Chitosan: An Overview of Its Properties and Applications
Abstract
:1. Introduction
2. Technological Chitosan Properties
2.1. Solubility
2.2. Viscosity
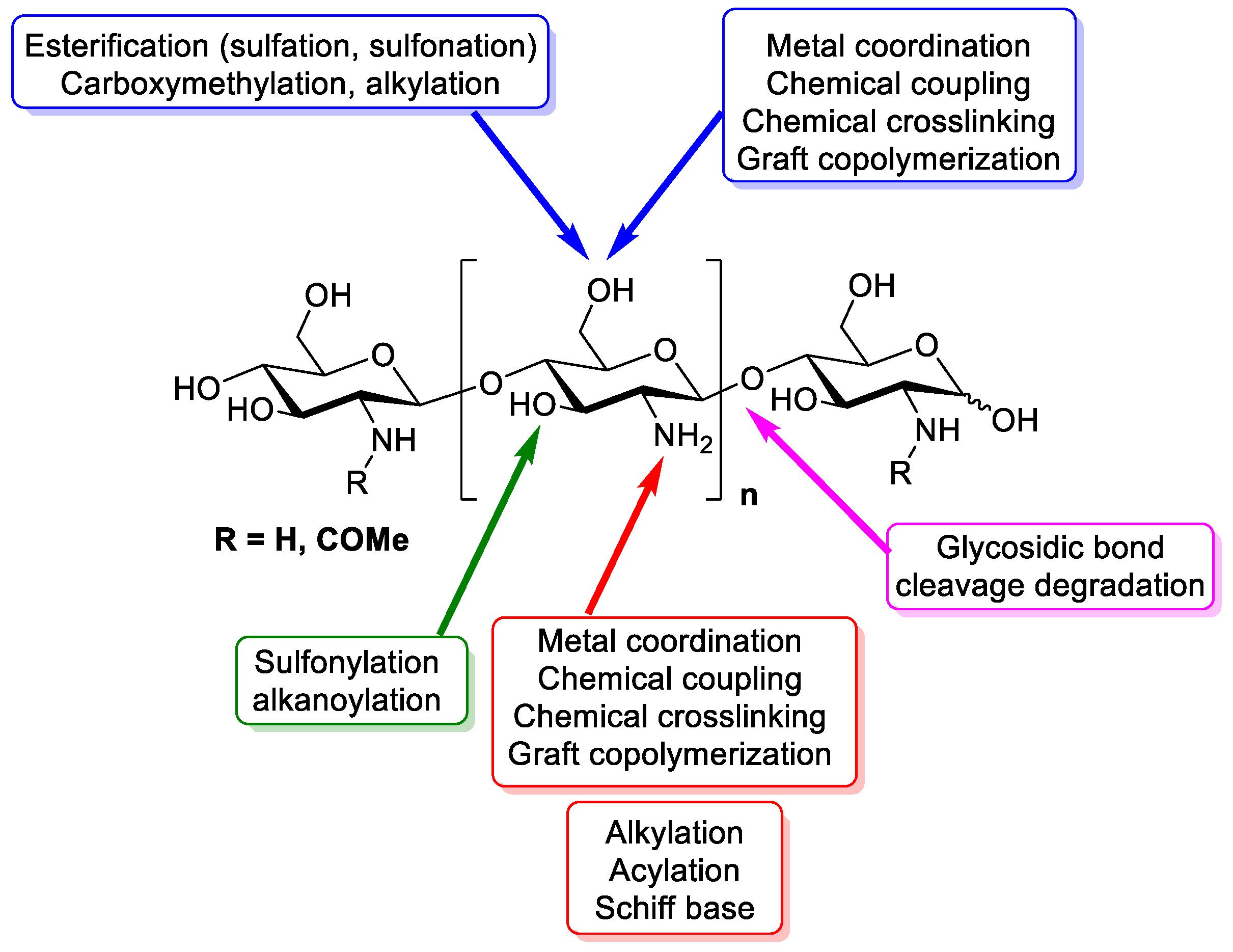
3. Chemistry of Chitosan
4. Biological Properties
4.1. Antimicrobial Activity
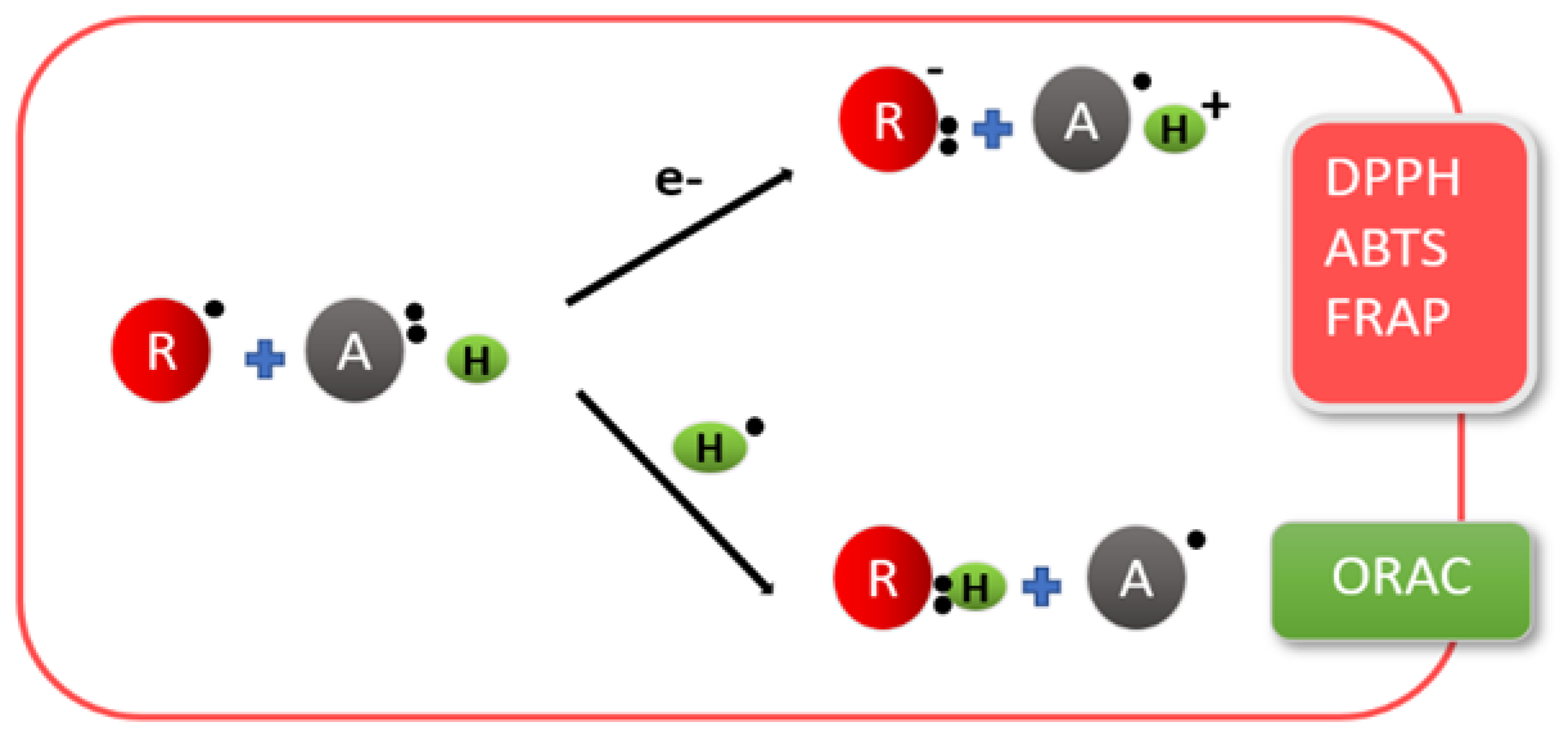
4.2. Antioxidant Activity
4.3. Anti-Inflammatory Properties
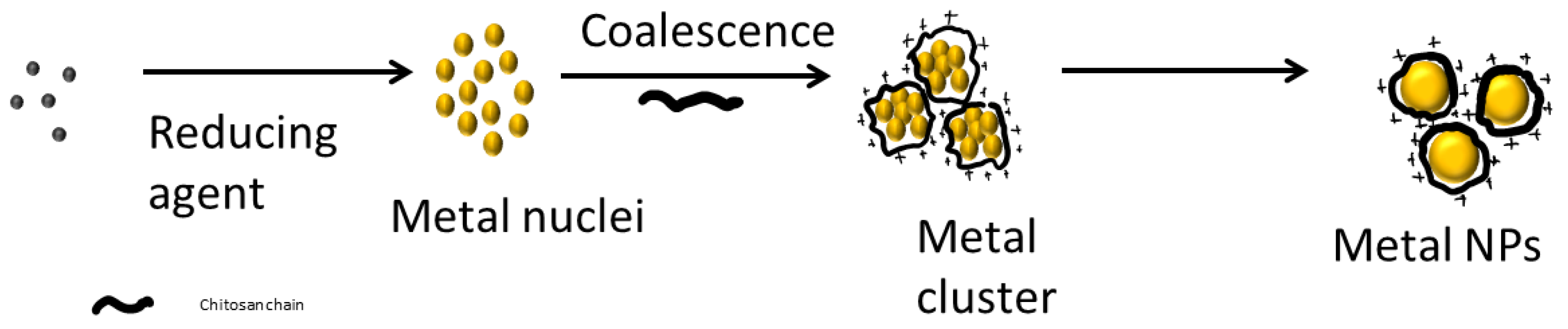
5. Metallic Nanoparticles and Chitosan
6. Chitosan in Biocatalysis
7. Chitosan in Drug Delivery
8. Conclusions and Prognosis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghormade, V.; Pathan, E.K.; Deshpande, M.V. Can fungi compete with marine sources for chitosan production? Int. J. Biol. Macromol. 2017, 104, 1415–1421. [Google Scholar] [CrossRef]
- Aranaz, I.; Mengíbar, M.; Harris, R.; Paños, I.; Miralles, B.; Acosta, N.; Galed, G.; Heras, A. Functional characterization of chitin and chitosan. Curr. Chem. Biol. 2009, 3, 203–230. [Google Scholar]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Jiang, Y.; Fu, C.; Wu, S.; Liu, G.; Guo, J.; Su, Z. Determination of the deacetylation degree of chitooligosaccharides. Mar. Drugs 2017, 15, 332. [Google Scholar] [CrossRef]
- Ways, T.M.M.; Lau, W.M.; Khutoryanskiy, V.V. Chitosan and its derivatives for application in mucoadhesive drug delivery systems. Polymers 2018, 10, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sizílio, R.H.; Galvão, J.G.; Trindade, G.G.G.; Pina, L.T.S.; Andrade, L.N.; Gonsalves, J.K.M.C.; Lira, A.A.M.; Chaud, M.V.; Alves, T.F.R.; Arguelho, M.L.P.M.; et al. Chitosan/pvp-based mucoadhesive membranes as a promising delivery system of betamethasone-17-valerate for aphthous stomatitis. Carbohydr. Polym. 2018, 190, 339–345. [Google Scholar] [CrossRef] [PubMed]
- Azuma, K.; Osaki, T.; Minami, S.; Okamoto, Y. Anticancer and Anti-Inflammatory Properties of Chitin and Chitosan Oligosaccharides. J. Funct. Biomater. 2015, 6, 33–49. [Google Scholar] [CrossRef] [PubMed]
- Avelelas, F.; Horta, A.; Pinto, L.F.V.; Marques, S.C.; Nunes, P.M.; Pedrosa, R.; Leandro, S.M. Antifungal and antioxidant properties of chitosan polymers obtained from nontraditional Polybius henslowii sources. Mar. Drugs 2019, 17, 239. [Google Scholar] [CrossRef] [Green Version]
- Ke, C.L.; Deng, F.S.; Chuang, C.Y.; Lin, C.H. Antimicrobial actions and applications of Chitosan. Polymers 2021, 13, 904. [Google Scholar] [CrossRef]
- Shih, P.Y.; Liao, Y.T.; Tseng, Y.K.; Deng, F.S.; Lin, C.H. A potential antifungal effect of chitosan against candida albicansis mediated via the inhibition of SAGA complex component expression and the subsequent alteration of cell surface integrity. Front. Microbiol. 2019, 10, 602. [Google Scholar] [CrossRef]
- Sarkar, S.; Das, D.; Dutta, P.; Kalita, J.; Wann, S.B.; Manna, P. Chitosan: A promising therapeutic agent and effective drug delivery system in managing diabetes mellitus. Carbohydr. Polym. 2020, 247, 116594. [Google Scholar] [CrossRef]
- Amirani, E.; Hallajzadeh, J.; Asemi, Z.; Mansournia, M.A.; Yousefi, B. Effects of chitosan and oligochitosans on the phosphatidylinositol 3-kinase-AKT pathway in cancer therapy. Int. J. Biol. Macromol. 2020, 164, 456–467. [Google Scholar] [CrossRef] [PubMed]
- Ueno, H.; Mori, T.; Fujinaga, T. Topical formulations and wound healing applications of chitosan. Adv. Drug Deliv. Rev. 2001, 52, 105–115. [Google Scholar] [CrossRef]
- Chitosan Market Size, Share & Trends Analysis Report by Application (Pharmaceutical & Biomedical, Water Treatment, Cosmetics, Food & Beverage), By Region (APAC, North America, Europe, MEA), and Segment Forecasts, 2020–2027); Report ID: 978-1-68038-798-8; Grand View Research: San Francisco, CA, USA, 2020; Available online: https://www.grandviewresearch.com/industry-analysis/global-chitosan-market (accessed on 31 August 2020).
