Surface Functionalization of Black Phosphorus via Amine Compounds and Its Impacts on the Flame Retardancy and Thermal Decomposition Behaviors of Epoxy Resin
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Preparation of BP
2.3. Preparation of NH2-Funtionalized of BP (BP-NH2)
2.4. Preparation of EP/BP-NH2 Nanocomposites
2.5. Characterizations
3. Results and Discussion
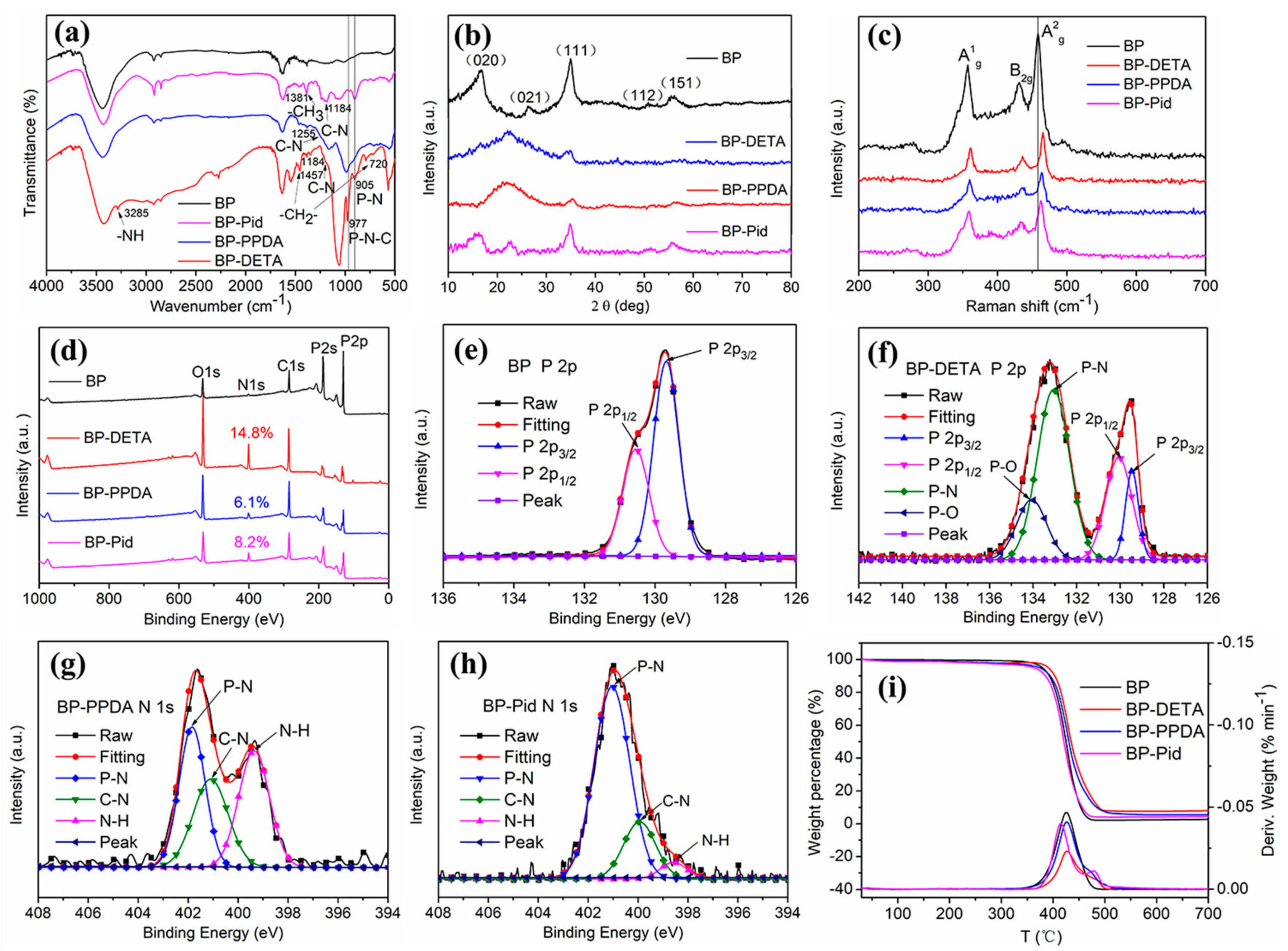
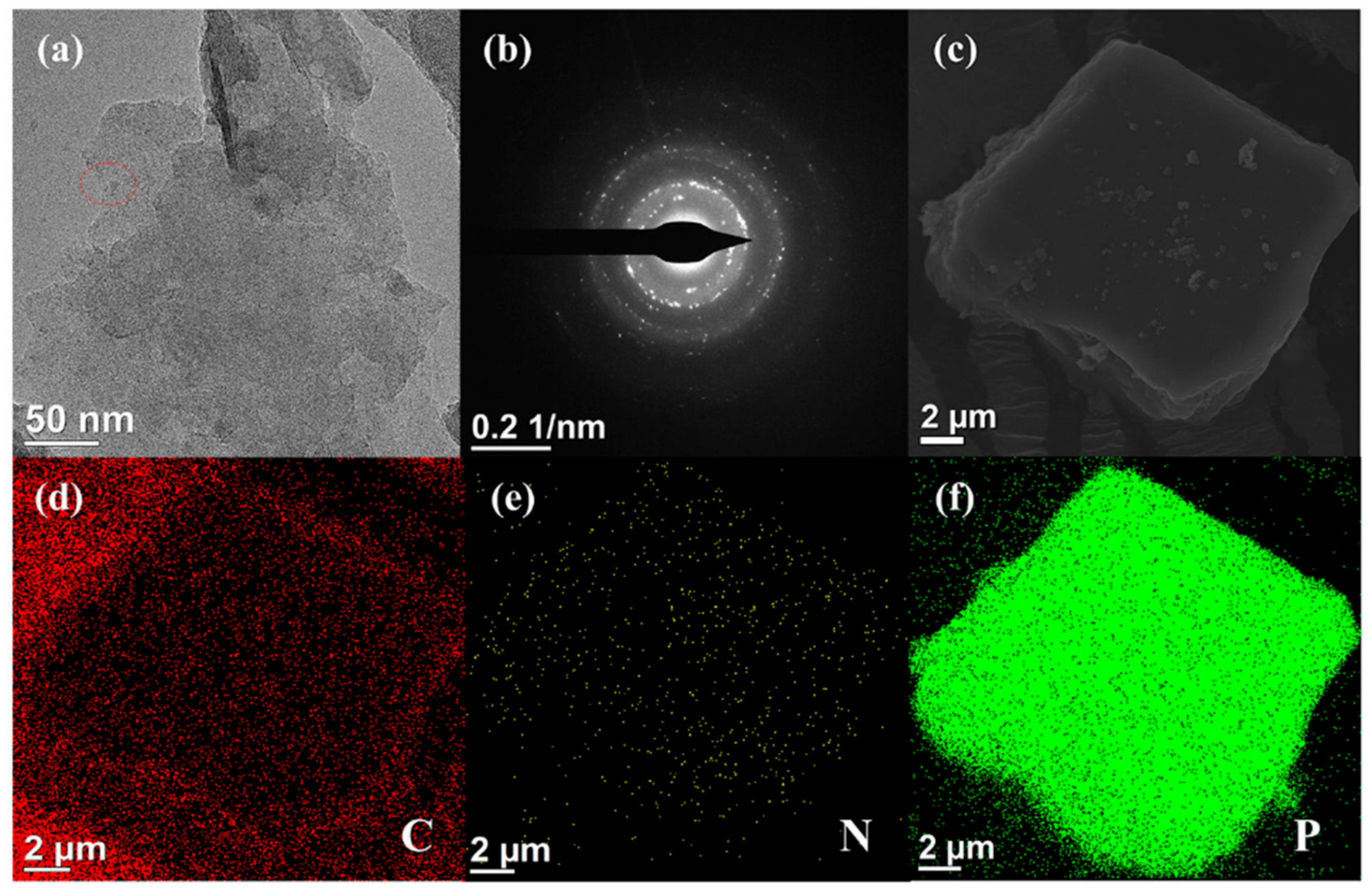
3.1. Characterizations of BP-NH2 Structure and Morphology
3.2. The Amino Reactivity of BP-NH2 with Epoxide Group and the Dispersibility in EP
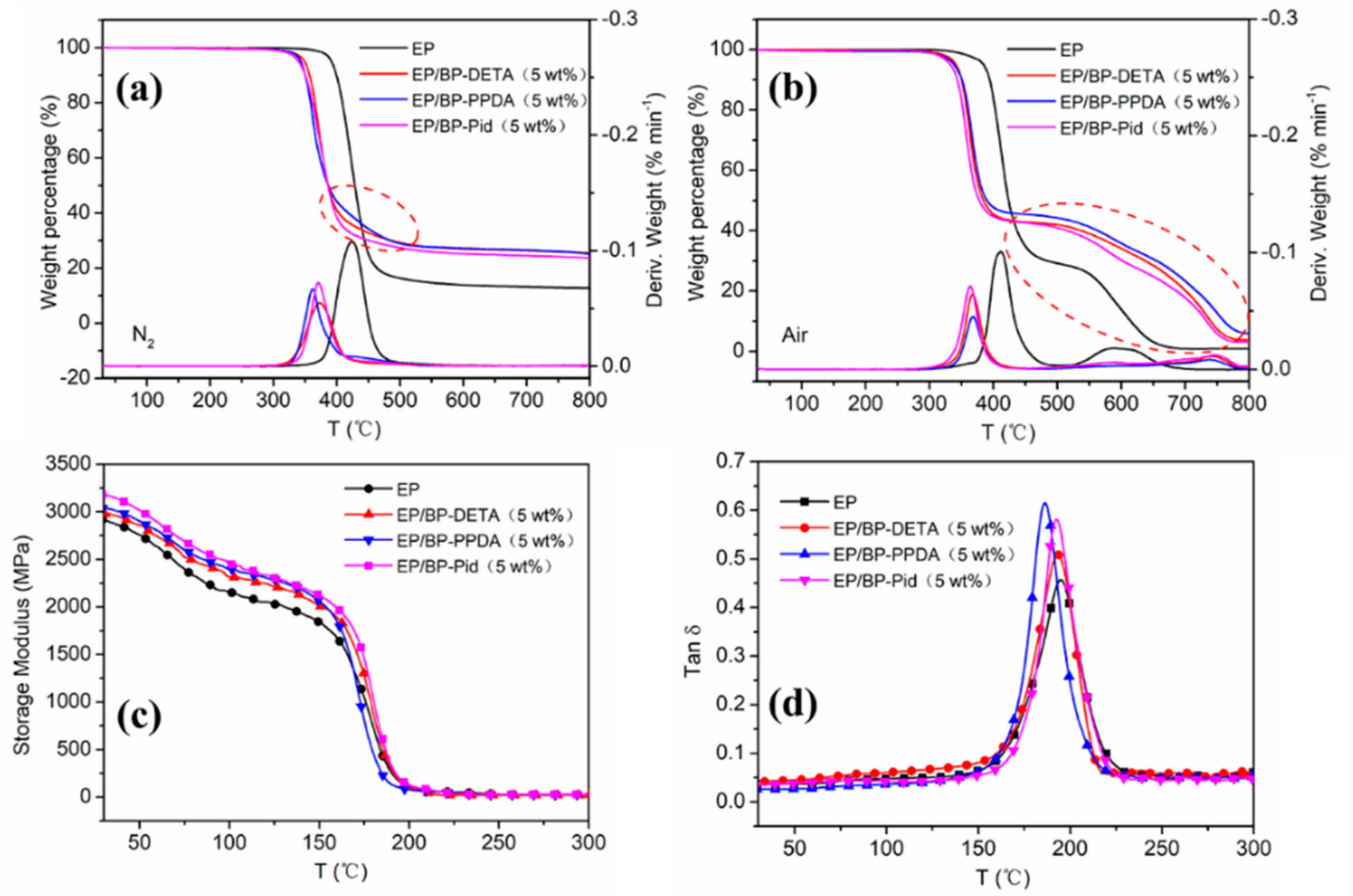
3.3. Thermal Stability and Thermal Dynamic Mechanical Properties of EP Composites
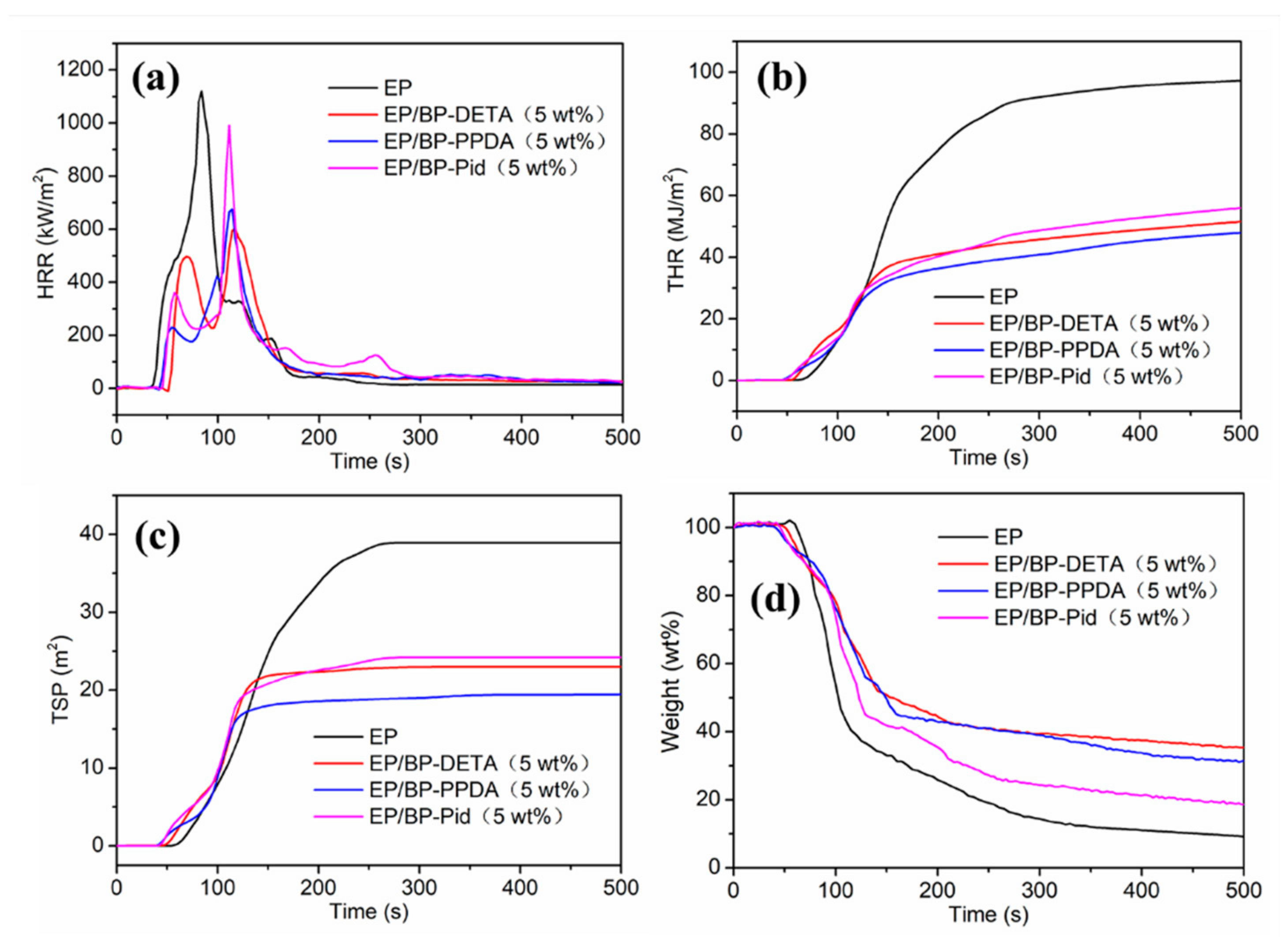
3.4. Flame Retardancy of EP Composites
3.5. Flame Retardant Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jiang, S.; Tang, G.; Chen, J.; Huang, Z.; Hu, Y. Biobased Polyelectrolyte Multilayer-Coated Hollow Mesoporous Silica as a Green Flame Retardant for Epoxy Resin. J. Hazard. Mater. 2017, 342, 689–697. [Google Scholar] [CrossRef]
- Ai, Y.; Xia, L.; Pang, F.; Xu, Y.; Zhao, H.; Jian, R. Mechanically Strong and Flame-Retardant Epoxy Resins with Anti-Corrosion Performance. Compos. Part B Eng. 2020, 193, 108019. [Google Scholar] [CrossRef]
- Ma, C.; Qiu, S.; Yu, B.; Wang, J.; Wang, C.; Zeng, W.; Hu, Y. Economical and Environment-Friendly Synthesis of a Novel Hyperbranched Poly(Aminomethylphosphine Oxide-Amine) as Co-Curing Agent for Simultaneous Improvement of Fire Safety, Glass Transition Temperature and Toughness of Epoxy Resins. Chem. Eng. J. 2017, 322, 618–631. [Google Scholar] [CrossRef]
- Täuber, K.; Marsico, F.; Wurm, F.; Schartel, B. Hyperbranched Poly(Phosphoester)s as Flame Retardants for Technical and High Performance Polymers. Polym. Chem. 2014, 5, 7042–7053. [Google Scholar] [CrossRef]
- Song, K.; Wang, Y.; Ruan, F.; Yang, W.; Fang, Z.; Zheng, D.; Li, X.; Li, N.; Qiao, M.; Liu, J. Synthesis of a Reactive Template-Induced Core-Shell PZS@ZIF-67 Composite Microspheres and Its Application in Epoxy Composites. Polymers 2021, 13, 2646. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; You, N.; Ku, B. Highly Efficient Halogen-Free Flame Retardants of Thermally-Oxidized Polyacrylonitrile Copolymers Containing Bio-Derived Caffeic Acid Derivatives. Polym. Chem. 2020, 11, 6658–6669. [Google Scholar] [CrossRef]
