Using Geopolymer Technology on Synthesizing Leucite Ceramics
Abstract
:1. Introduction
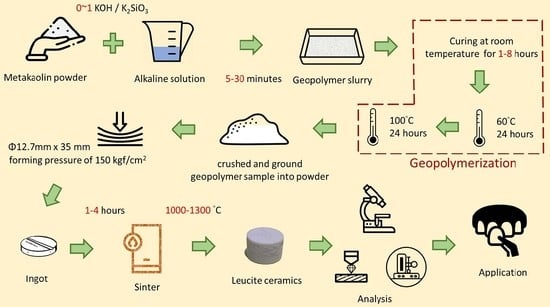
2. Materials and Methods
2.1. Materials
2.2. Methods
3. Results
3.1. Viscosity and Mechanical Properties
3.2. Microstructures
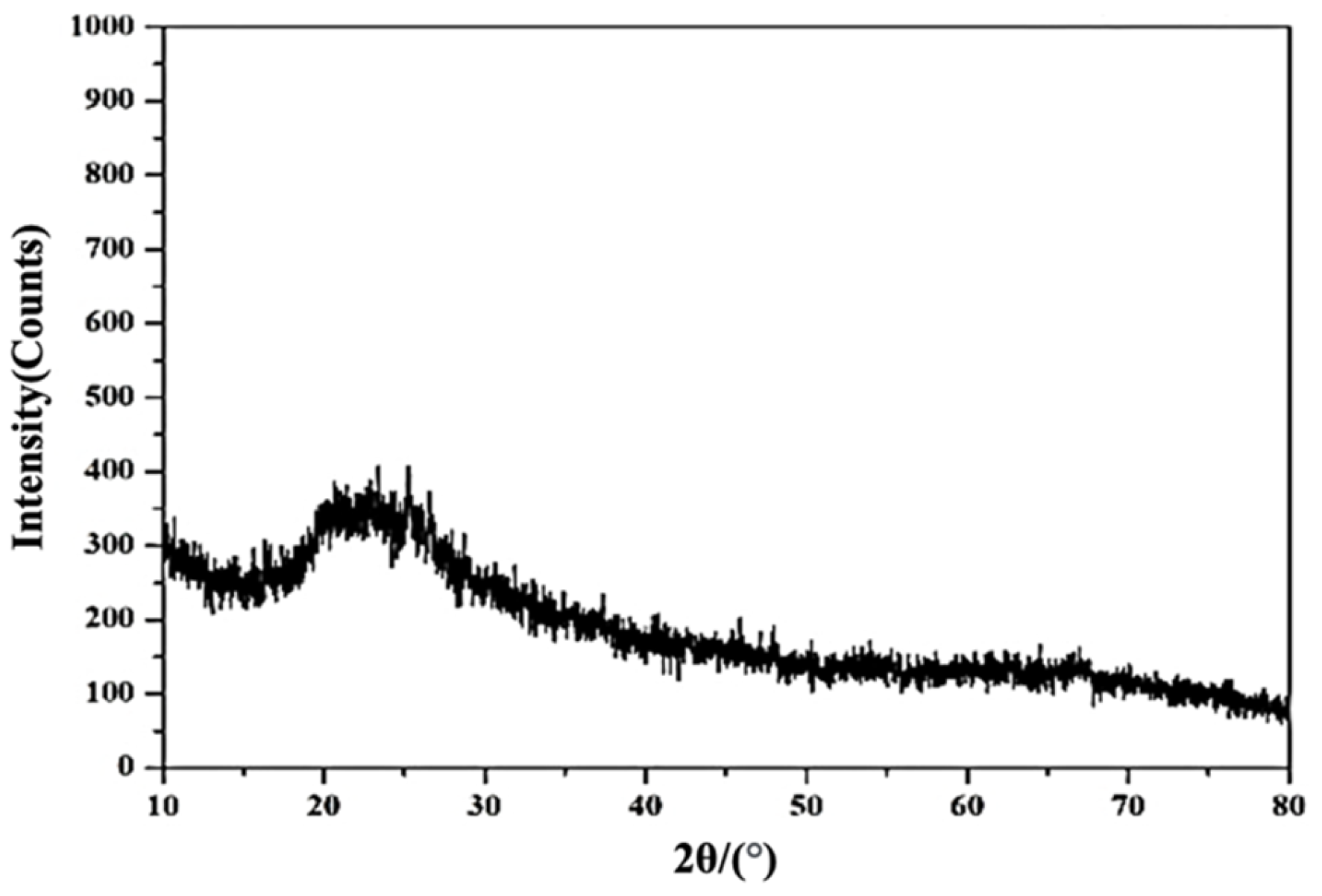
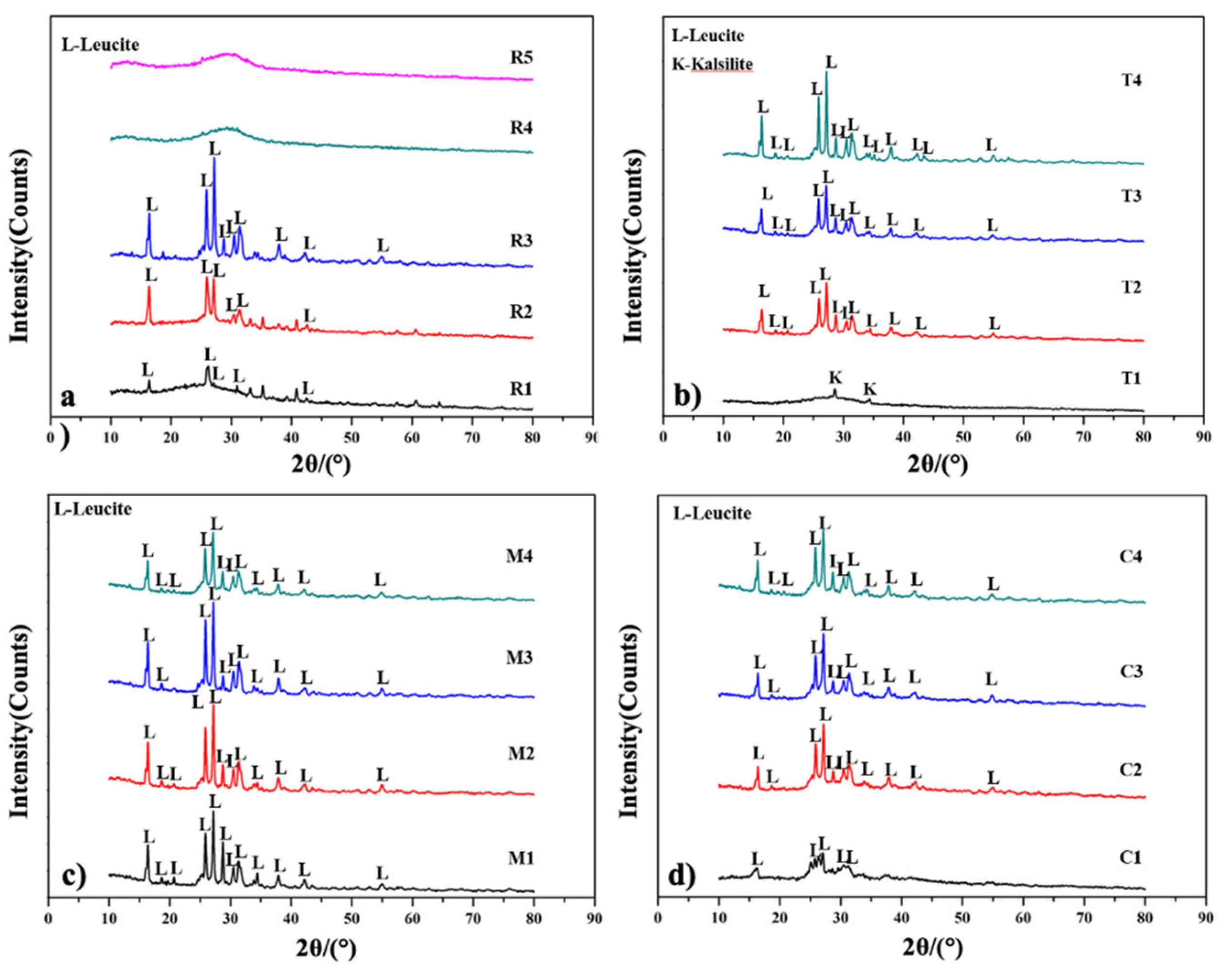
3.2.1. XRD
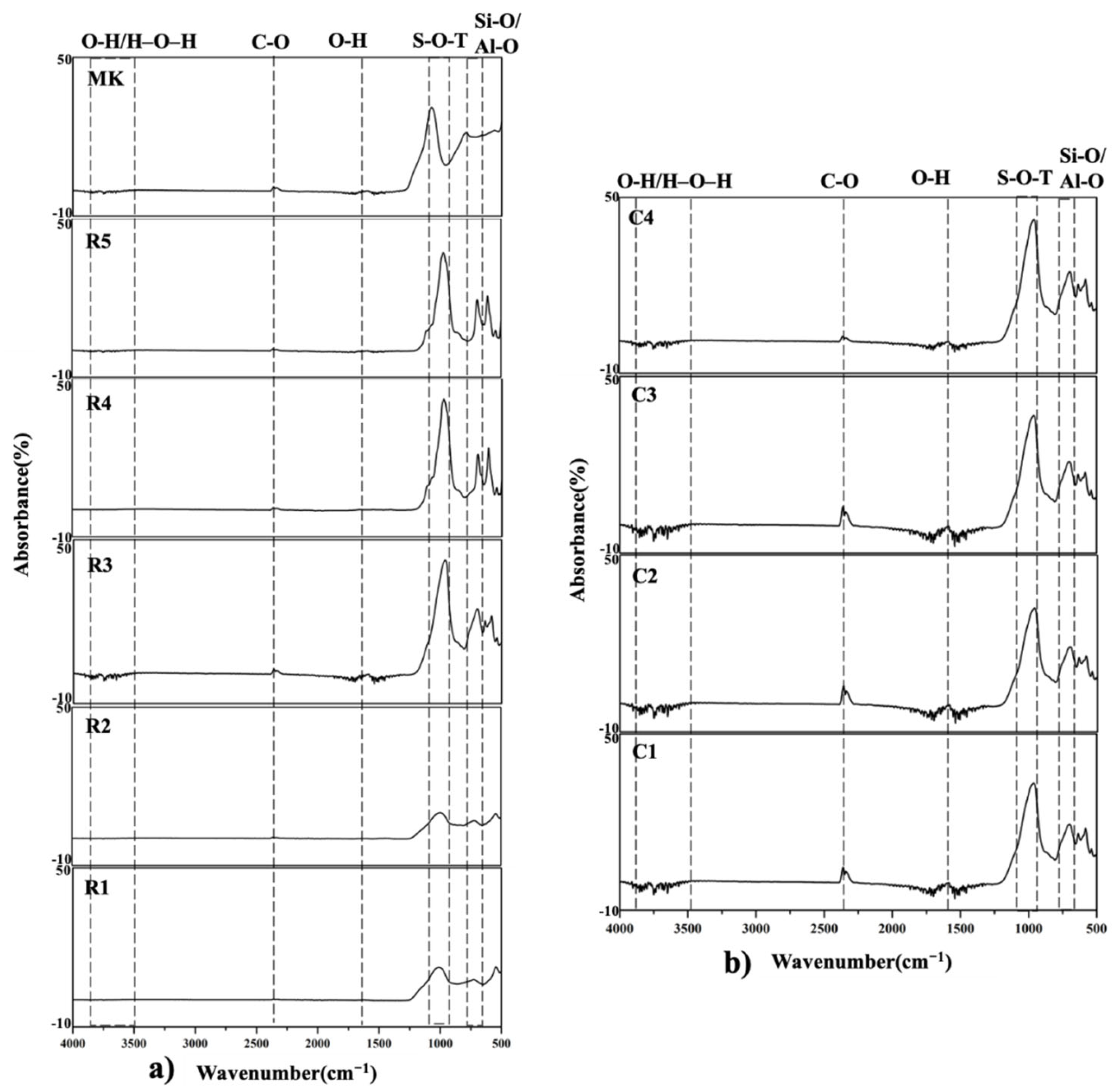
3.2.2. FTIR
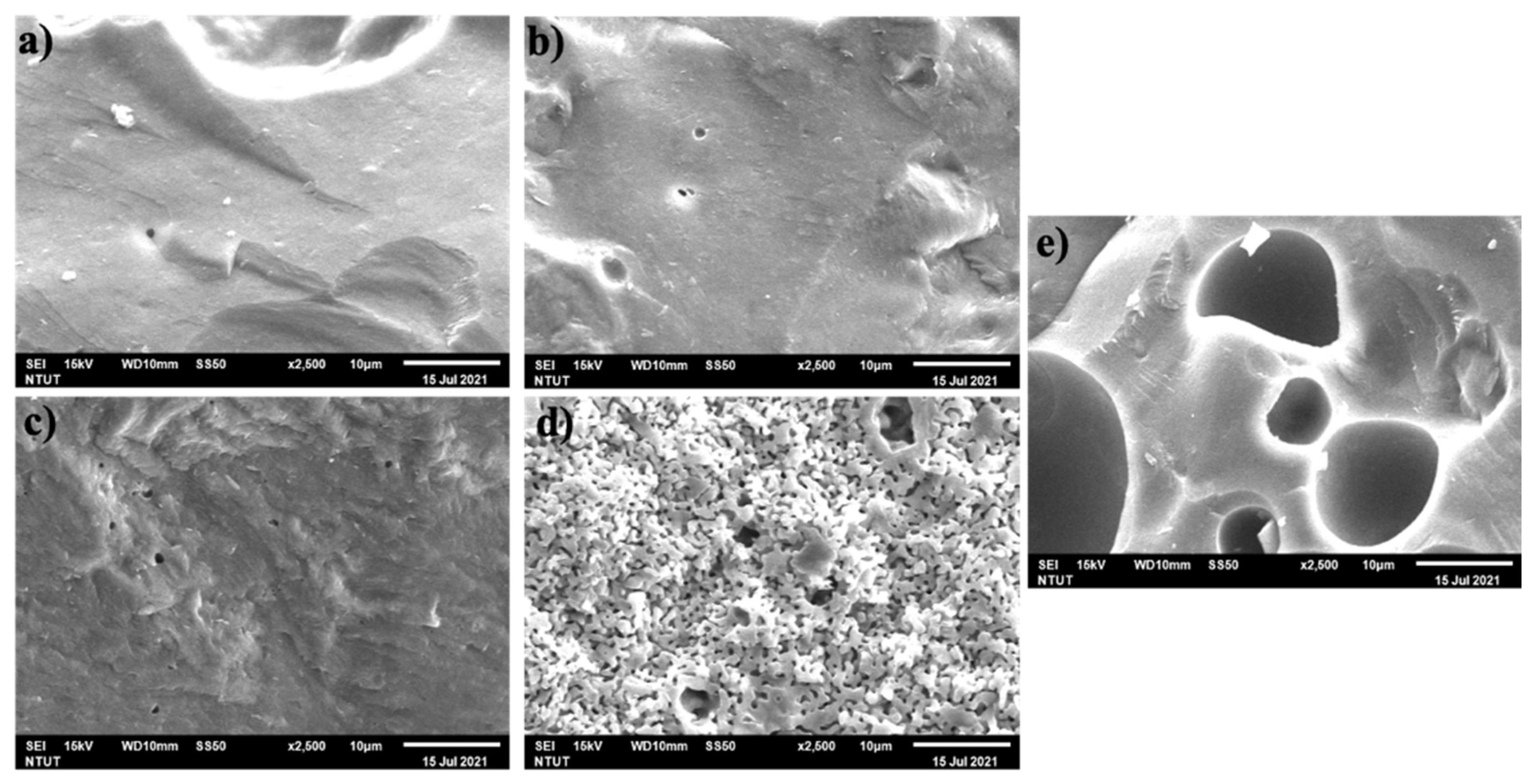
3.2.3. SEM
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Davidovits, J. Solid phase synthesis of a mineral block polymer by low temperature polycondensation of aluminosilicate polymers. In Proceedings of the IUPAC International Symposium on Macromolecules, Stockholm, Sweeden, 4–7 February 1976; pp. 3–15. [Google Scholar]
- Zhang, Z.; Provis, J.L.; Reid, A.; Wang, H. Geopolymer foam concrete: An emerging material for sustainable construction. Constr. Build. Mater. 2014, 56, 113–127. [Google Scholar] [CrossRef]
- Poggetto, G.D.; D’Angelo, A.; Blanco, I.; Piccolella, S.; Leonelli, C.; Catauro, M. FT-IR Study, Thermal Analysis, and Evaluation of the Antibacterial Activity of a MK-Geopolymer Mortar Using Glass Waste as Fine Aggregate. Polymers 2021, 13, 2970. [Google Scholar] [CrossRef] [PubMed]
- Catauro, M.; Tranquillo, E.; Barrino, F.; Poggetto, G.D.; Blanco, I.; Cicala, G.; Ognibene, G.; Recca, G. Mechanical and thermal properties of fly ash-filled geopolymers. J. Therm. Anal. Calorim. 2019, 138, 3267–3276. [Google Scholar] [CrossRef]
- Davidovits, J. Geopolymer Chemistry and Applications; Geopolymer Institute: Saint-Quentin, France, 2011. [Google Scholar]