- Kaczmarek, M.B.; Struszczyk-Swita, K.; Li, X.; Szczęsna-Antczak, M.; Daroch, M. Enzymatic modifications of chitin, chitosan, and chitooligosaccharides. Front. Bioeng. Biotechnol. 2019, 7, 243. [Google Scholar] [CrossRef] [Green Version]
- Domard, A. pH and c.d. measurements on a fully deacetylated chitosan: Application to CuII-polymer interactions. Int. J. Biol. Macromol. 1987, 9, 98–104. [Google Scholar] [CrossRef]
- Rinaudo, M.; Pavlov, G.; Desbrières, J. Solubilization of Chitosan in Strong Acid Medium. Int. J. Polym. Anal. Charact. 1999, 5, 267–276. [Google Scholar] [CrossRef]
- Cho, Y.W.; Jang, J.; Park, C.R.; Ko, S.W. Preparation and solubility in acid and water of partially deacetylated chitins. Biomacromolecules 2000, 1, 609–614. [Google Scholar] [CrossRef]
- Sogias, I.A.; Khutoryanskiy, V.V.; Williams, A.C. Exploring the factors affecting the solubility of chitosan in water. Macromol. Chem. Phys. 2010, 211, 426–433. [Google Scholar] [CrossRef]
- Kasaai, M.R.; Arul, J.; Charlet, G. Intrinsic viscosity-molecular weight relationship for chitosan. J. Polym. Sci. Part B 2000, 38, 2591–2598. [Google Scholar] [CrossRef]
- Chattopadhyay, D.P.; Inamdar, M.S. Aqueous behaviour of chitosan. Int. J. Polym. Sci. 2010, 2010, 1–7. [Google Scholar] [CrossRef]
- Wang, W.; Xu, D. Viscosity and flow properties of concentrated solutions of chitosan with different degrees of deacetylation. Int. J. Biol. Macromol. 1994, 16, 149–152. [Google Scholar] [CrossRef]
- Aranaz, I.; Harris, R.; Heras, A. Chitosan amphiphilic derivatives. Chemistry and applications. Curr. Org. Chem. 2010, 14, 308–330. [Google Scholar] [CrossRef]
- Ding, K.; Wang, Y.; Wang, H.; Yuan, L.; Tan, M.; Shi, X.; Lyu, Z.; Liu, Y.; Chen, H. 6-O-sulfated chitosan promoting the neural differentiation of mouse embryonic stem cells. ACS Appl. Mater. Interfaces 2014, 6, 20043–20050. [Google Scholar] [CrossRef]
- Coquery, C.; Negrell, C.; Caussé, N.; Pébère, N.; David, G. Synthesis of new high molecular weight phosphorylated chitosans for improving corrosion protection. Pure Appl. Chem. 2019, 91, 509–521. [Google Scholar] [CrossRef]
- Heras, A.; Rodríguez, N.M.; Ramos, V.M.; Agulló, E. N-methylene phosphonic chitosan: A novel soluble derivative. Carbohydr. Polym. 2001, 44, 1–8. [Google Scholar] [CrossRef]
- Ramos, V.M.; Rodríguez, N.M.; Rodríguez, M.S.; Heras, A.; Agulló, E. Modified chitosan carrying phosphonic and alkyl groups. Carbohydr. Polym. 2003, 51, 425–429. [Google Scholar] [CrossRef]
- Jayakumar, R.; Reis, R.L.; Mano, J.F. Synthesis and characterization of N-methylenephenyl phosphonic chitosan. J. Macromol. Sci. Part A Pure Appl. Chem. 2007, 44, 271–275. [Google Scholar] [CrossRef] [Green Version]
- Ramos, V.M.; Rodríguez, M.S.; Agulló, E.; Rodríguez, N.M.; Heras, A. Chitosan with phosphonic and carboxylic group: New multidentate ligands. Int. J. Polym. Mater. 2002, 51, 711–720. [Google Scholar] [CrossRef]
- Ramos, V.M.; Rodríguez, N.M.; Díaz, M.F.; Rodríguez, M.S.; Heras, A.; Agulló, E. N-methylene phosphonic chitosan. Effect of preparation methods on its properties. Carbohydr. Polym. 2003, 52, 39–46. [Google Scholar] [CrossRef]
- Zhu, D.; Yao, K.; Bo, J.; Zhang, H.; Liu, L.; Dong, X.; Song, L.; Leng, X. Hydrophilic/lipophilic N-methylene phosphonic chitosan as a promising non-viral vector for gene delivery. J. Mater. Sci. Mater. Med. 2010, 21, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Kasaai, M.R.; Arul, J.; Charlet, G. Fragmentation of chitosan by ultrasonic irradiation. Ultrason. Sonochem. 2008, 15, 1001–1008. [Google Scholar] [CrossRef]
- Wu, T.; Zivanovic, S.; Hayes, D.G.; Weiss, J. Efficient reduction of chitosan molecular weight by high-intensity ultrasound: Underlying mechanism and effect of process parameters. J. Agric. Food Chem. 2008, 56, 5112–5119. [Google Scholar] [CrossRef]
- Chang, K.L.B.; Tai, M.C.; Cheng, F.H. Kinetics and products of the degradation of chitosan by hydrogen peroxide. J. Agric. Food Chem. 2001, 49, 4845–4851. [Google Scholar] [CrossRef]
- Vårum, K.M.; Holme, H.K.; Izume, M.; Stokke, B.T.; Smidsrød, O. Determination of enzymatic hydrolysis specificity of partially N-acetylated chitosans. Biochim. Biophys. Acta Gen. Subj. 1996, 1291, 5–15. [Google Scholar] [CrossRef]
- Skjak-Braek, G.; Anthonsen, T.; Sandford, P. (Eds.) Chitin and Chitosan: Sources, Chemistry, Biochemistry, Physical Properties and Applications; Elsevier Applied Science: London, UK, 1989. [Google Scholar]
- Varum, K.M.; Ottoy, M.H.; Smidsrod, O. Acid hydrolysis of chitosans. Carbohydr. Polym. 2001, 46, 89–98. [Google Scholar] [CrossRef]
- Cabrera, J.C.; Van Cutsem, P. Preparation of chitooligosaccharides with degree of polymerization higher than 6 by acid or enzymatic degradation of chitosan. Biochem. Eng. J. 2005, 25, 165–172. [Google Scholar] [CrossRef]
- Thadathil, N.; Velappan, S.P. Recent developments in chitosanase research and its biotechnological applications: A review. Food Chem. 2014, 150, 392–399. [Google Scholar] [CrossRef]
- Aam, B.B.; Heggset, E.B.; Norberg, A.L.; Sørlie, M.; Vårum, K.M.; Eijsink, V.G.H. Production of chitooligosaccharides and their potential applications in medicine. Mar. Drugs 2010, 8, 1482–1517. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, A.B.V.; Tharanathan, R.N. A comparative study on depolymerization of chitosan by proteolytic enzymes. Carbohydr. Polym. 2004, 58, 275–283. [Google Scholar]
- Li, J.; Du, Y.; Yang, J.; Feng, T.; Li, A.; Chen, P. Preparation and characterisation of low molecular weight chitosan and chito-oligomers by a commercial enzyme. Polym. Degrad. Stab. 2005, 87, 441–448. [Google Scholar] [CrossRef]
- Li, J.; Du, Y.; Liang, H. Influence of molecular parameters on the degradation of chitosan by a commercial enzyme. Polym. Degrad. Stab. 2007, 92, 515–524. [Google Scholar] [CrossRef]
- Qin, C.; Du, Y.; Zong, L.; Zeng, F.; Liu, Y.; Zhou, B. Effect of hemicellulase on the molecular weight and structure of chitosan. Polym. Degrad. Stab. 2003, 80, 435–441. [Google Scholar] [CrossRef]
- Lund, K.E.; Nielsen, H.H. Proteolysis in salmon (Salmo salar) during cold storage; effects of storage time and smoking process. J. Food Biochem. 2001, 25, 379–395. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.X.; Xia, W.S.; Zhang, J.L. Enzymatic preparation of chitooligosaccharides by commercial lipase. Food Chem. 2008, 111, 291–295. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.S.; Lee, Y.C.; Chan, L. The degradation of chitosan with the aid of lipase from Rhizopus japonicus for the production of soluble chitosan. J. Food Biochem. 2001, 25, 307–321. [Google Scholar] [CrossRef]
- Raafat, D.; Sahl, H.G. Chitosan and its antimicrobial potential—A critical literature survey. Microb. Biotechnol. 2009, 2, 186–201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raafat, D.; Von Bargen, K.; Haas, A.; Sahl, H.G. Insights into the mode of action of chitosan as an antibacterial compound. Appl. Environ. Microbiol. 2008, 74, 3764–3773. [Google Scholar] [CrossRef] [Green Version]
- Yildirim-Aksoy, M.; Beck, B.H. Antimicrobial activity of chitosan and a chitosan oligomer against bacterial pathogens of warmwater fish. J. Appl. Microbiol. 2017, 122, 1570–1578. [Google Scholar] [CrossRef]