- Liu, C.; Qiao, H.; Xu, G.; Liang, Y.; Yang, J.; Hu, J. Phosphorous-Nitrogen Modification of Epoxy Grafted Poly-Acrylic Resin: Synergistic Flame Retardment Effect. Polymers 2021, 13, 2826. [Google Scholar] [CrossRef] [PubMed]
- Shiu, B.; Wu, K.; Lou, C.; Lin, Q.; Lin, J. Synthesis of a Compound Phosphorus-Nitrogen Intumescent Flame Retardant for Applications to Raw Lacquer. Polymers 2021, 13, 2858. [Google Scholar] [CrossRef]
- Wang, L.; Wei, Y.; Deng, H.; Lyu, R.; Zhu, J.; Yang, Y. Synergistic Flame Retardant Effect of Barium Phytate and Intumescent Flame Retardant for Epoxy Resin. Polymers 2021, 13, 2900. [Google Scholar] [CrossRef]
- Bridgman, P. Two New Modifications of Phosphorus. J. Am. Chem. Soc. 1914, 36, 1344–1363. [Google Scholar] [CrossRef] [Green Version]
- Keyes, R.W. The Electrical Properties of Black Phosphorus. Phys. Rev. 1953, 92, 580–584. [Google Scholar] [CrossRef]
- Du, Y.; Ouyang, C.; Shi, S.; Lei, M. Ab Initio Studies on Atomic and Electronic Structures of Black Phosphorus. Solid. State. Sci. 2010, 107, 2465–2469. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, R.; Pu, H.; Chang, J.; Chen, J. Field-Effect Transistor Biosensors with Two-Dimensional Black Phosphorus Nanosheets. Biosens. Bioelectron. 2017, 89, 505–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellanos-Gomez, A.; Vicarelli, L.; Prada, E.; Island, J.; Narasimha-Acharya, K.; Blanter, S.; Groenendijk, D.; Buscema, M.; Steele, G.; Alvarez, J. Isolation and Characterization of Few-Layer Black Phosphorus. 2D Mater. 2014, 1, 025001. [Google Scholar] [CrossRef]
- Late, D. Liquid Exfoliation of Black Phosphorus Nanosheets and Its Application as Humidity Sensor. Microporous Mesoporous Mater. 2016, 225, 494–503. [Google Scholar] [CrossRef]
- Li, L.; Yu, Y.; Ye, G.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.; Zhang, Y. Black Phosphorus Field-Effect Transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Zou, B.; Zhang, T.; Ren, X.; Yu, B.; Zhou, Y.; Kan, Y.; Hu, Y. Integrated Effect of NH2-Functionalized/Triazine Based Covalent Organic Framework Black Phosphorus on Reducing Fire Hazards of Epoxy Nanocomposites. Chem. Eng. J. 2020, 401, 126058. [Google Scholar] [CrossRef]