- Abdullah, M.M.A.; Hussin, K.; Bnhussain, M.; Ismail, K.N.; Ibrahim, W.M.W. Mechanism and Chemical Reaction of Fly Ash Geopolymer Cement—A Review. Int. J. Pure Appl. Sci. Technol. 2011, 6, 35–44. [Google Scholar]
- Schmücker, M.; MacKenzie, K. Microstructure of sodium polysialate siloxo geopolymer. Ceram. Int. 2005, 31, 433–437. [Google Scholar] [CrossRef]
- Liew, Y.-M.; Heah, C.-Y.; Mustafa, A.B.M.; Kamarudin, H. Structure and properties of clay-based geopolymer cements: A review. Prog. Mater. Sci. 2016, 83, 595–629. [Google Scholar] [CrossRef]
- Giergiczny, Z. Fly ash and slag. Cem. Concr. Res. 2019, 124, 105826. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; Lee, M.; Williams, I.; Van Riessen, A. Fly ash based geopolymer thin coatings on metal substrates and its thermal evaluation. J. Hazard. Mater. 2010, 180, 748–752. [Google Scholar] [CrossRef]
- Amran, Y.M.; Alyousef, R.; Alabduljabbar, H.; El-Zeadani, M. Clean production and properties of geopolymer concrete; A review. J. Clean. Prod. 2020, 251, 119679. [Google Scholar] [CrossRef]
- Glid, M.; Sobrados, I.; Ben Rhaiem, H.; Sanz, J.; Amara, A.B.H. Alkaline activation of metakaolinite-silica mixtures: Role of dissolved silica concentration on the formation of geopolymers. Ceram. Int. 2017, 43, 12641–12650. [Google Scholar] [CrossRef]
- He, C.; Makovicky, E.; Osbæck, B. Thermal stability and pozzolanic activity of calcined kaolin. Appl. Clay Sci. 1994, 9, 165–187. [Google Scholar] [CrossRef]