- Tayel, A.A.; Moussa, S.; El-Tras, W.F.; Knittel, D.; Opwis, K.; Schollmeyer, E. Anticandidal action of fungal chitosan against Candida albicans. Int. J. Biol. Macromol. 2010, 47, 454–457. [Google Scholar] [CrossRef] [PubMed]
- Hosseinnejad, M.; Jafari, S.M. Evaluation of different factors affecting antimicrobial properties of chitosan. Int. J. Biol. Macromol. 2016, 85, 467–475. [Google Scholar] [CrossRef]
- Seyfarth, F.; Schliemann, S.; Elsner, P.; Hipler, U.C. Antifungal effect of high- and low-molecular-weight chitosan hydrochloride, carboxymethyl chitosan, chitosan oligosaccharide and N-acetyl-D-glucosamine against Candida albicans, Candida krusei and Candida glabrata. Int. J. Pharm. 2008, 353, 139–148. [Google Scholar] [CrossRef]
- Bano, I.; Arshad, M.; Yasin, T.; Ghauri, M.A.; Younus, M. Chitosan: A potential biopolymer for wound management. Int. J. Biol. Macromol. 2017, 102, 380–383. [Google Scholar] [CrossRef]
- Younes, I.; Rinaudo, M. Chitin and chitosan preparation from marine sources. Structure, properties and applications. Mar. Drugs 2015, 13, 1133–1174. [Google Scholar] [CrossRef] [Green Version]
- Abd El-Hack, M.E.; El-Saadony, M.T.; Shafi, M.E.; Zabermawi, N.M.; Arif, M.; Batiha, G.E.; Khafaga, A.F.; Abd El-Hakim, Y.M.; Al-Sagheer, A.A. Antimicrobial and antioxidant properties of chitosan and its derivatives and their applications: A review. Int. J. Biol. Macromol. 2020, 164, 2726–2744. [Google Scholar] [CrossRef] [PubMed]
- Chien, R.C.; Yen, M.T.; Mau, J.L. Antimicrobial and antitumor activities of chitosan from shiitake stipes, compared to commercial chitosan from crab shells. Carbohydr. Polym. 2016, 138, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Sarhan, W.A.; Azzazy, H.M.E.; El-Sherbiny, I.M. Honey/Chitosan Nanofiber Wound Dressing Enriched with Allium sativum and Cleome droserifolia: Enhanced Antimicrobial and Wound Healing Activity. ACS Appl. Mater. Interfaces 2016, 8, 6379–6390. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Dong, Y.; Song, A.; Yin, R.; Li, S. Alginate/chitosan based bi-layer composite membrane as potential sustained-release wound dressing containing ciprofloxacin hydrochloride. Appl. Surf. Sci. 2014, 311, 626–634. [Google Scholar] [CrossRef]
- Mukhtar Ahmed, K.B.; Khan, M.M.A.; Siddiqui, H.; Jahan, A. Chitosan and its oligosaccharides, a promising option for sustainable crop production- a review. Carbohydr. Polym. 2020, 227. [Google Scholar] [CrossRef] [PubMed]
- No, H.K.; Kim, S.H.; Lee, S.H.; Park, N.Y.; Prinyawiwatkul, W. Stability and antibacterial activity of chitosan solutions affected by storage temperature and time. Carbohydr. Polym. 2006, 65, 174–178. [Google Scholar] [CrossRef]
- Lee, B.C.; Kim, M.S.; Choi, S.H.; Kim, K.Y.; Kim, T.S. In vitro and in vivo antimicrobial activity of water-soluble chitosan oligosaccharides against Vibrio vulnificus. Int. J. Mol. Med. 2009, 24, 327–333. [Google Scholar]
- Sun, Z.; Shi, C.; Wang, X.; Fang, Q.; Huang, J. Synthesis, characterization, and antimicrobial activities of sulfonated chitosan. Carbohydr. Polym. 2017, 155, 321–328. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, A.M.M.; Dorkoosh, F.A.; Avadi, M.R.; Saadat, P.; Rafiee-Tehrani, M.; Junginger, H.E. Preparation, characterization and antibacterial activities of chitosan, N-trimethyl chitosan (TMC) and N-diethylmethyl chitosan (DEMC) nanoparticles loaded with insulin using both the ionotropic gelation and polyelectrolyte complexation methods. Int. J. Pharm. 2008, 355, 299–306. [Google Scholar] [CrossRef]
- Si, Z.; Hou, Z.; Vikhe, Y.S.; Thappeta, K.R.V.; Marimuthu, K.; De, P.P.; Ng, O.T.; Li, P.; Zhu, Y.; Pethe, K.; et al. Antimicrobial effect of a novel chitosan derivative and its synergistic effect with antibiotics. ACS Appl. Mater. Interfaces 2021, 13, 3237–3245. [Google Scholar] [CrossRef]
- Hamed, A.A.; Abdelhamid, I.A.; Saad, G.R.; Elkady, N.A.; Elsabee, M.Z. Synthesis, characterization and antimicrobial activity of a novel chitosan Schiff bases based on heterocyclic moieties. Int. J. Biol. Macromol. 2020, 153, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Sirisinha, C.; Sittichokchuchai, W. Molecular weight effect on antimicrobial activity of chitosan treated cotton fabrics. J. Appl. Polym. Sci. 2001, 80, 2495–2501. [Google Scholar]
- No, H.K.; Young Park, N.; Ho Lee, S.; Meyers, S.P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int. J. Food Microbiol. 2002, 74, 65–72. [Google Scholar] [CrossRef]
- Omura, Y.; Shigemoto, M.; Akiyama, T.; Saimoto, H.; Shigemasa, Y.; Nakamura, I.; Tsuchido, T. Antimicrobial activity of chitosan with different degrees of acetylation and molecular weights. Biocontrol Sci. 2003, 8, 25–30. [Google Scholar] [CrossRef] [Green Version]
- Šimůnek, J.; Brandysová, V.; Koppová, I.; Šimůnek, J., Jr. The antimicrobial action of chitosan, low molar mass chitosan, and chitooligosaccharides on human colonic bacteria. Folia Microbiol. 2012, 57, 341–345. [Google Scholar] [CrossRef]
- Tokura, S.; Ueno, K.; Miyazaki, S.; Nishi, N. Molecular Weight Dependent Antimicrobial Activity by Chitosan. Macromol. Symp. 1997, 120, 1–9. [Google Scholar] [CrossRef]
- Liu, N.; Chen, X.G.; Park, H.J.; Liu, C.G.; Liu, C.S.; Meng, X.H.; Yu, L.J. Effect of MW and concentration of chitosan on antibacterial activity of Escherichia coli. Carbohydr. Polym. 2006, 64, 60–65. [Google Scholar] [CrossRef]
- Chang, S.H.; Lin, H.T.V.; Wu, G.J.; Tsai, G.J. pH Effects on solubility, zeta potential, and correlation between antibacterial activity and molecular weight of chitosan. Carbohydr. Polym. 2015, 134, 74–81. [Google Scholar] [CrossRef] [PubMed]
- Mellegård, H.; Strand, S.P.; Christensen, B.E.; Granum, P.E.; Hardy, S.P. Antibacterial activity of chemically defined chitosans: Influence of molecular weight, degree of acetylation and test organism. Int. J. Food Microbiol. 2011, 148, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Younes, I.; Sellimi, S.; Rinaudo, M.; Jellouli, K.; Nasri, M. Influence of acetylation degree and molecular weight of homogeneous chitosans on antibacterial and antifungal activities. Int. J. Food Microbiol. 2014, 185, 57–63. [Google Scholar] [CrossRef] [PubMed]
- Qin, C.; Li, H.; Xiao, Q.; Liu, Y.; Zhu, J.; Du, Y. Water-solubility of chitosan and its antimicrobial activity. Carbohydr. Polym. 2006, 63, 367–374. [Google Scholar] [CrossRef]
- Sánchez, Á.; Mengíbar, M.; Rivera-Rodríguez, G.; Moerchbacher, B.; Acosta, N.; Heras, A. The effect of preparation processes on the physicochemical characteristics and antibacterial activity of chitooligosaccharides. Carbohydr. Polym. 2017, 157, 251–257. [Google Scholar] [CrossRef]
- Giacco, F.; Brownlee, M. Oxidative stress and diabetic complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [Green Version]
- Singh, A.; Kukreti, R.; Saso, L.; Kukreti, S. Oxidative stress: A key modulator in neurodegenerative diseases. Molecules 2019, 24, 1583. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Kogan, G.; Skorik, Y.A.; Žitňanová, I.; Križková, L.; Ďuračková, Z.; Gomes, C.A.R.; Yatluk, Y.G.; Krajčovič, J. Antioxidant and antimutagenic activity of N-(2-carboxyethyl) chitosan. Toxicol. Appl. Pharmacol. 2004, 201, 303–310. [Google Scholar] [CrossRef]
- Xing, R.; Yu, H.; Liu, S.; Zhang, W.; Zhang, Q.; Li, Z.; Li, P. Antioxidant activity of differently regioselective chitosan sulfates in vitro. Bioorgan. Med. Chem. 2005, 13, 1387–1392. [Google Scholar] [CrossRef]
