Development and Analysis of the Physicochemical and Mechanical Properties of Diorite-Reinforced Epoxy Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
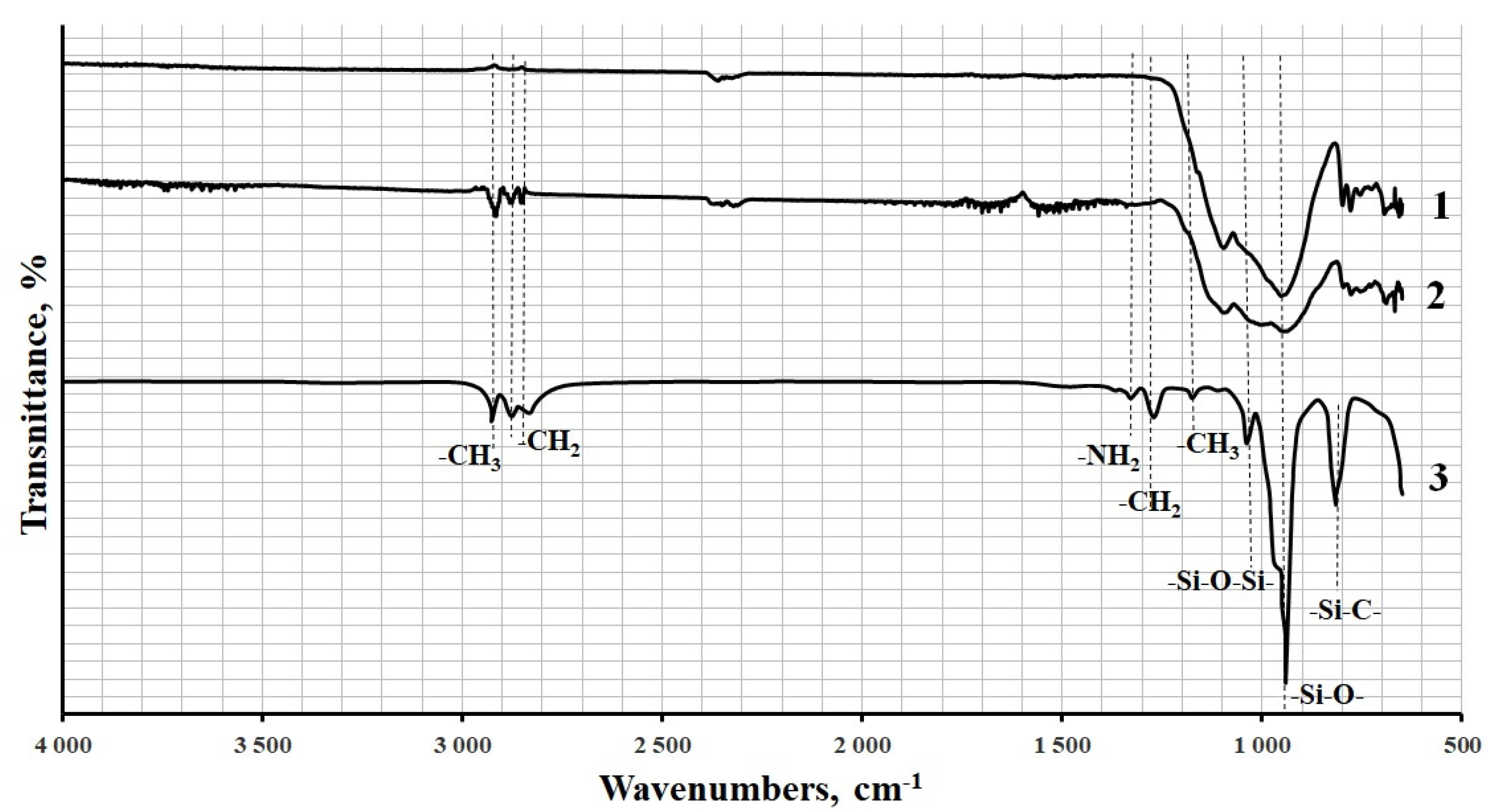
2.2. Functionalization of the Diorite Surface
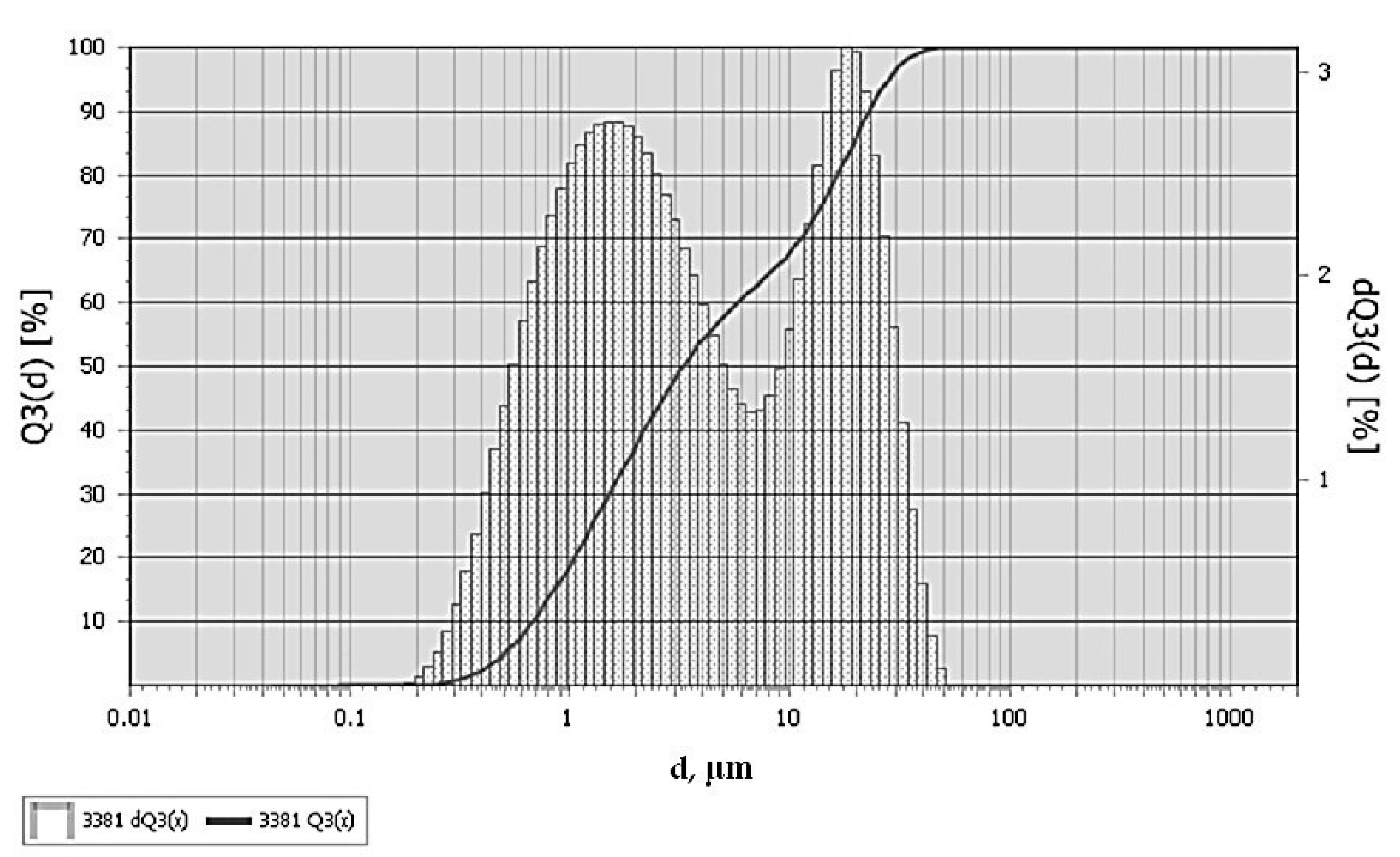
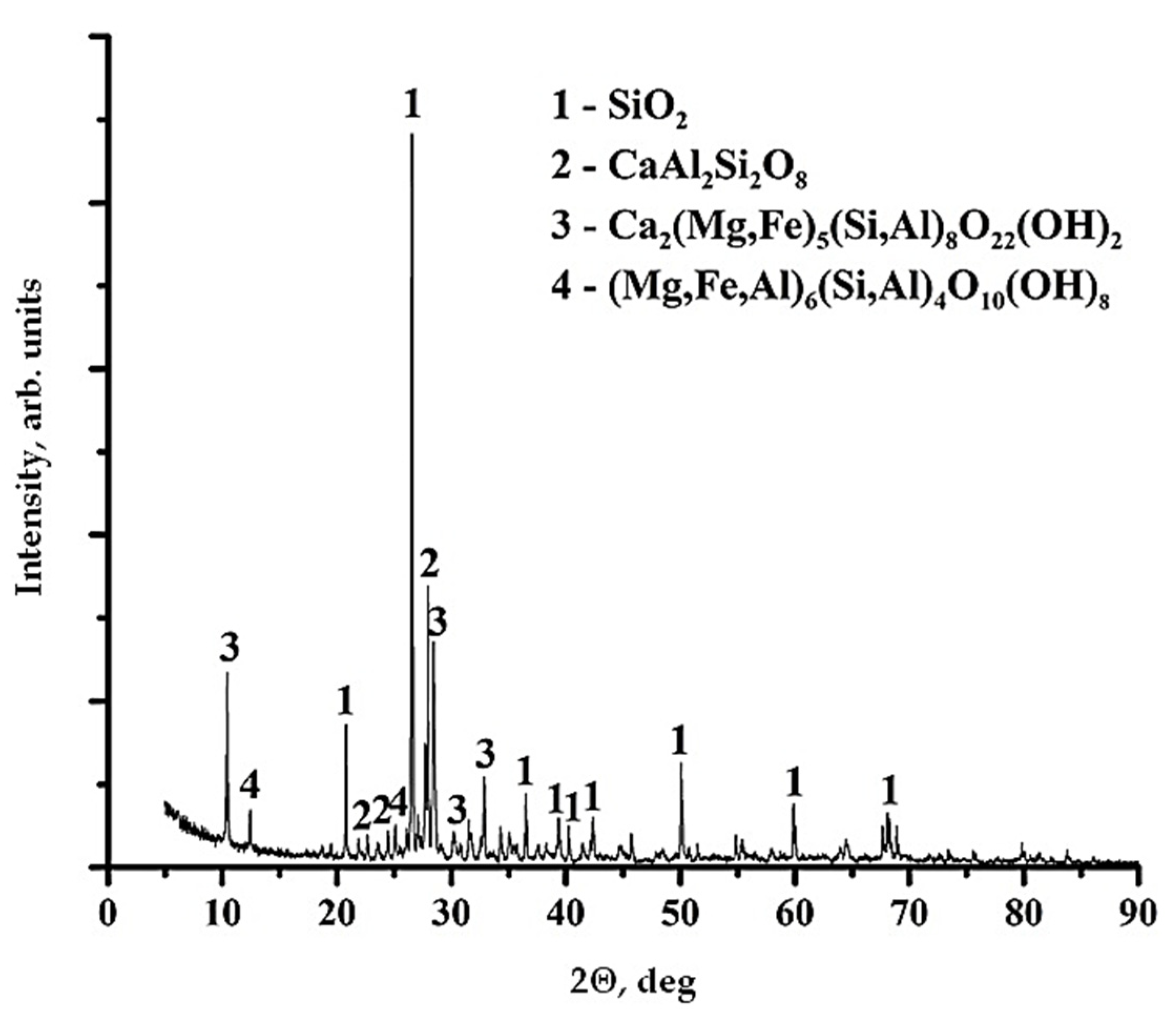
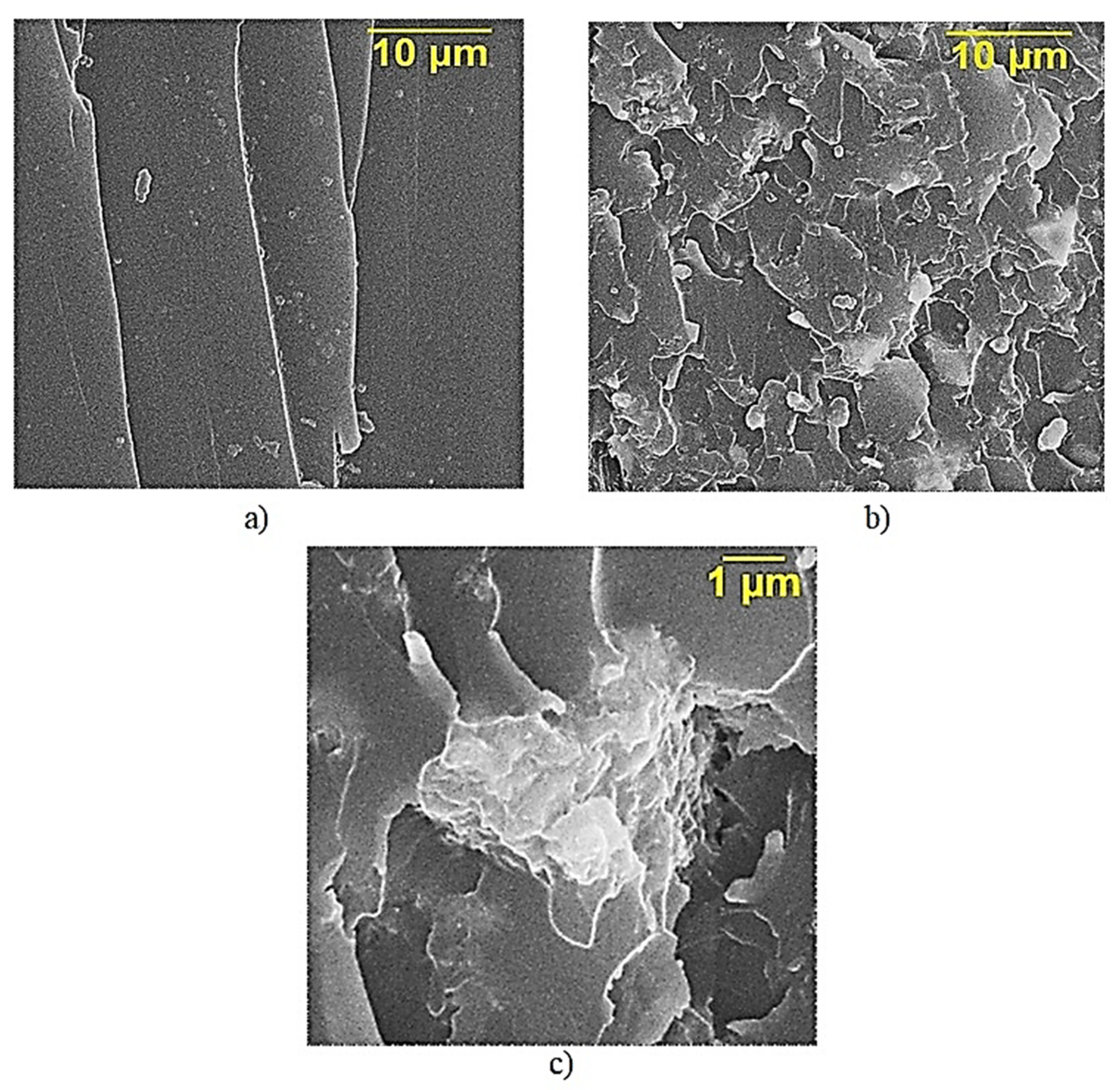
2.3. Characterization of Diorite
2.4. Preparation of Epoxy Composites
2.5. Testing of the Composites
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Starokadomskii, D.L. Epoxy composites with 10 and 50 wt% micronanoiron: Strength, microstructure, and chemical and thermal resistance. Russ. J. Appl. Chem. 2017, 90, 1337–1345. [Google Scholar] [CrossRef]
- Buketov, A.V.; Sapronov, A.A.; Buketova, N.N.; Brailo, M.V.; Marushak, P.O.; Panin, S.V.; Amelin, M.Y. Impact toughness of nanocomposite materials filled with fullerene C60 particles. Compos. Mech. Comput. Appl. 2018, 9, 141–161. [Google Scholar] [CrossRef]
- Mostovoi, A.S.; Plakunova, E.V.; Panova, L.G. New Epoxy Composites Based on Potassium Polytitanates. Int. Polym. Sci. Technol. 2013, 40, 49–51. [Google Scholar] [CrossRef]
- Buketov, A.V.; Sapronov, O.O.; Brailo, M.V.; Maruschak, P.O.; Yakushchenko, S.V.; Panin, S.V.; Nigalatiy, V.D. Dynamics of destruction of epoxy composites filled with ultra-dispersed diamond under impact conditions. Mech. Adv. Mater. Struct. 2020, 27, 725–733. [Google Scholar] [CrossRef]
- Hu, J.H.; Shan, J.Y.; Wen, D.H.; Liu, X.X.; Zhao, J.Q.; Tong, Z. Flame retardant, mechanical properties and curing kinetics of DOPO-based epoxy resins. Polym. Degrad. Stab. 2014, 109, 218–225. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Nurtazina, A.S.; Burmistrov, I.N.; Kadykova, Y.A. Effect of Finely Dispersed Chromite on the Physicochem-ical and Mechanical Properties of Modified Epoxy Composites. Russ. J. Appl. Chem. 2018, 91, 1758–1766. [Google Scholar] [CrossRef]