- Xu, H.; Van Deventer, J.S. Geopolymerisation of multiple minerals. Miner. Eng. 2002, 15, 1131–1139. [Google Scholar] [CrossRef]
- Cristóbal, A.S.; Castello, R.; Martin-Luengo, M.A.; Vizcayno, C. Zeolites prepared from calcined and mechanically modified kaolins: A comparative study. Appl. Clay Sci. 2010, 49, 239–246. [Google Scholar] [CrossRef]
- Ilic, B.; Mitrovic, A.; Milicic, L. Thermal treatment of kaolin clay to obtain metakaolin. Chem. Ind. 2010, 64, 351–356. [Google Scholar] [CrossRef] [Green Version]
- Siddique, R.; Klaus, J. Influence of metakaolin on the properties of mortar and concrete: A review. Appl. Clay Sci. 2009, 43, 392–400. [Google Scholar] [CrossRef]
- Dinakar, P.; Sahoo, P.K.; Sriram, G. Effect of Metakaolin Content on the Properties of High Strength Concrete. Int. J. Concr. Struct. Mater. 2013, 7, 215–223. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-H.; Wu, Y.-F.; Ding, Y.-C.; Cheng, T.-W. Fabrication of Ceramic Moulds Using Recycled Shell Powder and Sand with Geopolymer Technology in Investment Casting. Appl. Sci. 2020, 10, 4577. [Google Scholar] [CrossRef]
- Temuujin, J.; Minjigmaa, A.; Rickard, W.; Lee, M.; Williams, I.; van Riessen, A. Preparation of metakaolin based geopolymer coatings on metal substrates as thermal barriers. Appl. Clay Sci. 2009, 46, 265–270. [Google Scholar] [CrossRef]
- Rashad, A.M. Alkali-activated metakaolin: A short guide for civil Engineer—An overview. Constr. Build. Mater. 2013, 41, 751–765. [Google Scholar] [CrossRef]