- Zhou, J.; Wen, B.; Xie, H.; Zhang, C.; Bai, Y.; Cao, H.; Che, Q.; Guo, J.; Su, Z. Advances in the preparation and assessment of the biological activities of chitosan oligosaccharides with different structural characteristics. Food. Funct. 2021, 12, 926–951. [Google Scholar] [CrossRef]
- Ngo, D.H.; Qian, Z.J.; Vo, T.S.; Ryu, B.; Ngo, D.N.; Kim, S.K. Antioxidant activity of gallate-chitooligosaccharides in mouse macrophage RAW264.7 cells. Carbohydr. Polym. 2011, 84, 1282–1288. [Google Scholar] [CrossRef]
- Ngo, D.H.; Qian, Z.J.; Ngo, D.N.; Vo, T.S.; Wijesekara, I.; Kim, S.K. Gallyl chitooligosaccharides inhibit intracellular free radical-mediated oxidation. Food Chem. 2011, 128, 974–981. [Google Scholar] [CrossRef]
- Eom, T.K.; Senevirathne, M.; Kim, S.K. Synthesis of phenolic acid conjugated chitooligosaccharides and evaluation of their antioxidant activity. Environ. Toxicol. Pharmacol. 2012, 34, 519–527. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Zhou, D.; Xie, J.; Mao, F. Preparation of chitosan oligomers and their antioxidant activity. Eur. Food Res. Technol. 2007, 225, 451–456. [Google Scholar] [CrossRef]
- Pu, S.; Li, J.; Sun, L.; Zhong, L.; Ma, Q. An in vitro comparison of the antioxidant activities of chitosan and green synthesized gold nanoparticles. Carbohydr. Polym. 2019, 211, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Chang, S.H.; Lin, Y.Y.; Wu, G.J.; Huang, C.H.; Tsai, G.J. Effect of chitosan molecular weight on anti-inflammatory activity in the RAW 264.7 macrophage model. Int. J. Biol. Macromol. 2019, 131, 167–175. [Google Scholar] [CrossRef]
- Santos-Moriano, P.; Fernandez-Arrojo, L.; Mengibar, M.; Belmonte-Reche, E.; Peñalver, P.; Acosta, F.N.; Ballesteros, A.O.; Morales, J.C.; Kidibule, P.; Fernandez-Lobato, M.; et al. Enzymatic production of fully deacetylated chitooligosaccharides and their neuroprotective and anti-inflammatory properties. Biocatal. Biotransform. 2018, 36, 57–67. [Google Scholar] [CrossRef] [Green Version]
- Santos-Moriano, P.; Kidibule, P.; Míguez, N.; Fernández-Arrojo, L.; Ballesteros, A.O.; Fernández-Lobato, M.; Plou, F.J. Tailored enzymatic synthesis of chitooligosaccharides with different deacetylation degrees and their anti-inflammatory activity. Catalysts 2019, 9, 405. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Senevirathne, M.; Ahn, C.B.; Kim, S.K.; Je, J.Y. Factors affecting anti-inflammatory effect of chitooligosaccharides in lipopolysaccharides-induced RAW264.7 macrophage cells. Bioorg. Med. Chem. Lett. 2009, 19, 6655–6658. [Google Scholar] [CrossRef]
- Mao, S.; Wang, B.; Yue, L.; Xia, W. Effects of citronellol grafted chitosan oligosaccharide derivatives on regulating anti-inflammatory activity. Carbohydr. Polym. 2021, 262, 117972. [Google Scholar] [CrossRef]
- Sánchez, Á.; Mengíbar, M.; Fernández, M.; Alemany, S.; Heras, A.; Acosta, N. Influence of preparation methods of chitooligosaccharides on their physicochemical properties and their anti-inflammatory effects in mice and in RAW264.7 macrophages. Mar. Drugs 2018, 16, 430. [Google Scholar] [CrossRef] [Green Version]
- Watanabe, K. Photochemistry on nanoparticles. In Encyclopedia of Interfacial Chemistry: Surface Science and Electrochemistry; Elsevier: Amsterdam, The Netherlands, 2018; pp. 563–572. [Google Scholar] [CrossRef]
- Pal, D.; Saha, S. Current status and prospects of chitosan: Metal nanoparticles and their applications as nanotheranostic agents. In Nanotheranostics: Applications and Limitations; Springer International Publishing: Berlin/Heidelberg, Germany, 2019; pp. 79–114. [Google Scholar] [CrossRef]
- Esumi, K.; Takei, N.; Yoshimura, T. Antioxidant-potentiality of gold-chitosan nanocomposites. Colloids Surf. B Biointerfaces 2003, 32, 117–123. [Google Scholar] [CrossRef]
- Khan, Z. Chitosan capped Au@Pd@Ag trimetallic nanoparticles: Synthesis, stability, capping action and adsorbing activities. Int. J. Biol. Macromol. 2020, 153, 545–560. [Google Scholar] [CrossRef]
- Firoozi, S.; Jamzad, M.; Yari, M. Biologically synthesized silver nanoparticles by aqueous extract of Satureja intermedia C.A. Mey and the evaluation of total phenolic and flavonoid contents and antioxidant activity. J. Nanostruct. Chem. 2016, 6, 357–364. [Google Scholar] [CrossRef] [Green Version]
- Guo, Y.; Yan, H. Preparation and characterization of heparin-stabilized gold nanoparticles. J. Carbohydr. Chem. 2008, 27, 309–319. [Google Scholar] [CrossRef]
- Cheng, K.M.; Hung, Y.W.; Chen, C.C.; Liu, C.C.; Young, J.J. Green synthesis of chondroitin sulfate-capped silver nanoparticles: Characterization and surface modification. Carbohydr. Polym. 2014, 110, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.; Gupta, R.; Kumar, G.; Kumari, S.; Biswas, S.; Padmanabhan, P. Synthesis of silver nanoparticles by Bacillus clausii and computational profiling of nitrate reductase enzyme involved in production. J. Genet. Eng. Biotechnol. 2018, 16, 527–536. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.A.; Abyaneh, M.K.; Gosavi, S.W.; Kulkarni, S.K.; Pasricha, R.; Ahmad, A.; Khan, M.I. Nitrate reductase-mediated synthesis of silver nanoparticles from AgNO3. Biotechnol. Lett. 2007, 29, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Hansen, H.E.; Seland, F.; Sunde, S.; Burheim, O.S.; Pollet, B.G. Two routes for sonochemical synthesis of platinum nanoparticles with narrow size distribution. Mater. Adv. 2021, 2, 1962–1971. [Google Scholar] [CrossRef]
- Abedini, A.; Daud, A.R.; Hamid, M.A.A.; Othman, N.K.; Saion, E. A review on radiation-induced nucleation and growth of colloidal metallic nanoparticles. Nanoscale Res. Lett. 2013, 8, 474. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasretdinova, G.R.; Fazleeva, R.R.; Mukhitova, R.K.; Nizameev, I.R.; Kadirov, M.K.; Ziganshina, A.Y.; Yanilkin, V.V. Electrochemical synthesis of silver nanoparticles in solution. Electrochem. Commun. 2015, 50, 69–72. [Google Scholar] [CrossRef]
- Bonyár, A.; Csarnovics, I.; Veres, M.; Himics, L.; Csik, A.; Kámán, J.; Balázs, L.; Kökényesi, S. Investigation of the performance of thermally generated gold nanoislands for LSPR and SERS applications. Sens. Actuators B Chem. 2018, 255, 433–439. [Google Scholar] [CrossRef]
- Kang, H.; Buchman, J.T.; Rodriguez, R.S.; Ring, H.L.; He, J.; Bantz, K.C.; Haynes, C.L. Stabilization of Silver and Gold Nanoparticles: Preservation and Improvement of Plasmonic Functionalities. Chem. Rev. 2019, 119, 664–699. [Google Scholar] [CrossRef]
- Huang, H.; Yuan, Q.; Yang, X. Preparation and characterization of metal-chitosan nanocomposites. Colloids Surf. B Biointerfaces 2004, 39, 31–37. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Nguyen, Q.V.; Huynh, T.C. Simple, green, and low-temperature method for preparation of palladium nanoparticles with controllable sizes and their characterizations. J. Nanopart. Res. 2020, 22, 73. [Google Scholar] [CrossRef]
- Phan, T.T.V.; Hoang, G.; Nguyen, V.T.; Nguyen, T.P.; Kim, H.H.; Mondal, S.; Manivasagan, P.; Moorthy, M.S.; Lee, K.D.; Junghwan, O. Chitosan as a stabilizer and size-control agent for synthesis of porous flower-shaped palladium nanoparticles and their applications on photo-based therapies. Carbohydr. Polym. 2019, 205, 340–352. [Google Scholar] [CrossRef] [PubMed]
