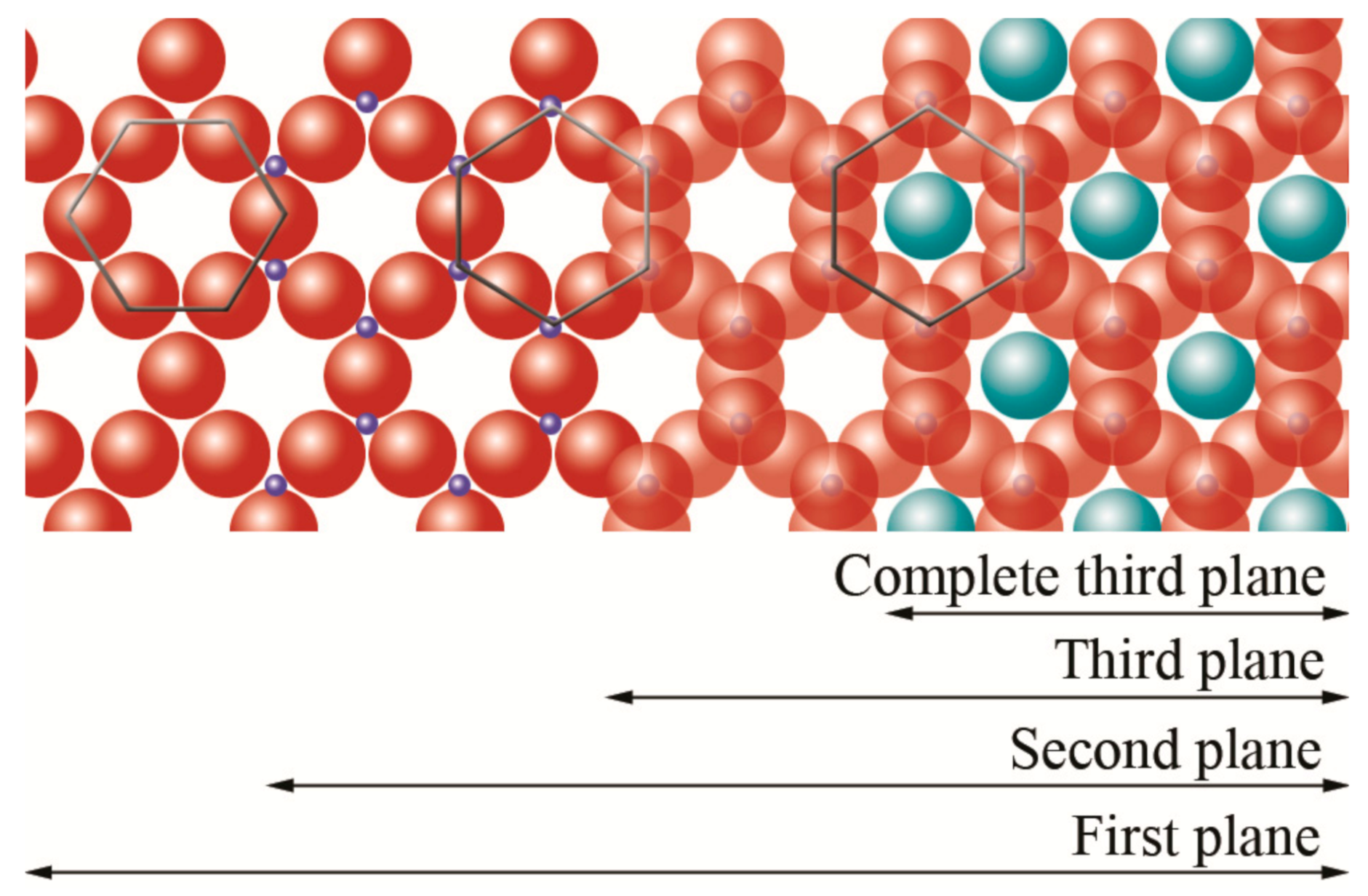
 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum,
magnesium/aluminum,  hydroxyl groups. Adapted from [9].
hydroxyl groups. Adapted from [9].