- Qu, Z.; Wu, K.; Meng, W.; Nan, B.; Hu, Z.; Xu, C.; Tan, Z.; Zhang, Q.; Meng, H.; Shi, J. Surface Coordination of Black Phosphorene for Excellent Stability, Flame Retardancy and Thermal Conductivity in Epoxy Resin. Chem. Eng. J. 2020, 397, 125416. [Google Scholar] [CrossRef]
- Walia, S.; Balendhran, S.; Ahmed, T.; Singh, M.; El-Badawi, C.; Brennan, M.; Weerathunge, P.; Karim, M.; Rahman, F.; Rassell, A.; et al. Ambient Protection of Few-Layer Black Phosphorus via Sequestration of Reactive Oxygen Species. Adv. Mater. 2017, 29, 1700152. [Google Scholar] [CrossRef]
- Lei, W.; Liu, G.; Zhang, J.; Liu, M. Black Phosphorus Nanostructures: Recent Advances in Hybridization, Doping and Functionalization. Chem. Soc. Rev. 2017, 46, 3492–3509. [Google Scholar] [CrossRef]
- Wu, S.; He, F.; Xie, G.; Bian, Z.; Luo, J.; Wen, S. Black Phosphorus: Degradation Favors Lubrication. Nano Lett. 2018, 18, 5618–5627. [Google Scholar] [CrossRef]
- Zhang, T.; Wan, Y.; Xie, H.; Mu, Y.; Du, P.; Wang, D.; Wu, X.; Ji, H.; Wan, L. Degradation Chemistry and Stabilization of Exfoliated Few-Layer Black Phosphorus in Water. J. Am. Chem. Soc. 2018, 140, 7561–7567. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Slough, W.; Pandey, R.; Karna, S. Degradation of Phosphorene in Air: Understanding at Atomic Level. 2D Mater. 2015, 3, 025011. [Google Scholar] [CrossRef] [Green Version]
- Van Druenen, M.; Davitt, F.; Collins, T.; Glynn, C.; O’Dwyer, C.; Holmes, J.; Collins, G. Evaluating the Surface Chemistry of Black Phosphorus during Ambient Degradation. Langmuir 2019, 35, 2172–2178. [Google Scholar] [CrossRef]
- Abellán, G.; Wild, S.; Lloret, V.; Scheuschner, N.; Gillen, R.; Mundloch, U.; Maultzsch, J.; Varela, M.; Hauke, F.; Hirsch, A. Fundamental Insights into the Degradation and Stabilization of Thin Layer Black Phosphorus. J. Am. Chem. Soc. 2017, 139, 10432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Qiao, J.; He, K.; Bliznakov, S.; Sutter, E.; Chen, X.; Luo, D.; Meng, F.; Dong, S.; Decker, J. Interaction of Black Phosphorus with Oxygen and Water. Chem. Mater. 2016, 28, 8330–8339. [Google Scholar] [CrossRef] [Green Version]