- Samal, S.; Kolinova, M.; Rahier, H.; Poggetto, G.D.; Blanco, I. Investigation of the Internal Structure of Fiber Reinforced Geopolymer Composite under Mechanical Impact: A Micro Computed Tomography (µCT) Study. Appl. Sci. 2019, 9, 516. [Google Scholar] [CrossRef] [Green Version]
- Samal, S.; Blanco, I. An Application Review of Fiber-Reinforced Geopolymer Composite. Fibers 2021, 9, 23. [Google Scholar] [CrossRef]
- Denry, I.L.; Holloway, J.A.; Colijn, H.O. Phase transformations in a leucite-reinforced pressable dental ceramic. J. Biomed. Mater. Res. 2001, 54, 351–359. [Google Scholar] [CrossRef]
- Taira, Y.; Sakai, M.; Sawase, T. Effects of primer containing silane and thiophosphate monomers on bonding resin to a leucite-reinforced ceramic. J. Dent. 2012, 40, 353–358. [Google Scholar] [CrossRef] [PubMed]
- Sattabanasuk, V.; Charnchairerk, P.; Punsukumtana, L.; Burrow, M.F. Effects of mechanical and chemical surface treatments on the resin-glass ceramic adhesion properties. J. Investig. Clin. Dent. 2017, 8, e12220. [Google Scholar] [CrossRef]
- De Melo, R.M.; Valandro, L.F.; Bottino, M.A. Microtensile bond strength of a repair composite to leucite-reinforced feldspathic ceramic. Braz. Dent. J. 2007, 18, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Rao, P.; Lu, M.; Wu, J. Mechanical Properties of Dental Porcelain with Different Leucite Particle Sizes. J. Am. Ceram. Soc. 2008, 91, 527–534. [Google Scholar] [CrossRef]
- Fabianelli, A.; Pollington, S.; Papacchini, F.; Goracci, C.; Cantoro, A.; Ferrari, M.; van Noort, R. The effect of different surface treatments on bond strength between leucite reinforced feldspathic ceramic and composite resin. J. Dent. 2010, 38, 39–43. [Google Scholar] [CrossRef]