 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum,
magnesium/aluminum,  hydroxyl groups. Adapted from [9].
hydroxyl groups. Adapted from [9].
 oxygen,
oxygen,  silicon,
silicon,  magnesium,
magnesium,  aluminum,
aluminum,  hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
 oxygen,
oxygen,  silicon,
silicon,  magnesium,
magnesium,  aluminum,
aluminum,  hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum,
magnesium/aluminum,  hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum,
magnesium/aluminum,  hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum,
magnesium/aluminum,  hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum,
magnesium/aluminum,  hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
hydroxyl groups. Adapted from https://www.edafologia.net/, last visited 12 May 2021.
 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum,
magnesium/aluminum,  hydroxyl groups. Schematic sketch based on Beyer [136] with permission from Elsevier.
hydroxyl groups. Schematic sketch based on Beyer [136] with permission from Elsevier.
 oxygen,
oxygen,  silicon,
silicon,  magnesium/aluminum,
magnesium/aluminum,  hydroxyl groups. Schematic sketch based on Beyer [136] with permission from Elsevier.
hydroxyl groups. Schematic sketch based on Beyer [136] with permission from Elsevier.













| Structure | Dioctahedral | Trioctahedral |
|---|---|---|
| T:O | Kaolinite group [10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26] | Serpentine group [27,28,29] |
| Pyrophyllite | Talc | |
| T:O:T | Smectite group [30,31,32,33,34,35,36,37,38,39,40,41,42,43,44] | |
| Montmorillonite [45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77] | Saponite | |
| Beidellite | Hectorite | |
| Nontronite | Stevensite | |
| Vermiculite group [14,78,79,80,81,82] | ||
| Illite | ||
| Mica group [81,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120,121,122,123,124,125,126] | ||
| Muscovite | Biotite | |
| Paragonite | Phlogopite | |
| Lepidolite | ||
| T:O:T:o | Chlorite group [127,128,129,130,131,132,133,134,135] | |
| Paligorskite | Sepiolite | |
| Mechanical Test | References |
|---|---|
| Tensile | [3,157,158,159,160,161,162,163,164,165,166,167,168,169,170,171,172,173,174,175,176,177,178,179,180,181,182,183,184,185,186,187] |
| Compression | [164,181] |
| Bending | [188,189,190,191,192,193] |
| Issues | Evaluation | References |
|---|---|---|
| Tested specimens | DDENT specimen dimensions. Use of a video extensometer. Notch sharpening. Ligament lengths | [223,224,225,226] |
| Test conditions | Test rate. Test temperature EWF in mode III. | [208,217,227,228,229,230,231,232,233,234] |
| Analysis of the results | Energy partitioning. Other geometries for β. we-J0 relationship | [235,236,237,238,239,240,241,242,243,244] |
| Polymer | Filler | References |
|---|---|---|
| Polypropylene | Montmorillonite clay | [232,259,262,263,264,265,266] |
| Boehmite clay | [267,268,269] | |
| mica | [270] | |
| Innosilicate | [271] | |
| Carbon nanotubes | [272,273,274,275,276] | |
| Graphene | [277] | |
| Other nanoparticles | [257,278,279] | |
| Polyethylene | Montmorillonite clay | [260,280,281,282] |
| Boehmite | [283] | |
| Mg–Al layered double hydroxide (LDH) | [284] | |
| Other nanoparticles | [285,286,287,288,289] | |
| Styrene | Montmorillonite clay | [290,261] |
| Carbon nanotubes | [291,292] | |
| Polyamide | Montmorillonite clay | [227,293,294,295,296,297,298,299] |
| Other nanoparticles | [300,301,302,303] | |
| Polycarbonate | Carbon nanotubes | [304,305] |
| Biodegradable polymers (PLA and others) | Monmorillonite clay | [224,306,307,308] |
| Boehmite | [307] | |
| Thermoplastic natural rubber (TPNR) | Montmorillonite clay | [309] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Franco-Urquiza, E.A. Clay-Based Polymer Nanocomposites: Essential Work of Fracture. Polymers 2021, 13, 2399. https://doi.org/10.3390/polym13152399
Franco-Urquiza EA. Clay-Based Polymer Nanocomposites: Essential Work of Fracture. Polymers. 2021; 13(15):2399. https://doi.org/10.3390/polym13152399
Chicago/Turabian StyleFranco-Urquiza, Edgar Adrian. 2021. "Clay-Based Polymer Nanocomposites: Essential Work of Fracture" Polymers 13, no. 15: 2399. https://doi.org/10.3390/polym13152399