- Gaikwad, G.; Bangde, P.; Rane, K.; Stenberg, J.; Borde, L.; Bhagwat, S.; Dandekar, P.; Jain, R. Continuous production and separation of new biocompatible palladium nanoparticles using a droplet microreactor. Microfluid. Nanofluid. 2021, 25, 27. [Google Scholar] [CrossRef]
- Adlim, M.; Abu Bakar, M.; Liew, K.Y.; Ismail, J. Synthesis of chitosan-stabilized platinum and palladium nanoparticles and their hydrogenation activity. J. Mol. Catal. A Chem. 2004, 212, 141–149. [Google Scholar] [CrossRef]
- Kleszcz, K.; Hebda, M.; Kyzioł, A.; Krawiec, H.; Kyzioł, K. Towards prevention of biofilm formation: Ti6Al7Nb modified with nanocomposite layers of chitosan and Ag/Au nanoparticles. Appl. Surf. Sci. 2021, 557, 149795. [Google Scholar] [CrossRef]
- Hortigüela, M.J.; Aranaz, I.; Gutiérrez, M.C.; Ferrer, M.L.; Del Monte, F. Chitosan gelation induced by the in situ formation of gold nanoparticles and its processing into macroporous scaffolds. Biomacromolecules 2011, 12, 179–186. [Google Scholar] [CrossRef]
- Abrica-González, P.; Zamora-Justo, J.A.; Sotelo-López, A.; Vázquez-Martínez, G.R.; Balderas-López, J.A.; Muñoz-Diosdado, A.; Ibáñez-Hernández, M. Gold nanoparticles with chitosan, N-acylated chitosan, and chitosan oligosaccharide as DNA carriers. Nanoscale Res. Lett. 2019, 14, 258. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Li, J.; Cai, J.; Zhong, L.; Ren, G.; Ma, Q. One pot synthesis of gold nanoparticles using chitosan with varying degree of deacetylation and molecular weight. Carbohydr. Polym. 2017, 178, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Twu, Y.K.; Chen, Y.W.; Shih, C.M. Preparation of silver nanoparticles using chitosan suspensions. Powder Technol. 2008, 185, 251–257. [Google Scholar] [CrossRef]
- Murugadoss, A.; Chattopadhyay, A. A ‘green’ chitosan-silver nanoparticle composite as a heterogeneous as well as micro-heterogeneous catalyst. Nanotechnology 2008, 19, 015603. [Google Scholar] [CrossRef] [PubMed]
- Kalaivani, R.; Maruthupandy, M.; Muneeswaran, T.; Hameedha Beevi, A.; Anand, M.; Ramakritinan, C.M.; Kumaraguru, A.K. Synthesis of chitosan mediated silver nanoparticles (Ag NPs) for potential antimicrobial applications. Front. Lab. Med. 2018, 2, 30–35. [Google Scholar] [CrossRef]
- Karthik, C.S.; Chethana, M.H.; Manukumar, H.M.; Ananda, A.P.; Sandeep, S.; Nagashree, S.; Mallesha, L.; Mallu, P.; Jayanth, H.S.; Dayananda, B.P. Synthesis and characterization of chitosan silver nanoparticle decorated with benzodioxane coupled piperazine as an effective anti-biofilm agent against MRSA: A validation of molecular docking and dynamics. Int. J. Biol. Macromol. 2021, 181, 540–551. [Google Scholar] [CrossRef]
- Pandey, I.; Chandra, A. Temperature-induced changes in polymer-based fractal patterns due to Ag metal aggregation. Appl. Phys. A 2021, 127, 369. [Google Scholar] [CrossRef]
- Aranaz, I.; Castro, C.; Heras, A.; Acosta, N. On the ability of low molecular weight chitosan enzymatically depolymerized to produce and stabilize silver nanoparticles. Biomimetics 2018, 3, 21. [Google Scholar] [CrossRef] [Green Version]
- Aranaz, I.; Alcántara, A.R.; Heras, A.; Acosta, N. Efficient reduction of Toluidine Blue O dye using silver nanoparticles synthesized by low molecular weight chitosans. Int. J. Biol. Macromol. 2019, 131, 682–690. [Google Scholar] [CrossRef]
- Romero-Fernández, M.; Paradisi, F. Protein immobilization technology for flow biocatalysis. Curr. Opin. Chem. Biol. 2020, 55, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Boudrant, J.; Woodley, J.M.; Fernandez-Lafuente, R. Parameters necessary to define an immobilized enzyme preparation. Process Biochem. 2020, 90, 66–80. [Google Scholar] [CrossRef]
- Thompson, M.P.; Penafiel, I.; Cosgrove, S.C.; Turner, N.J. Biocatalysis using immobilized enzymes in continuous flow for the synthesis of fine chemicals. Org. Process Res. Dev. 2019, 23, 9–18. [Google Scholar] [CrossRef]
- Thompson, M.P.; Derrington, S.R.; Heath, R.S.; Porter, J.L.; Mangas-Sanchez, J.; Devine, P.N.; Truppo, M.D.; Turner, N.J. A generic platform for the immobilisation of engineered biocatalysts. Tetrahedron 2019, 75, 327–334. [Google Scholar] [CrossRef]
- Reis, C.L.B.; de Sousa, E.Y.A.; Serpa, J.D.; Oliveira, R.C.; dos Santos, J.C.S. Design of immobilized enzyme biocatalysts: Drawbacks and opportunities. Quim. Nova 2019, 42, 768–783. [Google Scholar] [CrossRef]
- Ravindran, R.; Jaiswal, A.K. Current Advances in Immobilization Techniques of Enzymes. In Enzymatic Fuel Cells: Materials and Applications; Inamuddin, Ahmer, M.F., Ahamed, M.I., Asiri, A.M., Eds.; Materials Research Forum Llc: Millersville, PA, USA, 2019; Volume 44, pp. 51–72. [Google Scholar]
- Goncalves, M.C.P.; Kieckbusch, T.G.; Perna, R.F.; Fujimoto, J.T.; Morales, S.A.V.; Romanelli, J.P. Trends on enzyme immobilization researches based on bibliometric analysis. Process Biochem. 2019, 76, 95–110. [Google Scholar] [CrossRef]
- Fernandez-Lafuente, R. Editorial for Special Issue: Enzyme Immobilization and Its Applications. Molecules 2019, 24, 4. [Google Scholar] [CrossRef] [Green Version]
- Basso, A.; Serban, S. Industrial applications of immobilized enzymes—A review. Mol. Catal. 2019, 479, 110607. [Google Scholar] [CrossRef]
- Ahmad, A.; Javed, M.R.; Ibrahim, M.; Sajid, A.; Hussain, K.; Kaleem, M.; Fatima, H.M.; Nadeem, H. Methods of Enzyme Immobilization on Various Supports. In Enzymatic Fuel Cells: Materials and Applications; Inamuddin, Ahmer, M.F., Ahamed, M.I., Asiri, A.M., Eds.; Materials Research Forum Llc: Millersville, PA, USA, 2019; Volume 44, pp. 1–28. [Google Scholar]
- Grunwald, P. Immobilized Biocatalysts. Catalysts 2018, 8, 7. [Google Scholar] [CrossRef] [Green Version]
- Corici, L.; Ferrario, V.; Pellis, A.; Ebert, C.; Lotteria, S.; Cantone, S.; Voinovich, D.; Gardossi, L. Large scale applications of immobilized enzymes call for sustainable and inexpensive solutions: Rice husks as renewable alternatives to fossil-based organic resins. RSC Adv. 2016, 6, 63256–63270. [Google Scholar] [CrossRef]
- Bolivar, J.M.; Eisl, I.; Nidetzky, B. Advanced characterization of immobilized enzymes as heterogeneous biocatalysts. Catal. Today 2016, 259, 66–80. [Google Scholar] [CrossRef]
- Eş, I.; Vieira, J.D.G.; Amaral, A.C. Principles, techniques, and applications of biocatalyst immobilization for industrial application. Appl. Microbiol. Biotechnol. 2015, 99, 2065–2082. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, C.S.; Lemos, C.; de Sousa, M.; Goncalves, L.R.B. Enzyme immobilization onto renewable polymeric matrixes: Past, present, and future trends. J. Appl. Polym. Sci. 2015, 132, 15. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [Green Version]
- Mohamad, N.R.; Marzuki, N.H.C.; Buang, N.A.; Huyop, F.; Wahab, R.A. An overview of technologies for immobilization of enzymes and surface analysis techniques for immobilized enzymes. Biotechnol. Biotechnol. Equip. 2015, 29, 205–220. [Google Scholar] [CrossRef]
- Sirisha, V.L.; Jain, A.; Jain, A. Enzyme Immobilization: An Overview on Methods, Support Material, and Applications of Immobilized Enzymes. In Advances in Food and Nutrition Research; Academic Press Inc.: Cambridge, MA, USA, 2016; Volume 79, pp. 179–211. [Google Scholar]
- Krajewska, B. Application of chitin- and chitosan-based materials for enzyme immobilizations: A review. Enzyme Microb. Technol. 2004, 35, 126–139. [Google Scholar] [CrossRef]