- Bekeshev, A.; Mostovoy, A.; Tastanova, L.; Kadykova, Y.; Kalganova, S.; Lopukhova, M. Reinforcement of Epoxy Composites with Application of Finely-ground Ochre and Electrophysical Method of the Composition Modification. Polymers 2020, 12, 1437. [Google Scholar] [CrossRef]
- Pati, P.R.; Satpathy, M.P. Investigation on red brick dust filled epoxy composites using ant lion optimization approach. Polym. Compos. 2019, 40, 3877–3885. [Google Scholar] [CrossRef]
- Kumara, R.; Kumarb, K.; Sahooc, P.; Bhowmika, S. Study of mechanical properties of wood dust reinforced epoxy composite. Procedia Mater. Sci. 2014, 6, 551–556. [Google Scholar] [CrossRef] [Green Version]
- Behzadnasab, M.; Mirabedini, S.M.; Esfandeh, M. Corrosion protection of steel by epoxy nanocomposite coatings containing various combinations of clay and nanoparticulate zirconia. Corros. Sci. 2013, 75, 134–141. [Google Scholar] [CrossRef]
- Syugaev, A.V.; Maratkanova, A.N.; Shakov, A.A.; Nelyubov, A.V.; Lomayeva, S.F. Surface modification of iron particles with polystyrene and surfactants under high-energy ball milling. Surf. Coat. Technol. 2013, 236, 429–437. [Google Scholar] [CrossRef]
- Vahedi, H.; Pasbakhsh, P. Instrumented impact properties and fracture behaviour of epoxy/modified halloysitenanocompo-sites. Polym. Test. 2014, 39, 101–114. [Google Scholar] [CrossRef]
- Mostovoi, A.S.; Kurbatova, E.A. Controlling the properties of epoxy composites filled with brick dust. Russ. J. Appl. Chem. 2017, 90, 267–276. [Google Scholar] [CrossRef]