- Na Sui, L.; Yu, L.Y.; Dong, L.F. Study on Properties of Leucite for Dental Porcelain Compositions. Appl. Mech. Mater. 2011, 66-68, 1522–1527. [Google Scholar] [CrossRef]
- Ota, T.; Takahashi, M.; Yamai, I.; Suzuki, H. High-Thermal-Expansion Polycrystalline Leucite Ceramic. J. Am. Ceram. Soc. 1993, 76, 2379–2381. [Google Scholar] [CrossRef]
- Bell, J.L.; Driemeyer, P.E.; Kriven, W.M. Formation of Ceramics from Metakaolin-Based Geopolymers. Part II: K-Based Geopolymer. J. Am. Ceram. Soc. 2009, 92, 607–615. [Google Scholar] [CrossRef]
- Xie, N.; Bell, J.L.; Kriven, W.M. Fabrication of Structural Leucite Glass-Ceramics from Potassium-Based Geopolymer Precursors. J. Am. Ceram. Soc. 2010, 93, 2644–2649. [Google Scholar] [CrossRef]
- He, P.; Jia, D.; Wang, M.; Zhou, Y. Thermal evolution and crystallization kinetics of potassium-based geopolymer. Ceram. Int. 2011, 37, 59–63. [Google Scholar] [CrossRef]
- Ai, T.; Hong, F.-H.; Kang, Y.-N.; Zhang, H.-R.; Yan, X. Synthesis of Monolithic Potassium Geopolymer Ceramics Assisted by Molten Salt. Materials 2019, 12, 461. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.S.; Jia, D.C.; He, P.G.; Wang, M.R. Thermo-mechanical and Microstructural Characterization of Geopolymers with α-Al2O3 Particle Filler. Int. J. Thermophys. 2009, 30, 1568–1577. [Google Scholar] [CrossRef]
- CNS. Method of Test for Apparent Porosity, Water Absorption and Specific Gravity of Refractory Bricks. 2010. Available online: https://www.cnsonline.com.tw/ (accessed on 18 May 2010).