- Plutnar, J.; Sofer, Z.; Pumera, M. Products of Degradation of Black Phosphorus in Protic Solvents. ACS Nano 2018, 12, 8390–8396. [Google Scholar] [CrossRef] [PubMed]
- Abate, Y.; Akinwande, D.; Gamage, S.; Wang, H.; Snure, M.; Poudel, N.; Cronin, S. Recent Progress on Stability and Passivation of Black Phosphorus. Adv. Mater. 2018, 30, 1704749. [Google Scholar] [CrossRef]
- Favron, A.; Gaufres, E.; Fossard, F.; Phaneuf-L’Heureux, A.; Tang, N.; Levesque, P.; Loiseau, A.; Leonelli, R.; Francoeur, S.; Martel, R. Photooxidation and Quantum Confinement Effects in Exfoliated Black Phosphorus. Nat. Mater. 2015, 14, 826–832. [Google Scholar] [CrossRef]
- Zhu, H.; McDonnell, S.; Qin, X.; Azcatl, A.; Cheng, L.; Addou, R.; Kim, J.; Ye, P.; Wallace, R.M. Al2O3 on Black Phosphorus by Atomic Layer Deposition: An In Situ Interface Study. ACS Appl. Mater. Inter. 2015, 7, 13038–13043. [Google Scholar] [CrossRef] [PubMed]
- Wood, J.; Wells, S.; Jariwala, D.; Chen, K.; Cho, E.; Sangwan, V.; Liu, X.; Lauhon, L.; Marks, T.; Hersam, M. Effective Passivation of Exfoliated Black Phosphorus Transistors against Ambient Degradation. Nano Lett. 2014, 14, 6964–6970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Birowska, M.; Urban, J.; Baranowski, M.; Maude, D.; Plochocka, P.; Szwacki, N. The Impact of Hexagonal Boron Nitride Encapsulation on the Structural and Vibrational Properties of Few Layer Black Phosphorus. Nanotechnology 2019, 30, 120165. [Google Scholar] [CrossRef] [Green Version]
- Illarionov, Y.; Waltl, M.; Rzepa, G.; Kim, J.; Kim, S.; Dodabalapur, A.; Akinwande, D.; Grasser, T. Long-Term Stability and Reliability of Black Phosphorus Field-Effect Transistors. ACS Nano 2016, 10, 9543–9549. [Google Scholar] [CrossRef] [PubMed]
- Constantinescu, G.; Hine, N. Multipurpose Black-Phosphorus/hBN Heterostructures. Nano Lett. 2016, 16, 2586–2594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gamage, S.; Li, Z.; Yakovlev, V.; Lewis, C.; Wang, H.; Cronin, S.; Abate, Y. Nanoscopy of Black Phosphorus Degradation. Adv. Mater. Interfaces 2016, 3, 1600121. [Google Scholar] [CrossRef]