- Karnati, S.R.; Agbo, P.; Zhang, L. Applications of silica nanoparticles in glass/carbon fiber-reinforced epoxy nanocomposite. Compos. Commun. 2020, 17, 32–41. [Google Scholar] [CrossRef]
- Clifton, S.; Thimmappa, B.H.S.; Selvam, R.; Shivamurthy, B. Polymer nanocomposites for high-velocity impact applications—A review. Compos. Commun. 2020, 17, 72–86. [Google Scholar] [CrossRef]
- Sim, J.; Kang, Y.; Kim, B.J.; Park, Y.H.; Lee, Y.C. Preparation of Fly Ash/Epoxy Composites and Its Effects on Mechanical Properties. Polymers 2020, 12, 79. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, S.M. Kishore Effect of filler-fiber interactions on compressive strength of fly ash and short-fiber epoxy composites. J. Appl. Polym. Sci. 2003, 87, 836–841. [Google Scholar] [CrossRef]
- Sand Chee, S.; Jawaid, M. The Effect of Bi-Functionalized MMT on Morphology, Thermal Stability, Dynamic Mechanical, and Tensile Properties of Epoxy/Organoclay Nanocomposites. Polymers 2019, 11, 2012. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishna, H.V.; Priya, S.P.; Rai, S.K. Flexural, compression, chemical resistance, and morphology studies on granite powder-filled epoxy and acrylonitrile butadiene styrene-toughened epoxy matrices. J. Appl. Polym. Sci. 2007, 104, 171–177. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Nurtazina, A.S.; Kadykova, Y.A.; Bekeshev, A.Z. Highly Efficient Plasticizers-Antipirenes for Epoxy Polymers. Inorg. Mater. Appl. Res. 2019, 10, 1135–1139. [Google Scholar] [CrossRef]
- Fang, F.; Huo, S.; Shen, H.; Ran, S.; Wang, H.; Song, P.; Fang, Z. A bio-based ionic complex with different oxidation states of phosphorus for reducing flammability and smoke release of epoxy resins. Compos. Commun. 2020, 17, 104–108. [Google Scholar] [CrossRef]