- Benavent, V.; Steins, P.; Sobrados, I.; Sanz, J.; Lambertin, D.; Frizon, F.; Rossignol, S.; Poulesquen, A. Impact of aluminum on the structure of geopolymers from the early stages to consolidated material. Cem. Concr. Res. 2016, 90, 27–35. [Google Scholar] [CrossRef]
- Sithole, N.T.; Mashifana, T. Geosynthesis of building and construction materials through alkaline activation of granulated blast furnace slag. Constr. Build. Mater. 2020, 264, 120712. [Google Scholar] [CrossRef]
- Rehman, S.K.U.; Imtiaz, L.; Aslam, F.; Khan, M.K.; Haseeb, M.; Javed, M.F.; Alyousef, R.; Alabduljabbar, H. Experimental Investigation of NaOH and KOH Mixture in SCBA-Based Geopolymer Cement Composite. Materials 2020, 13, 3437. [Google Scholar] [CrossRef] [PubMed]
- Poulesquen, A.; Frizon, F.; Lambertin, D. Rheological behavior of alkali-activated metakaolin during geopolymerization. J. Non-Cryst. Solids 2011, 357, 3565–3571. [Google Scholar] [CrossRef]
- Mastura, W.W.; Kamarudin, H.; Nizar, I.K.; Abdullah, M.M.A.B.; Mohammed, H.; Al Bakri, A.M.M. The Effect of Curing Time on the Properties of Fly Ash-Based Geopolymer Bricks. Adv. Mater. Res. 2012, 626, 937–941. [Google Scholar] [CrossRef]
- Bell, J.L.; Sarin, P.; Driemeyer, P.E.; Haggerty, R.P.; Chupas, P.J.; Kriven, W.M. X-Ray pair distribution function analysis of a metakaolin-based, KAlSi2O6·5.5H2O inorganic polymer (geopolymer). J. Mater. Chem. 2008, 18, 5974–5981. [Google Scholar] [CrossRef]
- El-Maghraby, A.; Khaled, K.F.; Elsabawy, K.M. Formation of Leucite Crystals from Metakaolin-based Geopolymer using Kaolin and bentonite. Int. J. Chem. Sci. 2013, 11, 740. [Google Scholar]
- Sheu, T.-S.; O’Brien, W.J.; Rasmussen, S.T.; Tien, T.-Y. Mechanical properties and thermal expansion behaviour in leucite containing materials. J. Mater. Sci. 1994, 29, 125–128. [Google Scholar] [CrossRef]
- Huang, L.; Liu, Y.; Peng, C.; Wu, J. Synthesis and Stabilization of Cubic Leucite. J. Chin. Ceram. Soc. 2017, 45, 948–954. [Google Scholar] [CrossRef]
- Tchakouté, H.K.; Rüscher, C.H. Mechanical and microstructural properties of metakaolin-based geopolymer cements from sodium waterglass and phosphoric acid solution as hardeners: A comparative study. Appl. Clay Sci. 2017, 140, 81–87. [Google Scholar] [CrossRef]
- Rahier, H.; Van Mele, B.; Wastiels, J. Low-temperature synthesized aluminosilicate glasses. J. Mater. Sci. 1996, 31, 80–85. [Google Scholar] [CrossRef]
- Fine, G.; Stolper, E. Dissolved carbon dioxide in basaltic glasses: Concentrations and speciation. Earth Planet. Sci. Lett. 1986, 76, 263–278. [Google Scholar] [CrossRef]
- Jankeviciute, A.; Kareiva, A. Synthesis and characterization of leucite ceramics using sol–gel derived molecular precursors. Mendeleev Commun. 2011, 21, 287–288. [Google Scholar] [CrossRef]
- Hounsi, A.D.; Lecomte-Nana, G.; Djétéli, G.; Blanchart, P.; Alowanou, D.; Kpelou, P.; Napo, K.; Tchangbédji, G.; Praisler, M. How does Na, K alkali metal concentration change the early age structural characteristic of kaolin-based geopolymers. Ceram. Int. 2014, 40, 8953–8962. [Google Scholar] [CrossRef]
- Król, M.; Minkiewicz, J.; Mozgawa, W. IR spectroscopy studies of zeolites in geopolymeric materials derived from kaolinite. J. Mol. Struct. 2016, 1126, 200–206. [Google Scholar] [CrossRef]
- Alkan, M.; Hopa, Ç.; Yilmaz, Z.; Güler, H. The effect of alkali concentration and solid/liquid ratio on the hydrothermal synthesis of zeolite NaA from natural kaolinite. Microporous Mesoporous Mater. 2005, 86, 176–184. [Google Scholar] [CrossRef]