- Pei, J.; Gai, X.; Yang, J.; Wang, X.; Yu, Z.; Choi, D.; Luther-Davies, B.; Lu, Y. Producing Air-Stable Monolayers of Phosphorene and Their Defect Engineering. Nat. Commun. 2016, 7, 10450. [Google Scholar] [CrossRef] [Green Version]
- Gamage, S.; Fali, A.; Aghamiri, N.; Yang, L.; Ye, P.; Abate, Y. Reliable Passivation of Black Phosphorus by Thin Hybrid Coating. Nanotechnology 2017, 28, 113949. [Google Scholar] [CrossRef]
- Clark, N.; Nguyen, L.; Hamer, M.; Schedin, F.; Lewis, E.; Prestat, E.; Garner, A.; Cao, Y.; Zhu, M.; Kashtiban, R.; et al. Scalable Patterning of Encapsulated Black Phosphorus. Nano Lett. 2018, 18, 5373–5381. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Zhang, D.; Liu, J.; Chang, H.; Sun, Y.; Yin, N. Air-Stable Black Phosphorus Devices for Ion Sensing. ACS Appl. Mater. Inter. 2015, 7, 24396–24402. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, P.; Zhang, T.; Zhu, X.J.; Zhang, M.; Chen, M.; Du, P.; Wang, G.; Ji, H.; Yang, J.; et al. Azide Passivation of Black Phosphorus Nanosheets: Covalent Functionalization Affords Ambient Stability Enhancement. Angew. Chem. 2019, 58, 1479–1483. [Google Scholar] [CrossRef]
- Ryder, C.; Wood, J.; Wells, S.; Yang, Y.; Jariwala, D.; Marks, T.; Schatz, G.; Hersam, M. Covalent Functionalization and Passivation of Exfoliated Black Phosphorus via Aryl Diazonium Chemistry. Nat. Chem. 2016, 8, 597–602. [Google Scholar] [CrossRef]
- Cao, Y.; Tian, X.; Gu, J.; Liu, B.; Zhang, B.; Song, S.; Fan, F.; Chen, Y. Covalent Functionalization of Black Phosphorus with Conjugated Polymer for Information Storage. Angew. Chem. 2018, 57, 4543–4548. [Google Scholar] [CrossRef]
- Van Druenen, M.; Davitt, F.; Collins, T.; Glynn, C.; O’Dwyer, C.; Holmes, J.; Collins, G. Covalent Functionalization of Few-Layer Black Phosphorus Using lodonium Salts and Comparison to Diazonium Modified Black Phosphorus. Chem. Mater. 2018, 30, 4667–4674. [Google Scholar] [CrossRef]
- Shao, L.; Sun, H.; Miao, L.; Chen, X.; Han, M.; Sun, J.; Liu, S.; Li, L.; Cheng, F.; Chen, J. Facile Preparation of NH2-Functionalized Black Phosphorene for the Electrocatalytic Hydrogen Evolution Reaction. J. Mater. Chem. A 2018, 6, 2494–2499. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, T.; Jiang, D.; Duan, H.; Sun, Z.; Zhang, M.; Jin, H.; Guan, R.; Liu, Y.; Chen, M.; et al. Stabilizing Black Phosphorus Nanosheets via Edge-Selective Bonding of Sacrificial C-60 Molecules. Nat. Commun. 2018, 9, 4177–4185. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Tong, L.; Li, Z.; Yang, N.; Fu, H.; Wu, L.; Cui, H.; Zhou, W.; Wang, J.; Wang, H.; et al. Stable and Multifunctional Dye-Modified Black Phosphorus Nanosheets for Near-Infrared Imaging-Guided Photothermal Therapy. Chem. Mater. 2017, 29, 7131–7139. [Google Scholar] [CrossRef]
- Hu, H.; Gao, H.; Gao, L.; Li, F.; Xu, N.; Long, X.; Hu, Y.; Jin, J.; Ma, J. Covalent Functionalization of Black Phosphorus Nanoflakes by Carbon Free Radicals for Durable Air and Water Stability. Nanoscale 2018, 10, 5834–5839. [Google Scholar] [CrossRef]
- Sun, J.; Zheng, G.; Lee, H.; Liu, N.; Wang, H.; Yao, H.; Yang, W.; Cui, Y. Formation of Stable Phosphorus-Carbon Bond for Enhanced Performance in Black Phosphorus Nanoparticle-Graphite Composite Battery Anodes. Nano. Lett. 2014, 14, 4573–4580. [Google Scholar] [CrossRef]
- Qiu, S.; Zhou, Y.; Zhou, X.; Zhang, T.; Wang, C.; Yuen, R.; Hu, W.; Hu, Y. Air-Stable Polyphosphazene-Functionalized Few-Layer Black Phosphorene for Flame Retardancy of Epoxy Resins. Small 2019, 15, e1805175. [Google Scholar] [CrossRef]
- Zhao, X.; Babu, H.V.; Llorca, J.; Wang, D.-Y. Impact of Halogen-Free Flame Retardant with Varied Phosphorus Chemical Surrounding on the Properties of Diglycidyl Ether of Bisphenol-A Type Epoxy Resin: Synthesis, Fire Behaviour, Flame-Retardant Mechanism and Mechanical Properties. RSC Adv. 2016, 6, 59226–59236. [Google Scholar] [CrossRef] [Green Version]
- Qiu, S.; Wang, X.; Yu, B.; Feng, X.; Mu, X.; Yuen, R.; Hu, Y. Flame-Retardant-Wrappedpolyphosphazene Nanotubes: A Novel Strategy for Enhancing the Flame Retardancy and Smoke Toxicity Suppression of Epoxy Resins. J. Hazard. Mater. 2017, 325, 327–339. [Google Scholar] [CrossRef]