- Mostovoy, A.S.; Vikulova, M.A.; Lopukhova, M.I. Reinforcing effects of aminosilane-functionalized h-BN on the physico-chemical and mechanical behaviors of epoxy nanocomposites. Sci. Rep. 2020, 10, 10676. [Google Scholar] [CrossRef] [PubMed]
- Tikhani, F.; Moghari, S.; Jouyandeh, M.; Laoutid, F.; Vahabi, H.; Saeb, M.R.; Dubois, P. Curing Kinetics and Thermal Stability of Epoxy Composites Containing Newly Obtained Nano-Scale Aluminum Hypophosphite (AlPO2). Polymers 2020, 12, 644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hameed, A.; Islam, M.; Ahmad, I.; Mahmood, N.; Saeed, S.; Javed, H. Thermal and mechanical properties of carbon nano-tube/epoxy nanocomposites reinforced with pristine and functionalized multiwalled carbon nanotubes. Polym. Compos. 2015, 36, 1891–1898. [Google Scholar] [CrossRef]
- Sul, J.-H.; Prusty, B.G.; Crosky, A. Effect of the addition of multi-walled carbon nanotubes on the thermomechanical proper-ties of epoxy resin. Polym. Compos. 2017, 38, 1873–1880. [Google Scholar] [CrossRef]
- Choi, W.J.; Powell, R.L.; Kim, D.S. Curing behavior and properties of epoxy nanocomposites with amine functionalized mul-tiwall carbon nanotubes. Polym. Compos. 2009, 30, 415–421. [Google Scholar] [CrossRef]
- Rajaram, A.N.; Boay, C.G.; Srikanth, N. Effect of curing on the hygrothermal behaviour of epoxy and its carbon composite material. Compos. Commun. 2020, 22, 100507. [Google Scholar] [CrossRef]
- Qian, L.; Qiu, Y.; Sun, N.; Xu, M.; Xu, G.; Xin, F.; Chen, Y. Pyrolysis route of a novel flame retardant constructed by phos-phaphenanthrene and triazine-trione groups and its flame-retardant effect on epoxy resin. Polym. Degrad. Stab. 2014, 107, 98–105. [Google Scholar] [CrossRef]