- Peniche, C.; Arguelles-Monal, W.; Peniche, H.; Acosta, N. Chitosan: An attractive biocompatible polymer for microencapsulation. Macromol. Biosci. 2003, 3, 511–520. [Google Scholar] [CrossRef]
- Hoven, V.P.; Tangpasuthadol, V.; Angkitpaiboon, Y.; Vallapa, N.; Kiatkamjornwong, S. Surface-charged chitosan: Preparation and protein adsorption. Carbohydr. Polym. 2007, 68, 44–53. [Google Scholar] [CrossRef]
- Biro, E.; Nemeth, A.S.; Sisak, C.; Feczko, T.; Gyenis, J. Preparation of chitosan particles suitable for enzyme immobilization. J. Biochem. Biophys. Methods 2008, 70, 1240–1246. [Google Scholar] [CrossRef] [PubMed]
- Verma, M.L.; Kumar, S.; Das, A.; Randhawa, J.S.; Chamundeeswari, M. Chitin and chitosan-based support materials for enzyme immobilization and biotechnological applications. Environ. Chem. Lett. 2020, 18, 315–323. [Google Scholar] [CrossRef]
- Wang, D.Q.; Jiang, W.F. Preparation of chitosan-based nanoparticles for enzyme immobilization. Int. J. Biol. Macromol. 2019, 126, 1125–1132. [Google Scholar] [CrossRef]
- Malmiri, H.J.; Jahanian, M.A.G.; Berenjian, A. Potential applications of chitosan nanoparticles as novel support in enzyme immobilization. Am. J. Biochem. Biotechnol. 2012, 8, 203–219. [Google Scholar]
- Aranaz, I.; Acosta, N.; Heras, A. Encapsulation of an Agrobacterium radiobacter extract containing D-hydantoinase and D-carbamoylase activities into alginate-chitosan polyelectrolyte complexes. Preparation of the biocatalyst. J. Mol. Catal. B Enzym. 2009, 58, 54–64. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Heras, A. Enzymatic D-p-hydrophenyl glycine synthesis using chitin and chitosan as supports for biocatalyst immobilization. Biocatal. Biotransform. 2018, 36, 89–101. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Férnandez-Valle, M.E.; Heras, A. Optimization of D-amino acid production catalyzed by immobilized multi-enzyme system in polyelectrolyte complex gel capsules. J. Mol. Catal. B Enzym. 2015, 121, 45–52. [Google Scholar] [CrossRef]
- Aranaz, I.; Ramos, V.; De La Escalera, S.; Heras, A. Co-immobilization of D-hydantoinase and D-carboamylase on chitin: Application to the synthesis of p-hydroxyphenylglycine. Biocatal. Biotransform. 2003, 21, 349–356. [Google Scholar] [CrossRef]
- Volpato, G.; Rodrigues, R.C.; Fernandez-Lafuente, R. Use of enzymes in the production of semi-synthetic penicillins and cephalosporins: Drawbacks and perspectives. Curr. Med. Chem. 2010, 17, 3855–3873. [Google Scholar] [CrossRef] [PubMed]
- Mateo, C.; Abian, O.; Grazú, V.; Fernández-Lorente, G.; Palomo, J.M.; Fuentes, M.; Segura, R.L.; Montes, T.; López-Gallego, F.; Wilson, L.; et al. Recent advances in the industrial enzymatic synthesis of semi-synthetic β-lactam antibiotics. Med. Chem. Rev. Online 2005, 2, 207–218. [Google Scholar] [CrossRef]
- Rodriguez-Herrera, R.; Puc, L.E.C.; Sobrevilla, J.M.V.; Luque, D.; Cardona-Felix, C.S.; Aguilar-González, C.N.; Flores-Gallegos, A.C. Enzymes in the pharmaceutical industry for β-lactam antibiotic production. In Enzymes in Food Biotechnology: Production, Applications, and Future Prospects; Elsevier: Amsterdam, The Netherlands, 2018; pp. 627–643. [Google Scholar] [CrossRef]
- Farhadian, S.; Shareghi, B.; Tirgir, F.; Reiisi, S.; Dehkordi, N.G.; Momeni, L.; Heidari, E. Design, synthesis, and anti-gastric cancer activity of novel 2,5-diketopiperazine. J. Mol. Liq. 2019, 294, 111585. [Google Scholar] [CrossRef]
- Lukasevics, L.; Cizikovs, A.; Grigorjeva, L. Synthesis of 3-Hydroxymethyl Isoindolinones via Cobalt-Catalyzed C(sp2)-H Carbonylation of Phenylglycinol Derivatives. Org. Lett. 2020, 22, 2720–2723. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.X.; Xiang, J.C.; Cheng, Y.; Ma, J.T.; Wu, Y.D.; Wu, A.X. Direct Biomimetic Synthesis of β-Carboline Alkaloids from Two Amino Acids. J. Org. Chem. 2018, 83, 12247–12254. [Google Scholar] [CrossRef] [PubMed]
- Xiang, J.C.; Wang, Z.X.; Cheng, Y.; Xia, S.Q.; Wang, M.; Tang, B.C.; Wu, Y.D.; Wu, A.X. Divergent Synthesis of Functionalized Quinolines from Aniline and Two Distinct Amino Acids. J. Org. Chem. 2017, 82, 9210–9216. [Google Scholar] [CrossRef] [PubMed]
- Gräßle, S.; Vanderheiden, S.; Hodapp, P.; Bulat, B.; Nieger, M.; Jung, N.; Bräse, S. Solid Phase Synthesis of (Benzannelated) Six-Membered Heterocycles via Cyclative Cleavage of Resin-Bound Pseudo-Oxazolones. Org. Lett. 2016, 18, 3598–3601. [Google Scholar] [CrossRef] [PubMed]
- Clemente-Jiménez, J.M.; Martínez-Rodríguez, S.; Rodríguez-Vico, F.; Heras-Vázquez, F.J.L. Optically pure α-amino acids production by the “Hydantoinase Process”. Recent Pat. Biotechnol. 2008, 2, 35–46. [Google Scholar] [PubMed]
- Rodríguez-Alonso, M.J.; Clemente-Jiménez, J.M.; Rodríguez-Vico, F.; Las Heras- Vázquez, F.J. Rational re-design of the “double-racemase hydantoinase process” for optically pure production of natural and non-natural l-amino acids. Biochem. Eng. J. 2015, 101, 68–76. [Google Scholar] [CrossRef]
- Aranaz, I.; Acosta, N.; Heras, A. Synthesis of p-hydroxyphenylglicine by cell extract from Agrobaterium radiobacter encapsulated in alginate capsules. Enzym. Microb. Technol. 2006, 39, 215–221. [Google Scholar] [CrossRef]
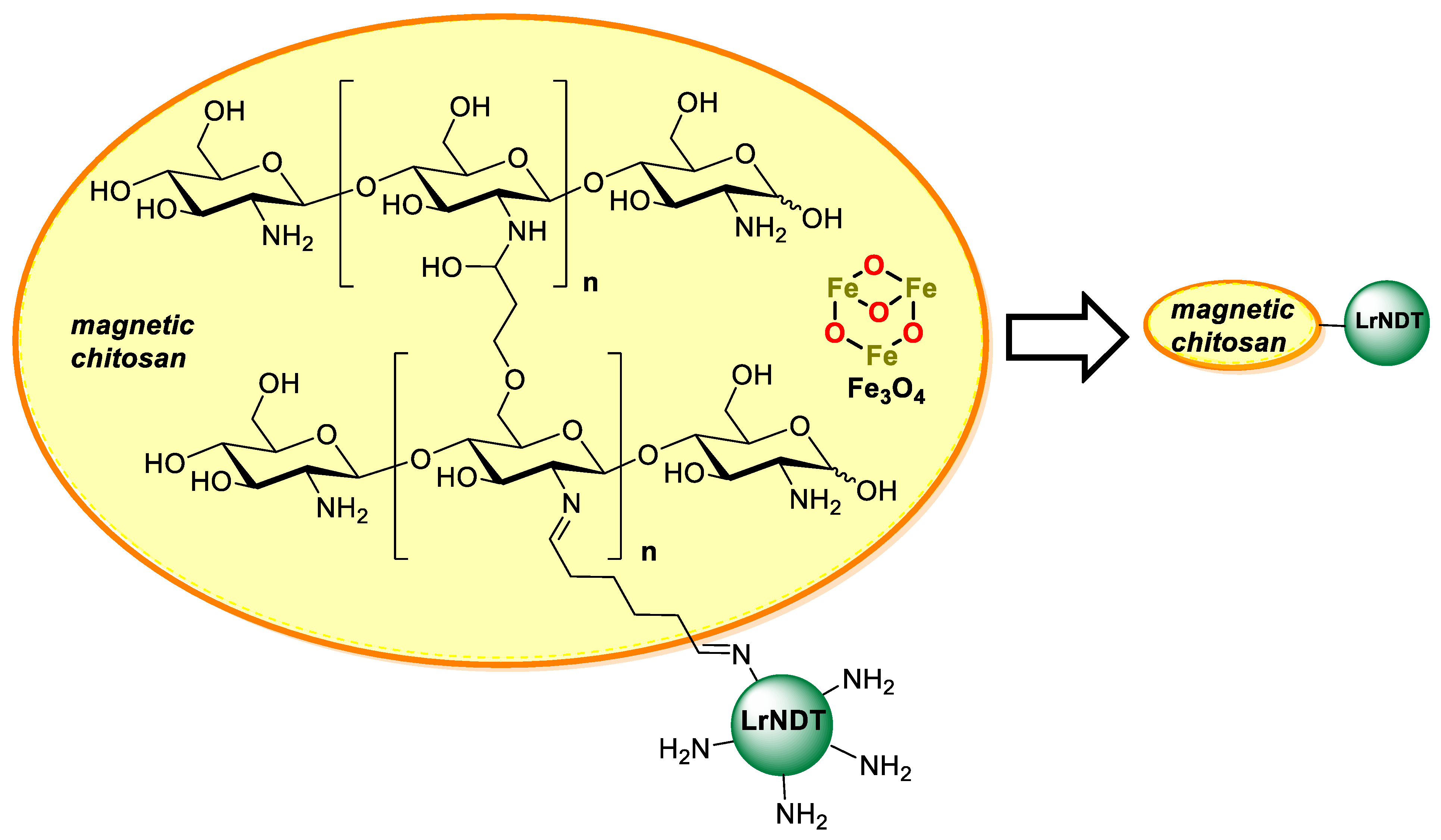
- Fernández-Lucas, J.; Harris, R.; Mata-Casar, I.; Heras, A.; De La Mata, I.; Arroyo, M. Magnetic chitosan beads for covalent immobilization of nucleoside 2′-deoxyribosyltransferase: Application in nucleoside analogues synthesis. J. Ind. Microbiol. Biotechnol. 2013, 40, 955–966. [Google Scholar] [CrossRef] [PubMed]
- Bernkop-Schnürch, A.; Dünnhaupt, S. Chitosan-based drug delivery systems. Eur. J. Pharm. Biopharm. 2012, 81, 463–469. [Google Scholar] [CrossRef]
- Ojeda-Hernández, D.D.; Canales-Aguirre, A.A.; Matias-Guiu, J.; Gomez-Pinedo, U.; Mateos-Díaz, J.C. Potential of Chitosan and Its Derivatives for Biomedical Applications in the Central Nervous System. Front. Bioeng. Biotechnol. 2020, 8, 389. [Google Scholar] [CrossRef]
- Noel, S.P.; Courtney, H.; Bumgardner, J.D.; Haggard, W.O. Chitosan films: A potential local drug delivery system for antibiotics. Clin. Orthop. Relat. Res. 2008, 466, 1377–1382. [Google Scholar] [CrossRef] [Green Version]
- Affes, S.; Aranaz, I.; Acosta, N.; Heras, Á.; Nasri, M.; Maalej, H. Chitosan derivatives-based films as pH-sensitive drug delivery systems with enhanced antioxidant and antibacterial properties. Int. J. Biol. Macromol. 2021, 182, 730–742. [Google Scholar] [CrossRef] [PubMed]
- Aranaz, I.; Harris, R.; Navarro-García, F.; Heras, A.; Acosta, N. Chitosan based films as supports for dual antimicrobial release. Carbohydr. Polym. 2016, 146, 402–410. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Navarro, J.I.; Díaz-Zavala, N.P.; Velasco-Santos, C.; Martínez-Hernández, A.L.; Tijerina-Ramos, B.I.; García-Hernández, M.; Rivera-Armenta, J.L.; Páramo-García, U.; Reyes-de la Torre, A.I. Antimicrobial, optical and mechanical properties of Chitosan–Starch films with natural extracts. Int. J. Mol. Sci. 2017, 18, 997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noel, S.P.; Courtney, H.S.; Bumgardner, J.D.; Haggard, W.O. Chitosan sponges to locally deliver amikacin and vancomycin: A pilot in vitro evaluation. Clin. Orthop. Relat. Res. 2010, 468, 2074–2080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stinner, D.J.; Noel, S.P.; Haggard, W.O.; Watson, J.T.; Wenke, J.C. Local antibiotic delivery using tailorable chitosan sponges: The future of infection control? J. Orthop. Trauma 2010, 24, 592–597. [Google Scholar] [CrossRef] [Green Version]