- Zhao, X.; Xiao, D.; Alonso, J.; Wang, D.-Y. Inclusion Complex between Beta-Cyclodextrin and Phenylphosphonicdiamide as Novel Bio-Based Flame Retardant to Epoxy: Inclusion Behavior, Characterization and Flammability. Mater. Design. 2017, 114, 623–632. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Xing, W.; Feng, X.; Yu, B.; Song, L.; Hu, Y. Functionalization of Graphene with Grafted Polyphosphamide for Flame Retardant Epoxy Composites: Synthesis, Flammability and Mechanism. Polym. Chem. 2014, 5, 1145–1154. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, T.; Sun, Z.J.; Chen, H.L.; Guan, J.; Chen, X.; Ji, H.X.; Du, P.; Yang, S. Black Phosphorus Revisited: A Missing Metal-Free Elemental Photocatalyst for Visible Light Hydrogen Evolution. Adv. Mater. 2017, 29, 1605776. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, J.; Li, M.; Cai, Q.; Li, W.; Huang, C.; Yuan, C.; Xu, Y.; Zeng, B.; Dai, L. Design of h-BN@boronate Polymer core-Shell Nanoplates to Simultaneously Enhance the Flame Retardancy and Mechanical Properties of Epoxy Resin through the Interficial Regulation. Compos. Part A Appl. Sci. Manuf. 2020, 130, 105751. [Google Scholar] [CrossRef]
- Xu, Z.; Lin, S.; Onofrio, N.; Zhou, L.; Shi, F.; Lu, W.; Kang, K.; Zhang, Q.; Lau, S. Exceptional Catalytic Effects of Black Phosphorus Quantum Dots in Shuttling-Free Lithium Sulfur Batteries. Nat. Commun. 2018, 9, 4164–4174. [Google Scholar] [CrossRef]
- Wang, X.; Xing, W.; Feng, X.; Song, L.; Hu, Y. MoS2/Polymer Nanocomposites: Preparation, Properties, and Applications. Polym. Rev. 2017, 57, 440–466. [Google Scholar] [CrossRef]
- Tan, Y.; Shao, Z.; Yu, L.; Long, J.; Qi, M.; Chen, L.; Wang, Y. Piperazine-Modified Ammonium Polyphosphate as Monocomponent Flame-Retardant Hardener for Epoxy Resin: Flame Retardance, Curing Behavior and Mechanical Property. Polym. Chem. 2016, 7, 3003–3012. [Google Scholar] [CrossRef]
- Qu, Z.; Wu, K.; Jiao, E.; Chen, W.; Hu, Z.; Xu, C.; Shi, J.; Wang, S.; Tan, Z. Surface Functionalization of Few-Layer Black Phosphorene and Its Flame Retardancy in Epoxy Resin. Chem. Eng. J. 2019, 382, 122991. [Google Scholar] [CrossRef]
- Zhao, X.; De Juan, S.; Guerrero, F.; Li, Z.; Llorca, J.; Wang, D.-Y. Effect of N,N′-Diallyl-Phenylphosphoricdiamide on Ease of Ignition, Thermal Decomposition Behavior and Mechanical Properties of Poly(Lactic Acid). Polym. Degrad. Stabil. 2016, 127, 2–10. [Google Scholar] [CrossRef] [Green Version]