- Li, W.; Song, B.; Zhang, S.; Zhang, F.; Liu, C.; Zhang, N.; Yao, H.; Shi, Y. Using 3-Isocyanatopropyltrimethoxysilane to Decorate Graphene Oxide with Nano-Titanium Dioxide for Enhancing the Anti-Corrosion Properties of Epoxy Coating. Polymers 2020, 12, 837. [Google Scholar] [CrossRef] [Green Version]
- Buketov, A.V.; Dolgov, N.A.; Sapronov, A.A.; Nigalatii, V.D.; Babich, N.V. Mechanical Characteristics of Epoxy Nanocom-posite Coatings with Ultradisperse Diamond Particles. Strength Mater. 2017, 49, 464–471. [Google Scholar] [CrossRef]
- Wong, T.-T.; Lau, K.-T.; Tam, W.-Y.; Etches, J.A.; Kim, J.-K.; Wu, Y. Effects of silane surfactant on Nano-ZnO and rheology properties of nano-ZnO/epoxy on the UV absorbability of nano-ZnO/epoxy/micron-HGF composite. Compos. Part B 2016, 90, 378–385. [Google Scholar] [CrossRef]
- Lavorgna, M.; Romeo, V.; Martone, A.; Zarrelli, M.; Giordano, M.; Buonocore, G.G.; Qu, M.Z.; Fei, G.X.; Xia, H.S. Silanization and silica enrichment of multiwalled carbon nanotubes: Synergistic effects on the thermal-mechanical properties of epoxy nanocomposites. Eur. Polym. J. 2013, 49, 428–438. [Google Scholar] [CrossRef]
- Shen, J.F.; Huang, W.S.; Wu, L.P.; Hu, Y.Z.; Ye, M.X. The reinforcement role of different amino-functionalized multi-walled carbon nanotubes in epoxy nanocomposites. Compos. Sci. Technol. 2007, 67, 3041–3050. [Google Scholar] [CrossRef]
- Wang, M.; Ma, L.; Li, B.; Zhang, W.; Zheng, H.; Wu, G.; Huang, Y.; Song, G. One-step generation of silica particles onto graphene oxide sheets for superior mechanical properties of epoxy composite and scale application. Compos. Commun. 2020, 22, 100514. [Google Scholar] [CrossRef]



| The Qualitative Characteristics | Value |
|---|---|
| Properties of epoxy resin ED-20 | |
| Content of epoxy groups, % | 20.0–22.5 |
| Viscosity, Pa × s | 13–20 |
| Epoxy equivalent, g/mol | 195–216 |
| Density at 25 °C, kg/m3 | 1166 |