- Palomo, A.; Alonso, S.; Jimenez, A.M.F.; Sobrados, I.; Sanz, J. Alkaline Activation of Fly Ashes: NMR Study of the Reaction Products. J. Am. Ceram. Soc. 2004, 87, 1141–1145. [Google Scholar] [CrossRef]
- Lee, W.K.W.; Van Deveter, J.S.J. Use of Infrared Spectroscopy to Study Geopolymerization of Heterogeneous Amorphous Aluminosilicates. Langmuir 2003, 19, 8726–8734. [Google Scholar] [CrossRef]
- Hajimohammadi, A.; Provis, J.; van Deventer, J.S. Time-resolved and spatially-resolved infrared spectroscopic observation of seeded nucleation controlling geopolymer gel formation. J. Colloid Interface Sci. 2011, 357, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Kljajevic, L.; Nenadović, S.S.; Nenadovic, M.; Bundaleski, N.; Todorovic, B.; Pavlović, V.; Rakočević, Z.L. Structural and chemical properties of thermally treated geopolymer samples. Ceram. Int. 2017, 43, 6700–6708. [Google Scholar] [CrossRef]
- Autef, A.; Joussein, E.; Gasgnier, G.; Pronier, S.; Sobrados, M.I.; Sanz, J.; Rossignol, S. Role of metakaolin dehydroxylation in geopolymer synthesis. Powder Technol. 2013, 250, 33–39. [Google Scholar] [CrossRef]
- Gharzouni, A.; Vidal, L.; Essaidi, N.; Joussein, E.; Rossignol, S. Recycling of geopolymer waste: Influence on geopolymer formation and mechanical properties. Mater. Des. 2016, 94, 221–229. [Google Scholar] [CrossRef]
- Ivanovic, M.; Kljajevic, L.; Gulicovski, J.; Petkovic, M.; Jankovic-Castvan, I.; Bucevac, D.; Nenadovic, S. The effect of the concentration of alkaline activator and aging time on the structure of metakaolin based geopolymer. Sci. Sinter. 2020, 52, 219–229. [Google Scholar] [CrossRef]
- Sitarz, M.; Mozgawa, W.; Handke, M. Rings in the structure of silicate glasses. J. Mol. Struct. 1999, 511-512, 281–285. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, H.; Provis, J.; Bullen, F.; Reid, A.; Zhu, Y. Quantitative kinetic and structural analysis of geopolymers. Part 1. The activation of metakaolin with sodium hydroxide. Thermochim. Acta 2012, 539, 23–33. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, W.; Li, Z. Infrared spectroscopy study of structural nature of geopolymeric products. J. Wuhan Univ. Technol. Sci. Ed. 2008, 23, 522–527. [Google Scholar] [CrossRef]
- Mozgawa, W.; Sitarz, M. Vibrational spectra of aluminosilicate ring structures. J. Mol. Struct. 2002, 614, 273–279. [Google Scholar] [CrossRef]
- González-García, D.; Téllez-Jurado, L.; Jiménez-Álvarez, F.; Balmori-Ramírez, H. Structural study of geopolymers obtained from alkali-activated natural pozzolan feldspars. Ceram. Int. 2017, 43, 2606–2613. [Google Scholar] [CrossRef]
- Ahmad, R.; Abdullah, M.M.A.B.; Hussin, K.; Sandu, A.V. XRD and FTIR study of the effect of ultra high molecular weight polyethylene (UHMWPE) as binder on kaolin geopolymer ceramics. AIP Conf. Proc. 2017, 1835, 20030. [Google Scholar]
- Heah, C.; Kamarudin, H.; Al Bakri, A.M.; Binhussain, M.; Musa, L.; Nizar, I.K.; Ghazali, C.M.R.; Liew, Y. Curing Behavior on Kaolin-Based Geopolymers. Adv. Mater. Res. 2012, 548, 42–47. [Google Scholar] [CrossRef]
- Tippayasam, C.; Balyore, P.; Thavorniti, P.; Kamseu, E.; Leonelli, C.; Chindaprasirt, P.; Chaysuwan, D. Potassium alkali concentration and heat treatment affected metakaolin-based geopolymer. Constr. Build. Mater. 2016, 104, 293–297. [Google Scholar] [CrossRef]
- Villaquirán-Caicedo, M.; de Gutiérrez, R.M. Synthesis of ceramic materials from ecofriendly geopolymer precursors. Mater. Lett. 2018, 230, 300–304. [Google Scholar] [CrossRef]

| Oxide (wt.%) | Metakaolin |
|---|---|
| SiO2 | 49.8 |
| Al2O3 | 19.3 |
| TiO2 | 10.2 |
| Fe2O3 | 6.3 |
| K2O | 2.4 |
| CaO | 2.2 |