- Yilgor, P.; Tuzlakoglu, K.; Reis, R.L.; Hasirci, N.; Hasirci, V. Incorporation of a sequential BMP-2/BMP-7 delivery system into chitosan-based scaffolds for bone tissue engineering. Biomaterials 2009, 30, 3551–3559. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Li, W.; Liu, H.; Yuan, T.; Yang, Y.; Zhou, W.; Hu, Y.; Yang, Z. Preparation of 3D Printed Chitosan/Polyvinyl Alcohol Double Network Hydrogel Scaffolds. Macromol. Biosci. 2021, 21, 2000398. [Google Scholar] [CrossRef]
- Ma, S.; Moser, D.; Han, F.; Leonhard, M.; Schneider-Stickler, B.; Tan, Y. Preparation and antibiofilm studies of curcumin loaded chitosan nanoparticles against polymicrobial biofilms of Candida albicans and Staphylococcus aureus. Carbohydr. Polym. 2020, 241, 116254. [Google Scholar] [CrossRef]
- Bartos, C.; Varga, P.; Szabó-Révész, P.; Ambrus, R. Physico-chemical and in vitro characterization of chitosan-based microspheres intended for nasal administration. Pharmaceutics 2021, 13, 608. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Chang, Z.; Liu, Z.; Zhu, L.; Wang, M.; Hao, D.; He, B. Controlled delivery of bioactive BDNF for potential treatment of peripheral nerve injury. Polym. Degrad. Stab. 2020, 181, 109296. [Google Scholar] [CrossRef]
- Aranaz, I.; Paños, I.; Peniche, C.; Heras, Á.; Acosta, N. Chitosan spray-dried microparticles for controlled delivery of venlafaxine hydrochloride. Molecules 2017, 22, 1980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gull, N.; Khan, S.M.; Butt, O.M.; Islam, A.; Shah, A.; Jabeen, S.; Khan, S.U.; Khan, A.; Khan, R.U.; Butt, M.T.Z. Inflammation targeted chitosan-based hydrogel for controlled release of diclofenac sodium. Int. J. Biol. Macromol. 2020, 162, 175–187. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Liu, R.; Wu, J.; Liu, Z.; Li, J.; Zhou, J.; Hao, T.; Wang, Y.; Du, Z.; Duan, C.; et al. The use of chitosan based hydrogel for enhancing the therapeutic benefits of adipose-derived MSCs for acute kidney injury. Biomaterials 2012, 33, 3673–3681. [Google Scholar] [CrossRef] [PubMed]
- Acosta, N.; Sánchez, E.; Calderón, L.; Cordoba-Diaz, M.; Cordoba-Diaz, D.; Dom, S.; Heras, A. Physical stability studies of semi-solid formulations from natural compounds loaded with Chitosan Microspheres. Mar. Drugs 2015, 13, 5901–5919. [Google Scholar] [CrossRef] [Green Version]
- Guo, L.; Xia, J.; Yu, S.; Yan, J.; He, F.; Zhang, M.; Fan, Q.; Yang, R.; Zhao, W. Natural edible materials made of protein-functionalized aerogel particles for postprandial hyperglycemia management. Int. J. Biol. Macromol. 2021, 167, 279–288. [Google Scholar] [CrossRef]
- López-Iglesias, C.; Barros, J.; Ardao, I.; Monteiro, F.J.; Alvarez-Lorenzo, C.; Gómez-Amoza, J.L.; García-González, C.A. Vancomycin-loaded chitosan aerogel particles for chronic wound applications. Carbohydr. Polym. 2019, 204, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Ching, Y.C.; Chuah, C.H. Synthesis of chitosan aerogels as promising carriers for drug delivery: A review. Carbohydr. Polym. 2020, 231, 115744. [Google Scholar] [CrossRef]
- Chen, M.; Li, L.; Xia, L.; Jiang, S.; Kong, Y.; Chen, X.; Wang, H. The kinetics and release behaviour of curcumin loaded pH-responsive PLGA/chitosan fibers with antitumor activity against HT-29 cells. Carbohydr. Polym. 2021, 265, 118077. [Google Scholar] [CrossRef]
- Kalalinia, F.; Taherzadeh, Z.; Jirofti, N.; Amiri, N.; Foroghinia, N.; Beheshti, M.; Bazzaz, B.S.F.; Hashemi, M.; Shahroodi, A.; Pishavar, E.; et al. Evaluation of wound healing efficiency of vancomycin-loaded electrospun chitosan/poly ethylene oxide nanofibers in full thickness wound model of rat. Int. J. Biol. Macromol. 2021, 177, 100–110. [Google Scholar] [CrossRef]
- Gorantla, S.; Dabholkar, N.; Sharma, S.; Rapalli, V.K.; Alexander, A.; Singhvi, G. Chitosan-based microneedles as a potential platform for drug delivery through the skin: Trends and regulatory aspects. Int. J. Biol. Macromol. 2021, 184, 438–453. [Google Scholar] [CrossRef]
- Chi, J.; Zhang, X.; Chen, C.; Shao, C.; Zhao, Y.; Wang, Y. Antibacterial and angiogenic chitosan microneedle array patch for promoting wound healing. Bioact. Mater. 2020, 5, 253–259. [Google Scholar] [CrossRef]
- Imam, S.S.; Alshehri, S.; Altamimi, M.A.; Hussain, A.; Qamar, W.; Gilani, S.J.; Zafar, A.; Alruwaili, N.K.; Alanazi, S.; Almutairy, B.K. Formulation of piperine–chitosan-coated liposomes: Characterization and in vitro cytotoxic evaluation. Molecules 2021, 26, 3281. [Google Scholar] [CrossRef] [PubMed]
- Shukla, S.K.; Chan, A.; Parvathaneni, V.; Gupta, V. Metformin-loaded chitosomes for treatment of malignant pleural mesothelioma—A rare thoracic cancer. Int. J. Biol. Macromol. 2020, 160, 128–141. [Google Scholar] [CrossRef]
- Prakash, J.; Prema, D.; Venkataprasanna, K.S.; Balagangadharan, K.; Selvamurugan, N.; Venkatasubbu, G.D. Nanocomposite chitosan film containing graphene oxide/hydroxyapatite/gold for bone tissue engineering. Int. J. Biol. Macromol. 2020, 154, 62–71. [Google Scholar] [CrossRef]
- Kumar, A.; Kaur, H. Sprayed in-situ synthesis of polyvinyl alcohol/chitosan loaded silver nanocomposite hydrogel for improved antibacterial effects. Int. J. Biol. Macromol. 2020, 145, 950–964. [Google Scholar] [CrossRef] [PubMed]
- Menazea, A.A.; Eid, M.M.; Ahmed, M.K. Synthesis, characterization, and evaluation of antimicrobial activity of novel Chitosan/Tigecycline composite. Int. J. Biol. Macromol. 2020, 147, 194–199. [Google Scholar] [CrossRef] [PubMed]
- European Pharmacopoeia, 10th ed.; Council of Europe, European Directorate for the Quality of Medicines Healthcare: Strasbourg, France, 2019.
- The United States Pharmacopeial Convention. The United States Pharmacopeia: The National Formulary; The United States Pharmacopeial Convention: Rockville, MD, USA, 2018. [Google Scholar]
- Salama, A.H.; Elmotasem, H.; Salama, A.A.A. Nanotechnology based blended chitosan-pectin hybrid for safe and efficient consolidative antiemetic and neuro-protective effect of meclizine hydrochloride in chemotherapy induced emesis. Int. J. Pharm. 2020, 584, 119411. [Google Scholar] [CrossRef]
- Saracogullari, N.; Gundogdu, D.; Ozdemir, F.N.; Soyer, Y.; Erel-Goktepe, I. The effect of polyacid on the physical and biological properties of chitosan based layer-by-layer films. Colloids Surf. A Physicochem. Eng. Asp. 2021, 617, 126313. [Google Scholar] [CrossRef]
- Mohebali, A.; Abdouss, M. Layered biocompatible pH-responsive antibacterial composite film based on HNT/PLGA/chitosan for controlled release of minocycline as burn wound dressing. Int. J. Biol. Macromol. 2020, 164, 4193–4204. [Google Scholar] [CrossRef]
- Pilicheva, B.; Uzunova, Y.; Bodurov, I.; Viraneva, A.; Exner, G.; Sotirov, S.; Yovcheva, T.; Marudova, M. Layer-by-layer self-assembly films for buccal drug delivery: The effect of polymer cross-linking. J. Drug Deliv. Sci. Technol. 2020, 59, 101897. [Google Scholar] [CrossRef]