| Properties of PEPA | |
| Molecular mass, g/mol | 230–250 |
| Viscosity, Pa × s | 0.6–0.9 |
| Density at 25 °C, kg/m3 | 1020 |
| Amine number, mg KOH/g | 1250 |
| Nitrogen content, % by weight | 30.0 |
| Properties of ORPP | |
| Viscosity, Pa × s | 0.4–0.6 |
| Density at 25 °C, kg/m3 | 1250–1295 |
| Phosphorus content, % by weight | 10.6–10.8 |
| Acidity, mg KOH/g | 0.10–0.12 |
| Boiling temperature, °C | ≥300 |
| Decomposition temperature, °C | 370 |
| Component | Concentration, % |
|---|---|
| Fe2O3 | 41.2 |
| SiO2 | 22.9 |
| CaO | 20.3 |
| Al2O3 | 10.8 |
| TiO2 | 2.4 |
| CuO | 1.2 |
| Mn2O7 | 0.7 |
| K2O | 0.3 |
| P | 0.1 |
| S | 0.06 |
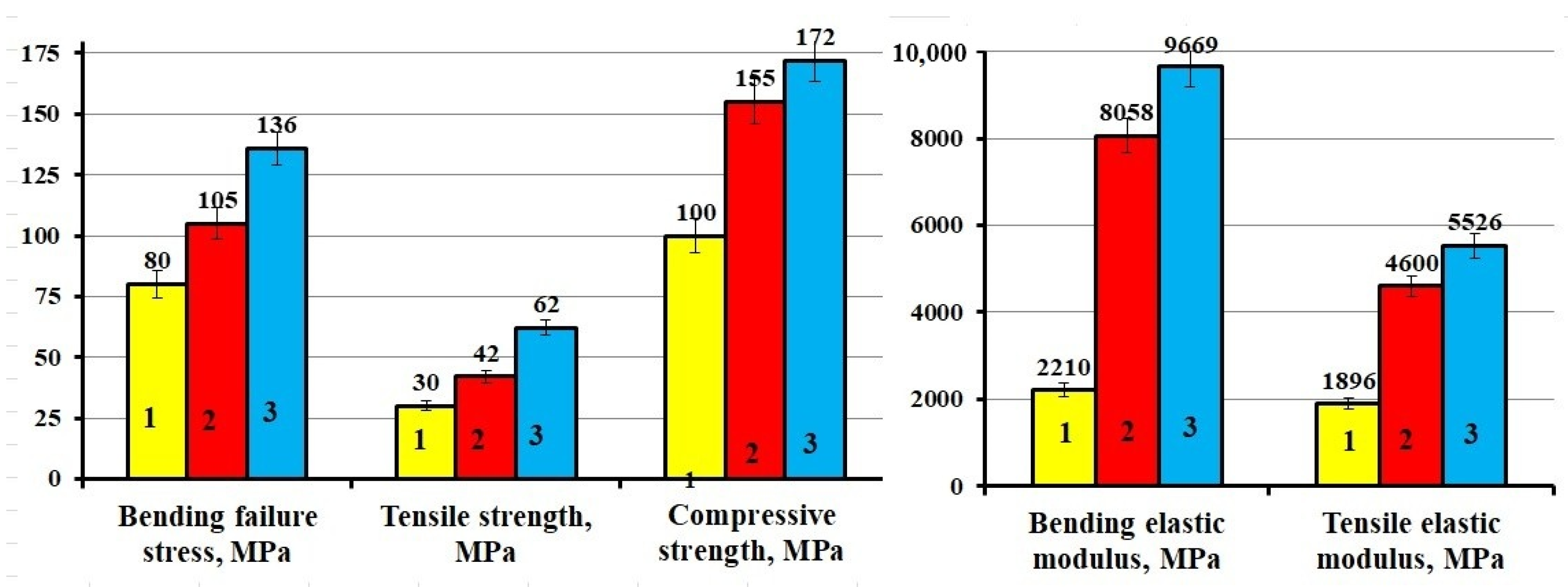
| Composition, Parts by Mass, Cured by 15 Parts by Mass of PEPA | σben, MPa | Eben, MPa | σcom, MPa | σten, MPa | Eten, MPa | aim, kJ/m2 | Hb, MPa | Hv, MPa |
|---|---|---|---|---|---|---|---|---|
| 100ED-20 + 40 ORPP | 80 ± 3.2 | 2210 ± 88 | 100 ± 4.0 | 30 ± 1.5 | 1896 ± 94 | 6 ± 0.3 | 175 ± 10 | 174 ± 10 |
| 100 ED-20 + 40 ORPP + 0.05 Diorite | 82 ± 3.3 | 2255 ± 90 | 105 ± 4.2 | 40 ± 1.8 | 2115 ± 105 | 7 ± 0.4 | 185 ± 11 | 184 ± 11 |
| 100 ED-20 + 40 ORPP + 0.1 Diorite | 95 ± 3.8 | 2343 ± 92 | 115 ± 4.5 | 54 ± 2.4 | 2490 ± 120 | 12 ± 0.7 | 210 ± 12 | 209 ± 12 |
| 100 ED-20 + 40 ORPP + 0.5 Diorite | 90 ± 3.5 | 2506 ± 98 | 120 ± 4.8 | 41 ± 1.8 | 2952 ± 142 | 7 ± 0.4 | 240 ± 14 | 240 ± 14 |
| 100 ED-20 + 40 ORPP + 10 Diorite | 90 ± 3.6 | 3556 ± 140 | 130 ± 5.0 | 40 ± 1.8 | 3100 ± 150 | 5 ± 0.2 | 250 ± 15 | 250 ± 15 |
| 100 ED-20 + 40 ORPP + 30 Diorite | 95 ± 3.7 | 6540 ± 261 | 140 ± 5.2 | 42 ± 2.0 | 4110 ± 202 | 5 ± 0.2 | 275 ± 16 | 278 ± 16 |
| 100 ED-20 + 40 ORPP + 50 Diorite | 105 ± 3.9 | 8058 ± 322 | 155 ± 6.2 | 42 ± 2.0 | 4600 ± 220 | 5 ± 0.2 | 295 ± 17 | 299 ± 17 |