| Others | 6.8 |
| L.O.I | 3.0 |
| Specimen Label | KOH | K2O/SiO2 | Sintering Time (h) | Temperature (°C) | Mixing Time (min) | Curing Time (h) |
|---|---|---|---|---|---|---|
| R1 | 0 | 1 | 2 | 1200 | 10 | 8 |
| R2 | 0.25 | 0.75 | ||||
| R3 | 0.5 | 0.5 | ||||
| R4 | 0.75 | 0.25 | ||||
| R5 | 1 | 0 |
| Specimen Label | KOH | K2O/SiO2 | Sintering Time (h) | Temperature (°C) | Mixing Time (min) | Curing Time (h) |
|---|---|---|---|---|---|---|
| S1 | 0.5 | 0.5 | 0.5 | 1200 | 10 | 8 |
| S2 | 1 | |||||
| S3 | 2 | |||||
| S4 | 4 |
| Specimen Label | KOH | K2O/SiO2 | Sintering Time (h) | Temperature (°C) | Mixing Time (min) | Curing Time (h) |
|---|---|---|---|---|---|---|
| T1 | 0.5 | 0.5 | 2 | 1000 | 10 | 8 |
| T2 | 1100 | |||||
| T3 | 1200 | |||||
| T4 | 1300 |
| Specimen Label | KOH | K2O/SiO2 | Sintering Time (h) | Temperature (°C) | Mixing Time (min) | Curing Time (h) |
|---|---|---|---|---|---|---|
| M1 | 0.5 | 0.5 | 2 | 1200 | 5 | 8 |
| M2 | 10 | |||||
| M3 | 15 | |||||
| M4 | 30 |
| Specimen Label | KOH | K2O/SiO2 | Sintering Time (h) | Temperature (°C) | Mixing Time (min) | Curing Time (h) |
|---|---|---|---|---|---|---|
| C1 | 0.5 | 0.5 | 2 | 1200 | 10 | 1 |
| C2 | 2 | |||||
| C3 | 4 | |||||
| C4 | 8 |
| Rotor Speed | Rotor | ||||||
|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | |
| 10 | 10 | 40 | 100 | 200 | 400 | 1000 | 4000 |
| 20 | 5 | 20 | 50 | 100 | 200 | 500 | 2000 |
| 50 | 2 | 8 | 20 | 40 | 80 | 200 | 800 |
| 100 | 1 | 4 | 10 | 20 | 40 | 100 | 400 |
| Specimen Label | Viscosity (MPa.s) | Water Absorption (%) | Porosity (%) | Bulk Density (g/cm3) | Rockwell Hardness (GPa) |
|---|---|---|---|---|---|
| R1 | 44,000 | 0.4 | 0.3 | 2.52 | 2.62 |
| R2 | 12,000 | 1 | 2 | 2.34 | 2.67 |
| R3 | 5250 | 1 | 2 | 3.21 | 6.02 |
| R4 | 9000 | 18 | 32 | 2.02 | none |
| R5 | 9850 | 18 | 32 | 1.73 | none |
| Specimen Label | Viscosity (MPa.s) | Water Absorption (%) | Porosity (%) | Bulk Density (g/cm3) | Rockwell Hardness (GPa) |
|---|---|---|---|---|---|
| M1 | 3250 | 13 | 25 | 2.06 | 4.04 |
| M2 | 5250 | 1 | 2 | 3.21 | 6.02 |
| M3 | 14,500 | 1 | 2 | 2.55 | 4.98 |
| M4 | 24,000 | 1 | 2 | 2.39 | 4.85 |
| Specimen Label | Viscosity (MPa.s) | Water Absorption (%) | Porosity (%) | Bulk Density (g/cm3) | Rockwell Hardness (GPa) |
|---|---|---|---|---|---|
| S1 | - | 1 | 3 | 2.34 | 5.18 |
| S2 | - | 1 | 3 | 2.40 | 5.03 |
| S3 | 5250 | 1 | 2 | 3.21 | 6.02 |
| S4 | - | 1 | 2 | 3.38 | 6.15 |
| Specimen Label | Viscosity (MPa.s) | Water Absorption (%) | Porosity (%) | Bulk Density (g/cm3) | Rockwell Hardness (GPa) |
|---|---|---|---|---|---|
| T1 | - | 2 | 4 | 2.12 | 4.41 |
| T2 | - | 2 | 2 | 2.82 | 4.81 |
| T3 | 5250 | 1 | 2 | 3.21 | 6.02 |
| T4 | - | 0.7 | 1 | 3.46 | 5.54 |
| Specimen Label | Viscosity (MPa.s) | Water Absorption (%) | Porosity (%) | Bulk Density (g/cm3) | Rockwell Hardness (GPa) |
|---|---|---|---|---|---|
| C1 | - | 2 | 7 | 2.28 | 3.805 |
| C2 | - | 1 | 5 | 2.47 | 3.88 |
| C3 | - | 1 | 4 | 3.08 | 4.51 |
| C4 | 5250 | 1 | 2 | 3.21 | 6.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hsieh, Y.-C.; Lee, W.-H.; Liao, P.-H. Using Geopolymer Technology on Synthesizing Leucite Ceramics. Polymers 2021, 13, 3621. https://doi.org/10.3390/polym13213621
Hsieh Y-C, Lee W-H, Liao P-H. Using Geopolymer Technology on Synthesizing Leucite Ceramics. Polymers. 2021; 13(21):3621. https://doi.org/10.3390/polym13213621
Chicago/Turabian StyleHsieh, Yi-Che, Wei-Hao Lee, and Pin-Hsun Liao. 2021. "Using Geopolymer Technology on Synthesizing Leucite Ceramics" Polymers 13, no. 21: 3621. https://doi.org/10.3390/polym13213621