- Khare, T.; Mahalunkar, S.; Shriram, V.; Gosavi, S.; Kumar, V. Embelin-loaded chitosan gold nanoparticles interact synergistically with ciprofloxacin by inhibiting efflux pumps in multidrug-resistant Pseudomonas aeruginosa and Escherichia coli. Environ. Res. 2021, 199, 111321. [Google Scholar] [CrossRef] [PubMed]
- Basu, S.K.; Kavitha, K.; Rupeshkumar, M. Evaluation of ionotropic cross-linked chitosan/gelatin B microspheres of tramadol hydrochloride. AAPS PharmSciTech 2011, 12, 28–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dawoud, M. Chitosan coated solid lipid nanoparticles as promising carriers for docetaxel. J. Drug Deliv. Sci. Technol. 2021, 62, 102409. [Google Scholar] [CrossRef]
- Öztürk, A.A.; Kıyan, H.T. Treatment of oxidative stress-induced pain and inflammation with dexketoprofen trometamol loaded different molecular weight chitosan nanoparticles: Formulation, characterization and anti-inflammatory activity by using in vivo HET-CAM assay. Microvasc. Res. 2020, 128, 103961. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.C.; Huang, S.F.; Lai, K.Y.; Ling, M.H. Fully embeddable chitosan microneedles as a sustained release depot for intradermal vaccination. Biomaterials 2013, 34, 3077–3086. [Google Scholar] [CrossRef]
- Chandrasekar, S.S.; Phanse, Y.; Hildebrand, R.E.; Hanafy, M.; Wu, C.W.; Hansen, C.H.; Osorio, J.E.; Suresh, M.; Talaat, A.M. Localized and systemic immune responses against SARS-COV-2 following mucosal immunization. Vaccines 2021, 9, 132. [Google Scholar] [CrossRef]
- Gao, X.; Gong, J.; Cai, Y.; Wang, J.; Wen, J.; Peng, L.; Ji, H.; Jiang, S.; Guo, D. Chitosan modified squalene nanostructured lipid carriers as a promising adjuvant for freeze-dried ovalbumin vaccine. Int. J. Biol. Macromol. 2021, 188, 855–862. [Google Scholar] [CrossRef]
- Moniz, T.; Costa Lima, S.A.; Reis, S. Marine polymeric microneedles for transdermal drug delivery. Carbohydr. Polym. 2021, 266, 118098. [Google Scholar] [CrossRef]
- Kurakula, M.; Raghavendra, N. Prospection of recent chitosan biomedical trends: Evidence from patent analysis (2009–2020). Int. J. Biol. Macromol. 2020, 165, 1924–1938. [Google Scholar] [CrossRef]
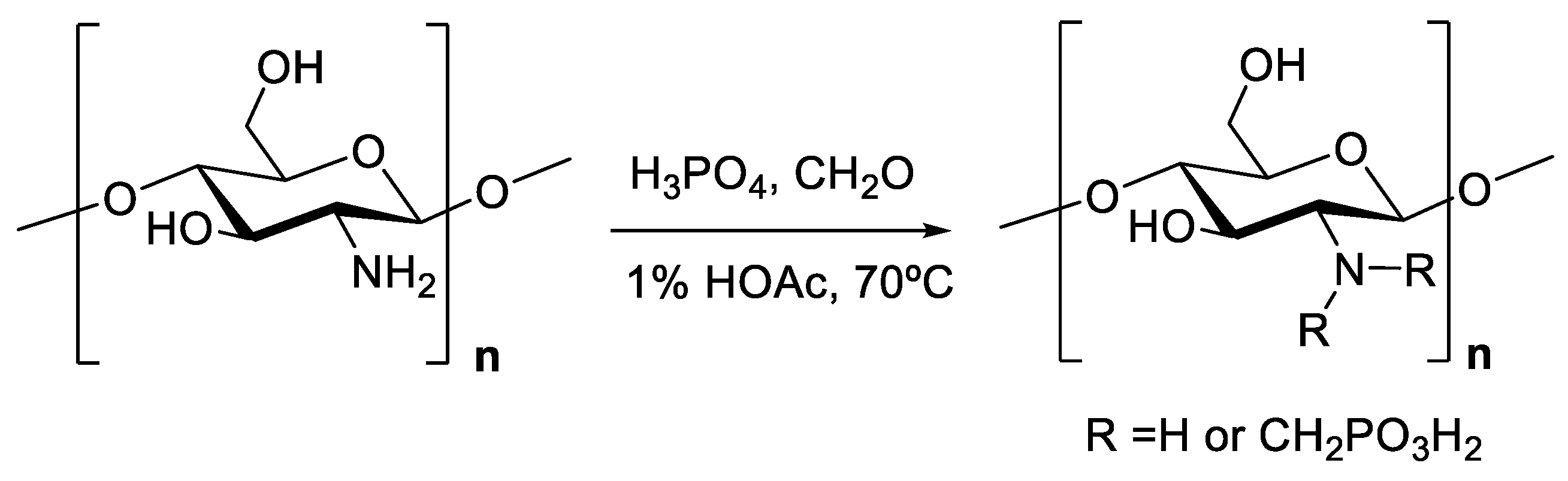





| Property/Activity | Reference |
|---|---|
| Mucoadhesive | [5,6] |
| Anti-inflammatory | [7] |
| Antioxidant | [8] |
| Antimicrobial | [9] |
| Antifungal | [10] |
| Antihyperglycemic | [11] |
| Antitumoral | [7,8,9,10,11,12] |
| Wound healing | [13] |
| Enzyme | Main Product |
|---|---|
| Chitosanase | Oligomers DP 2–3 |
| Hemicellulase | Dimers, trimers, tetramers, pentamers and hexamers |
| Pepsine | Glucosamine, N-acetylglucosamine oligomers with DP 2–6 |
| Pronase | 4–10 kDa |
| Papain | Glucosamine, N-acetylglucosamine oligomers with DP 2–6 |
| Lipase | High DP |
| System | Target | Inhibition | References |
|---|---|---|---|
| Chitosan | Aeromonas hydrophila Edwardsiella ictalurid Flavobacterium columnare | Complete 0.4% (E I, F C) 0.8% (A. H) | [50] |
| Chitosan | Candida albicans Gram-positive bacteria (such as Bacillus cereus, S. aureus, Bacillus megaterium, Lactobacillus plantarum, Listeria monocytogenes, Lactobacillus brevis, and Lactobacillus bulgaricus) Gram-negative bacteria (such as Salmonella typhimurium, E. coli, Pseudomonas aeruginosa, Pseudomonas fluorescens, Vibrio parahaemolyticus, Enterobacter aerogenes, and Vibrio cholera) | Strong and safe effect | [51,52] |
| Chitosan hydrochloride Carboxymethyl chitosan Chitosan oligosaccharide N-acetyl-D-glucosamine | Candida krusei, C. albicans, C. glabrata | No effect: chitosan oligosaccharide and N-acetyl-D-glucosamine. Weak effect: Carboxymethyl chitosan. Strong effect: Chitosan hydrochlorides. | [53] |
| Chitosan wound dressing | P. aeruginosa, B. cereus, L. monocytogenes | Strong effect: wound management due to their antimicrobial nature, ability to accelerate wound contraction and healing, haemostatic and analgesic | [54,55,56,57] |
| Chitosan sponges | S. aureus, E. coli | [58,59] | |
| Chitosan microparticles and nanoparticles | E. coli, Vibrio cholerae, S. enterica, Streptococcus uberis, S. uberis, S. enterica, K. pneumonia, S. aureus, V. cholerae, Salmonella choleraesuis, S. typhimurium | Strong effect | [60,61,62] |
| Metal | Reducing Agent | Stabilizer Chitosan Mw and DD | NPs Size | Morphology | Ref. |
|---|---|---|---|---|---|
| Palladium | Ascorbic acid | Cs 180 kDa, 75–85% DD | 5–20 | Spherical | [98] |
| Ascorbic acid | Cs 50 to 190 kDa, 75–85% | 50–70 | Flower-spherical | [110] | |
| Ascorbic acid | Cs, 50 to 190 kDa, 75–85% | 30–150 | Flower | [111] | |
| Ascorbic acid | TMCs 20 kDa | 55–120 | Spherical | [112] | |
| NaBH4 | Cs, 400 kDa DD 100% | nd | nd | [109] | |
| NaBH4 | Cs, (~400 kDa) | 2 | Spherical | [113] | |
| MeOH | Cs, (~400 kDa) | 2–5 | Spherical, large aggregate (Pd:MeOH 10:1) | [113] | |
| Hydrazine N2H4 | Cs, (~400 kDa) | 20 * | Highly aggregate | [113] | |
| Platinum | NaBH4 | Cs, 400 kDa DD 100% | 2–5 | spherical | [109] |
| NaBH4 | Cs, (~400 kDa) | 2–3 | spherical | [113] | |
| MeOH | Cs, (~400 kDa) | 2 | spherical | [113] | |
| Hydrazine N2H4 | Cs, (~400 kDa) | 17–25 * | aggregates | [113] | |
| Gold | Cs, 1278 kDa | Cs, 1278 kDa | 16 | [114] | |
| Cs 817 KDa | Cs, 817 KDa | 5 | Spherical | [115] | |
| NaBH4 | Cs, 400 kDa DD 100% | [109] | |||
| Cs DD > 85%; >200,000 cps | Cs, DD > 85%; >200,000 | 5–20 | Spherical | [101] | |
| NaBH4 | Cs n.c. | 6–20 | Spherical; polyhedral | [97] | |
| COS 5 kDa | COS 5 kDa | 7–15 | Spherical | [116] | |
| Cs, | Cs, DD 53–95%, Mw 2.6–490 kDa | 5–200 nm | Spherical, triangles, polyhedral | [117] | |
| Silver | Cs | Cs 1240 kDa, DA 0.13 | 10–150 | Spherical Triangles in long storage | [118] |
| Cs | Cs, high Mw, DA 0.25 | 5 | Spherical | [119] | |
| Cs DD > 85%; >200,000 cps | Cs DD > 85%; >200,000 cps | 20–200 | Spherical, fractal | [101] | |
| Ascorbic acid | Cs 180 kDa, 75–85% DD | 5–20 | Spherical | [98] | |
| NaBH4 | Cs 400 kDa DD 100% | 30–200 | Spherical clusters | [109] | |
| Gamma radiation | Cs n.c. | 4–5 | Spherical | [101] | |
| Cs n.c. | Cs n.c. | 10–60 | Spherical | [120] | |
| Ascorbic acid/Cs 1278 kDa | Cs 1278 kDa | 8 | [114] | ||
| Cs n.c. | Cs n.c. | [121] | |||
| Cs | Cs (50–190 kDa DD 75–85%) | Fractal patterns | [122] |
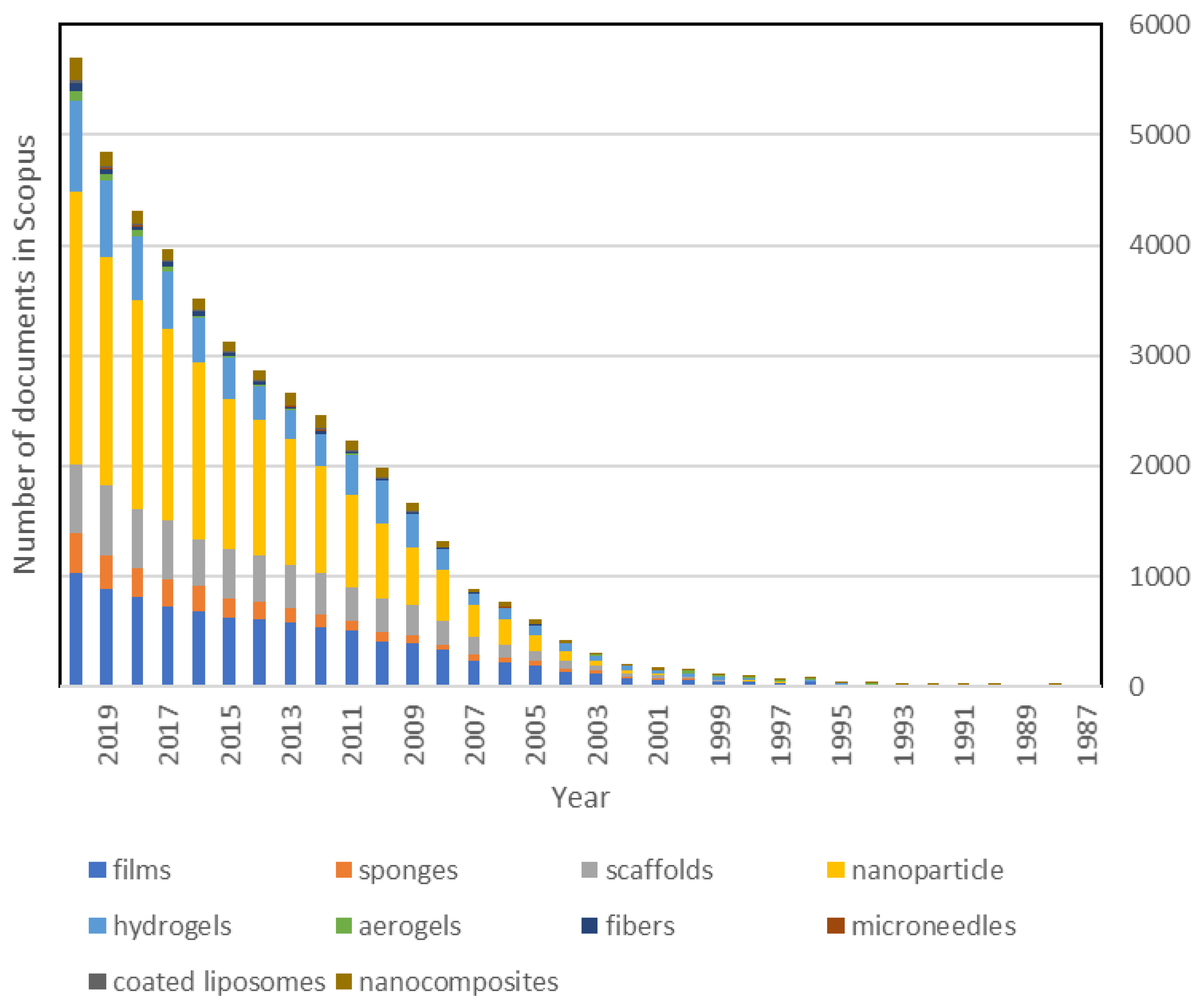
| Presentation | References |
|---|---|
| Films | [168,169,170,171] |
| Sponges | [172,173] |
| Scaffolds | [174,175] |
| Nanoparticles | [176] |
| Microspheres | [177,178,179] |
| Hydrogels | [180,181,182] |
| Aerogels | [183,184,185] |
| Fibers | [186,187] |
| Microneedles | [188,189] |
| Coated Liposomes | [190,191] |
| Nanocomposites | [192,193] |
| Composites | [194] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aranaz, I.; Alcántara, A.R.; Civera, M.C.; Arias, C.; Elorza, B.; Heras Caballero, A.; Acosta, N. Chitosan: An Overview of Its Properties and Applications. Polymers 2021, 13, 3256. https://doi.org/10.3390/polym13193256
Aranaz I, Alcántara AR, Civera MC, Arias C, Elorza B, Heras Caballero A, Acosta N. Chitosan: An Overview of Its Properties and Applications. Polymers. 2021; 13(19):3256. https://doi.org/10.3390/polym13193256
Chicago/Turabian StyleAranaz, Inmaculada, Andrés R. Alcántara, Maria Concepción Civera, Concepción Arias, Begoña Elorza, Angeles Heras Caballero, and Niuris Acosta. 2021. "Chitosan: An Overview of Its Properties and Applications" Polymers 13, no. 19: 3256. https://doi.org/10.3390/polym13193256