| 100 ED-20 + 40 ORPP + 75 Diorite | 70 ± 2.6 | 8950 ± 355 | 150 ± 6.0 | 38 ± 1.5 | 4920 ± 245 | 4 ± 0.2 | 310 ± 18 | 315 ± 18 |
| 100 ED-20 + 40 ORPP + 100 Diorite | 58 ± 2.1 | 9812 ± 389 | 125 ± 4.8 | 35 ± 1.4 | 5200 ± 260 | 4 ± 0.2 | 340 ± 20 | 351 ± 20 |
| Composition, Parts by Mass | Viscosity, P × s |
|---|---|
| 100 ED-20 + 40 ORPP | 1.50 ± 0.07 |
| 100 ED-20 + 40 ORPP + 0.05 Diorite | 1.50 ± 0.07 |
| 100 ED-20 + 40 ORPP + 0.1 Diorite | 1.55 ± 0.08 |
| 100 ED-20 + 40 ORPP + 0.5 Diorite | 1.60 ± 0.09 |
| 100 ED-20 + 40 ORPP + 10 Diorite | 3.90 ± 0.18 |
| 100 ED-20 + 40 ORPP + 30 Diorite | 15.20 ± 0.65 |
| 100 ED-20 + 40 ORPP + 50 Diorite | 24.50 ± 1.10 |
| 100 ED-20 + 40 ORPP + 75 Diorite | 32.30 ± 1.40 |
| 100 ED-20 + 40 ORPP + 100 Diorite | 40.60 ± 1.80 |
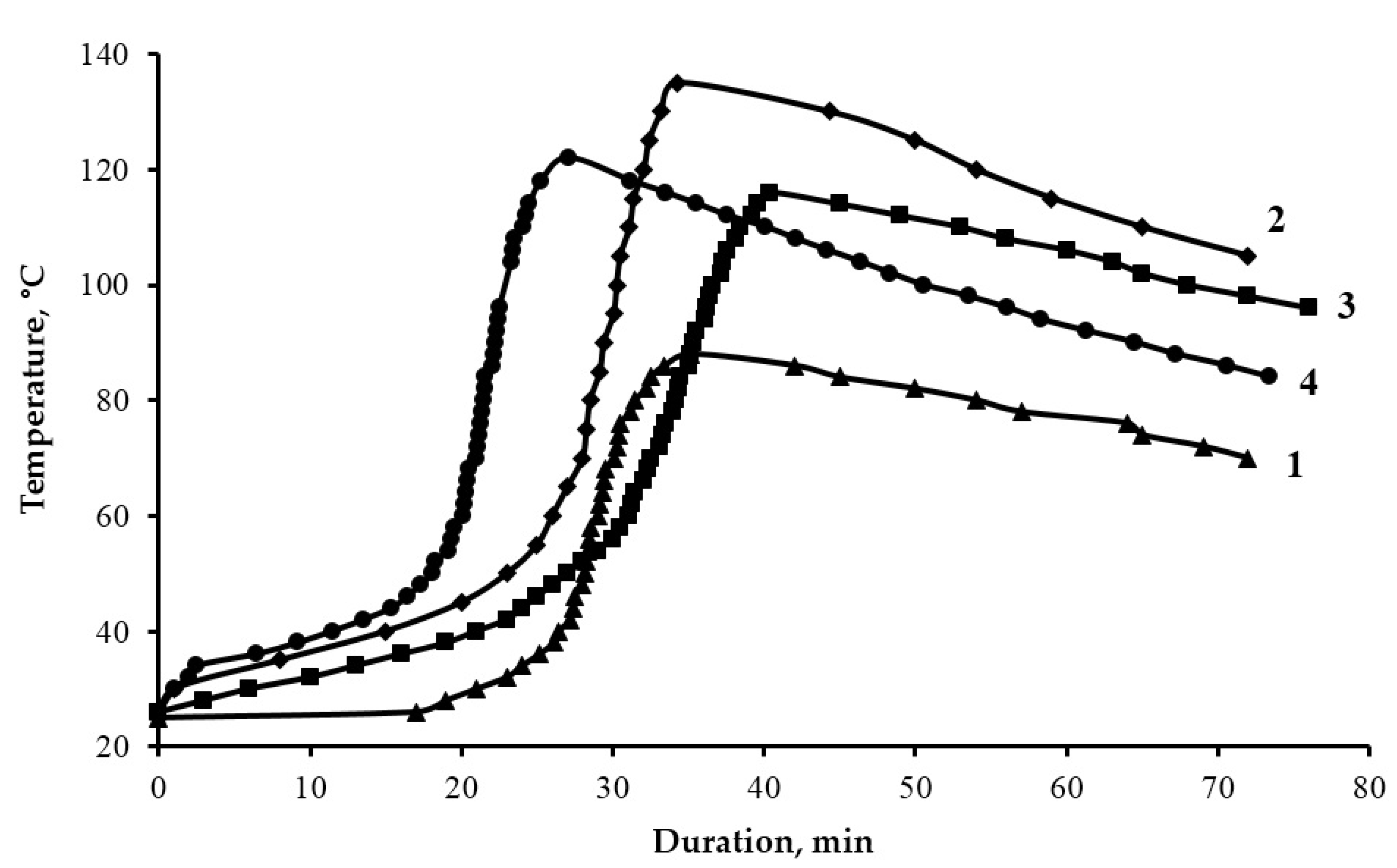
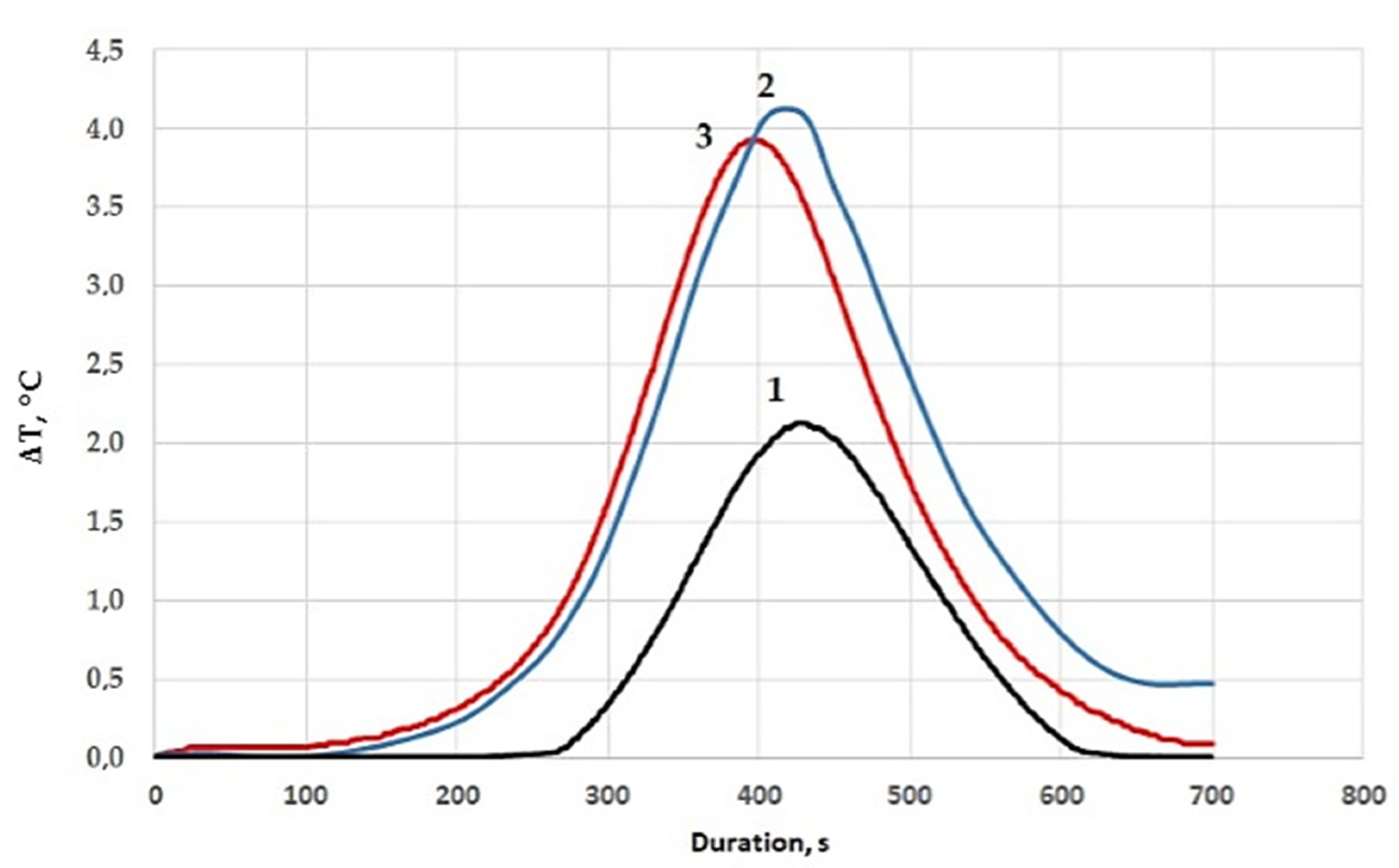
| Composition, Parts by Mass, Cured by 15 Parts by Mass of PEPA | τgel, min | τcur, min | Tmax, °C | X, % |
|---|---|---|---|---|
| 100 ED-20 + 40 ORPP | 27 | 38 | 88 | 90.0 |
| 100 ED-20 + 40 ORPP + 0.1 Diorite | 24 | 34 | 135 | 94.0 |
| 100 ED-20 + 40 ORPP + 50 Diorite | 29 | 40 | 116 | 98.0 |
| 100 ED-20 + 40 ORPP + 50 Diorite(APTES) | 19 | 28 | 122 | 98.6 |
| Composition, Parts by Mass, Cured by 15 Parts by Mass of PEPA | Tstart–Tend Tmax °C | Tglass, °C | H, J/g |
|---|---|---|---|
| 100 ED-20 + 40 ORPP | 46–167 108 | 78 | 415 |
| 100 ED-20 + 40 ORPP + 0.1 Diorite | 36–175 110 | 81 | 893 |
| 100 ED-20 + 40 ORPP + 50 Diorite | 34–211 109 | 84 | 703 |
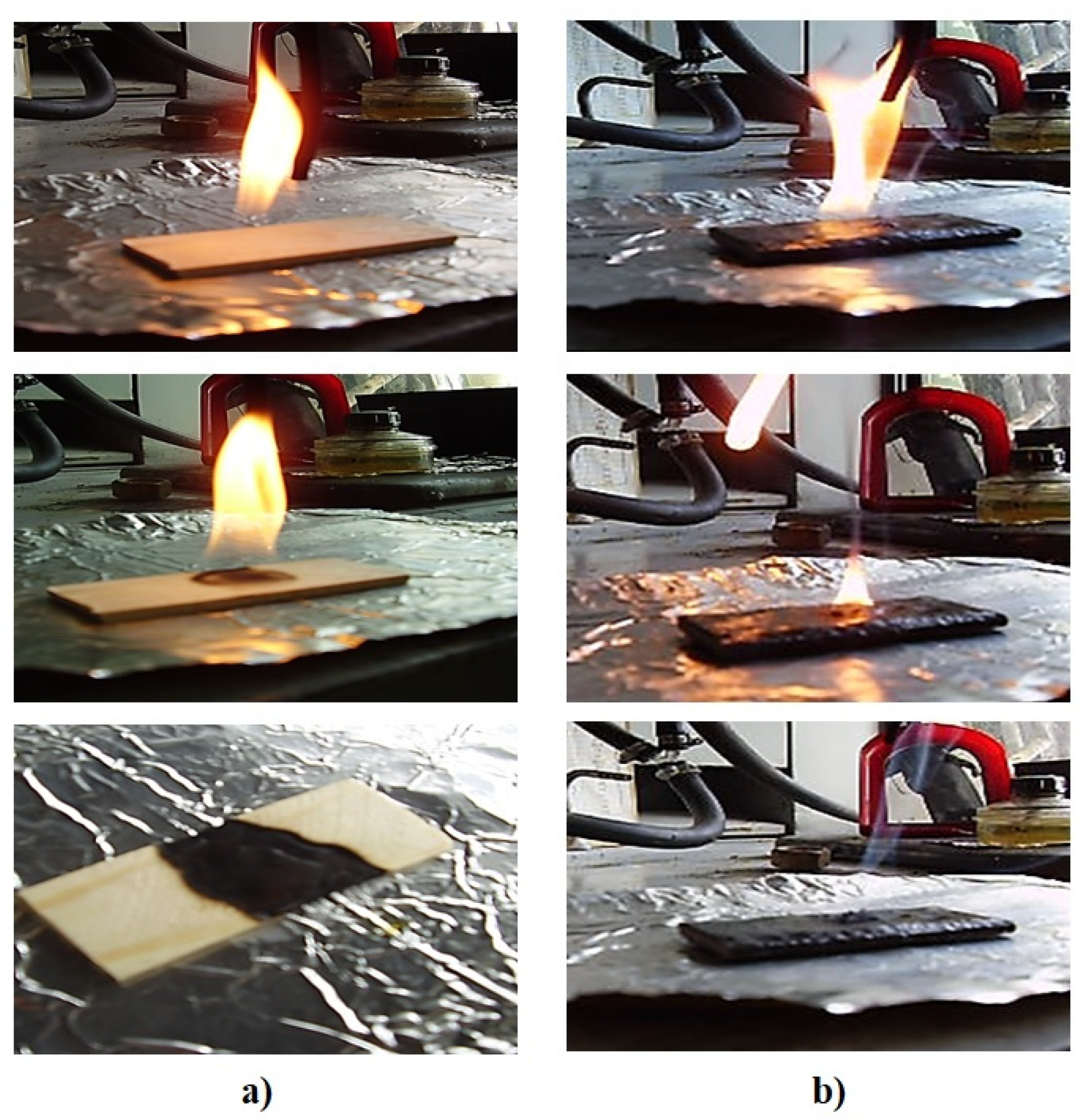
| Composition, Parts by Mass, Cured by 15 Parts by Mass of PEPA | Tin, °C | Tf °C | Yield of CS at Tf, % Mass | Δm, % | OI, % | Tv, °C |
|---|---|---|---|---|---|---|
| 100 ED-20 | 200 | 390 | 40 (390 °C) | 78 | 19 | 86 |
| 100 ED-20 + 40 ORPP | 230 | 370 | 54 (370 °C) | 4.7 | 28 | 132 |
| 100 ED-20 + 40 ORPP + 0.1 Diorite | 240 | 380 | 56 (380 °C) | 4.4 | 28 | 140 |
| 100 ED-20 + 40 ORPP + 50 Diorite | 245 | 380 | 70 (380 °C) | 2.2 | 30 | 180 |
| 100 ED-20 + 40 ORPP + 100 Diorite | 245 | 370 | 77 (370 °C) | 1.8 | 32 | 188 |
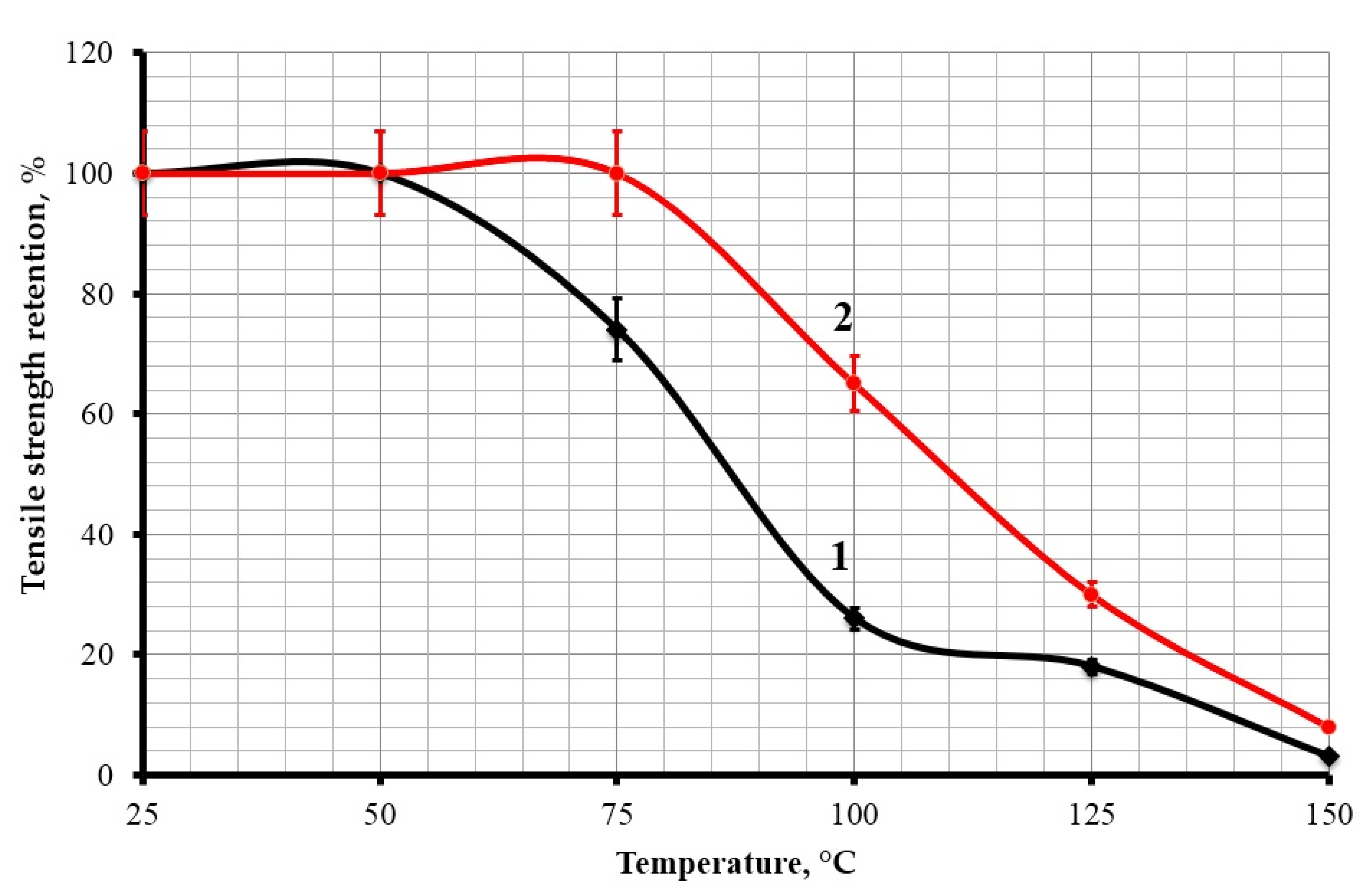
| Composition | σben, MPa | Eben, MPa | σcom, MPa | σten, MPa | Eten, MPa |
|---|---|---|---|---|---|
| ED-20 + ORPP + PEPA + Diorite(APTES) | 136 | 9669 | 172 | 62 | 5526 |
| Analogs | |||||
| Epoxy resin + TETA + Fly ash [16] | - | - | 160 | 48 | - |
| DER-331 + JOINTMINE 905-3s + MMT [18] | - | - | - | 50 | 1500 |
| ED-20 + ORPP + PEPA + Ocher [7] | 102 | 10,163 | 156 | 45 | 4110 |
| ED-20 + PEPA + UDD [4] | 102 | 3200 | - | - | - |
| Epoxy resin + TETA + Granite(TEMS) [19] | 79 | 5126 | 107 | - | - |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekeshev, A.; Mostovoy, A.; Kadykova, Y.; Akhmetova, M.; Tastanova, L.; Lopukhova, M. Development and Analysis of the Physicochemical and Mechanical Properties of Diorite-Reinforced Epoxy Composites. Polymers 2021, 13, 2421. https://doi.org/10.3390/polym13152421
Bekeshev A, Mostovoy A, Kadykova Y, Akhmetova M, Tastanova L, Lopukhova M. Development and Analysis of the Physicochemical and Mechanical Properties of Diorite-Reinforced Epoxy Composites. Polymers. 2021; 13(15):2421. https://doi.org/10.3390/polym13152421
Chicago/Turabian StyleBekeshev, Amirbek, Anton Mostovoy, Yulia Kadykova, Marzhan Akhmetova, Lyazzat Tastanova, and Marina Lopukhova. 2021. "Development and Analysis of the Physicochemical and Mechanical Properties of Diorite-Reinforced Epoxy Composites" Polymers 13, no. 15: 2421. https://doi.org/10.3390/polym13152421