- Xiao, D.; Li, Z.; Gohs, U.; Wagenknecht, U.; Voit, B.; Wang, D.-Y. Functionalized Allylamine Polyphosphate as a Novel Multifunctional Highly Efficient Fire Retardant for Polypropylene. Polym. Chem. 2017, 8, 6309–6318. [Google Scholar] [CrossRef]
- Hong, J.; Wu, T.; Wu, H.; Zeng, B.; Zeng, S.; Chen, T.; Wang, X.; Lu, Z.; Yuan, C.; Balaji, K.; et al. Nanohybrid Silver Nanoparticles@halloysite Nanotubes Coated with Polyphosphazene for Effectively Enhancing the Fire Safety of Epoxy Resin. Chem. Eng. J. 2021, 407, 127087. [Google Scholar] [CrossRef]




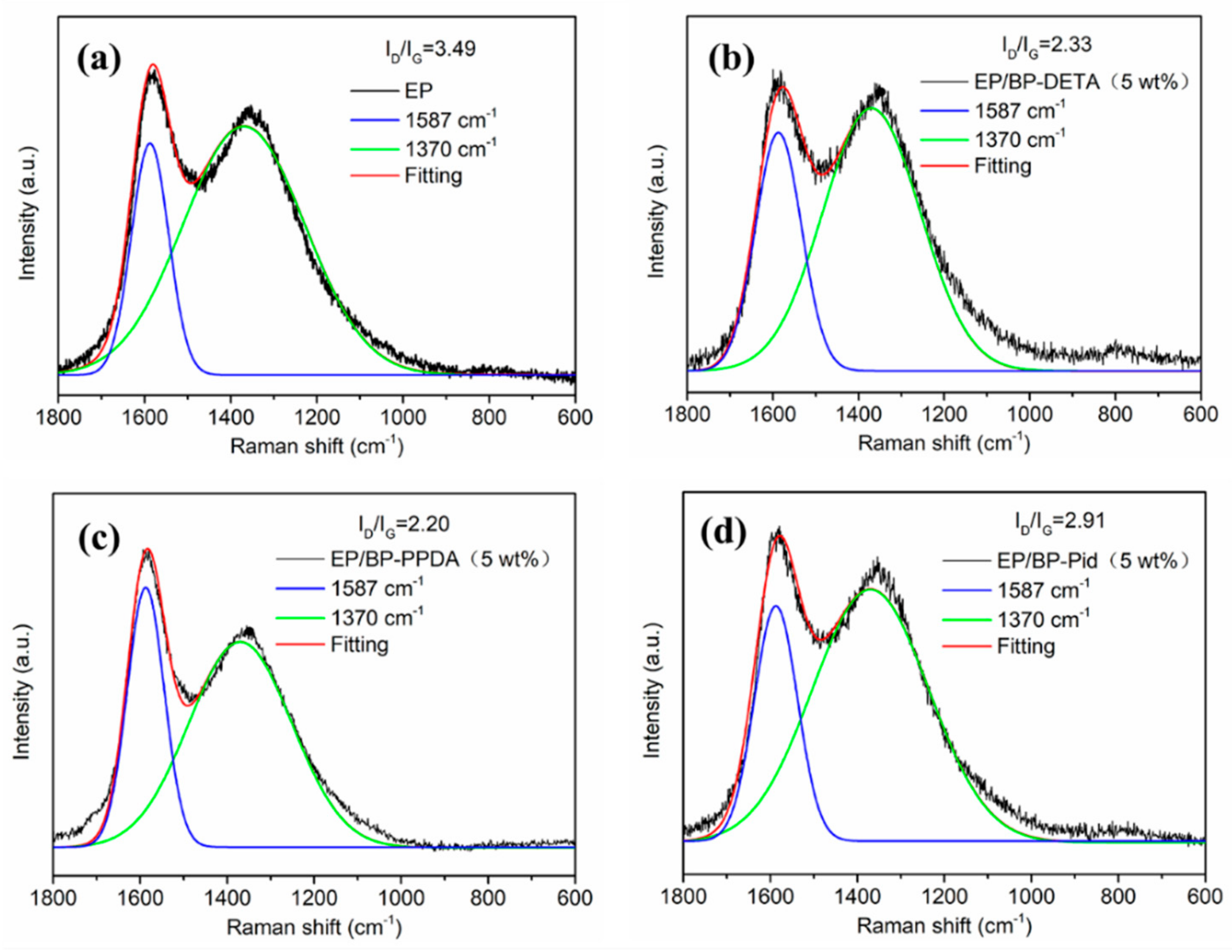
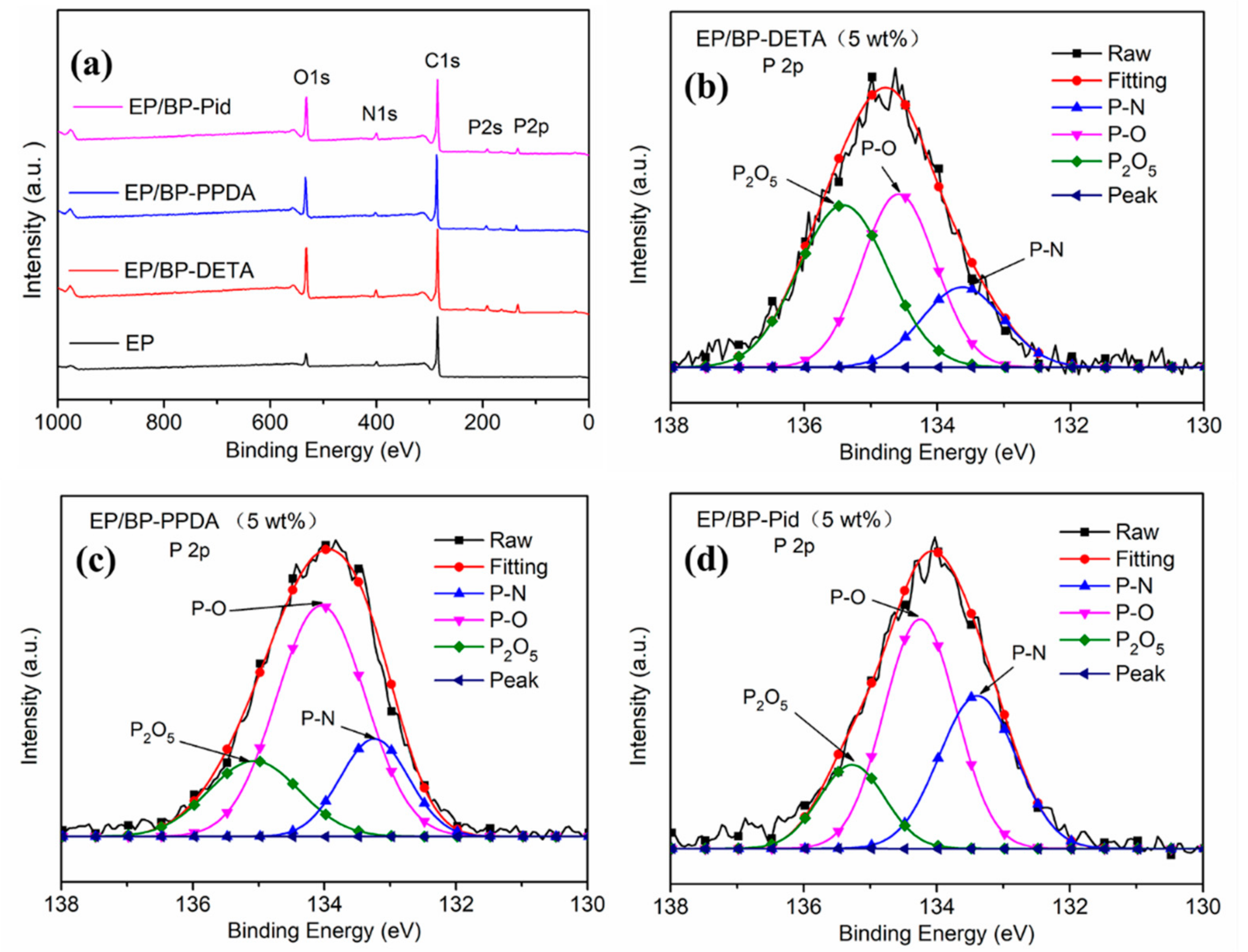
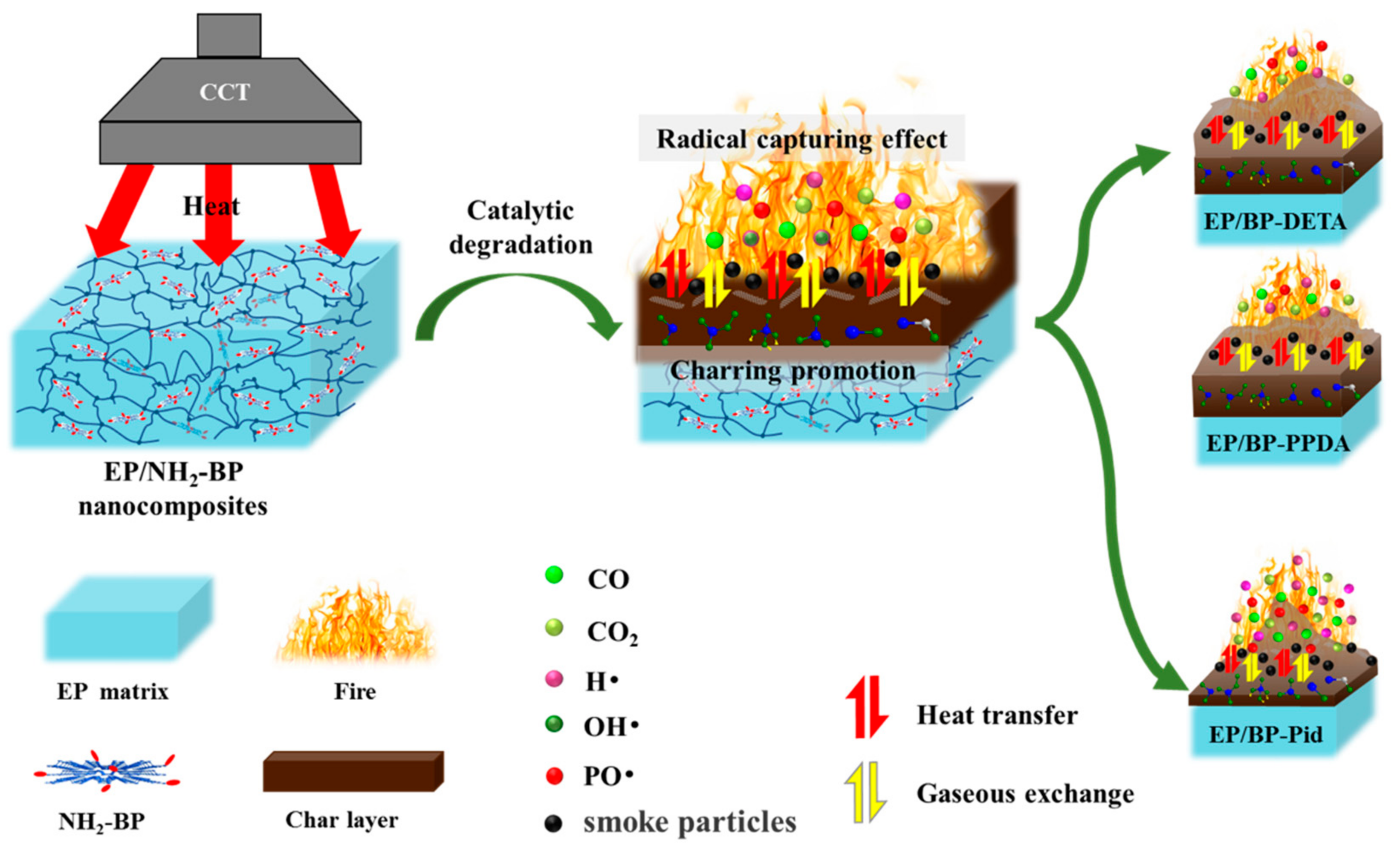
| Sample | T5% a (°C) | Tmax1 b (°C) | Tmax2 c (°C) | Residue (800 °C, %) | |||
|---|---|---|---|---|---|---|---|
| N2 | Air | N2 | Air | Air | N2 | Air | |
| 391 | 385 | 425 | 411 | 588 | 12.8 | 0.9 | |
| 349 | 342 | 374 | 365 | 739 | 25.5 | 3.8 | |
| 343 | 339 | 363 | 366 | 739 | 25.2 | 6.0 | |
| 339 | 333 | 371 | 363 | 747 | 23.7 | 3.1 | |
| Sample | LOI (%) | UL 94 | ||
|---|---|---|---|---|
| Rating | ||||
| EP | 19.8 | >30 | >60 | NR |
| EP/BP-DETA (1 wt%) | 26.1 | >30 | >60 | NR |
| EP/BP-DETA (3 wt%) | 28.2 | 7.3 | 11.8 | V 1 |
| EP/BP-DETA (5 wt%) | 30.1 | 1.8 | 3.6 | V 0 |
| EP/BP-PPDA (1 wt%) | 27.9 | >30 | >60 | NR |
| EP/BP-PPDA (3 wt%) | 30.1 | 2.0 | 10.6 | V 1 |
| EP/BP-PPDA (5 wt%) | 32.3 | 1.6 | 1.8 | V 0 |
| EP/BP-Pid (1 wt%) | 26.2 | >30 | >60 | NR |
| EP/BP-Pid (3 wt%) | 27.8 | 15.0 | 42.3 | NR |
| EP/BP-Pid (5 wt%) | 31.9 | 3.0 | 4.8 | V 0 |
| Sample | TTI a (s) | TPHRR b (s) | PHRR c (kW/m2) | FIGRA (kW/m2/s) | THR d (MJ/m2) | av-EHC e (kJ/kg) | TSP f (m2) | Residue (wt %) |
|---|---|---|---|---|---|---|---|---|
| EP | 40 | 84 | 1120 | 13.3 | 97.3 | 15.2 | 38.9 | 9.2 |
| EP/BP-DETA (5 wt%) | 40 | 117 | 605 | 5.2 | 51.6 | 13.5 | 23.0 | 35.1 (↑73.8%) |
| EP/BP-PPDA (5 wt%) | 37 | 114 | 674 | 5.9 | 47.0 | 13.2 | 20.2 | 31.3 (↑70.6%) |
| EP/BP-Pid (5 wt%) | 38 | 111 | 991 | 8.9 | 56.0 | 12.4 | 24.2 | 18.7 (↑50.8%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, S.; Tao, B.; Zhao, X.; Chen, G.; Wang, D.-Y. Surface Functionalization of Black Phosphorus via Amine Compounds and Its Impacts on the Flame Retardancy and Thermal Decomposition Behaviors of Epoxy Resin. Polymers 2021, 13, 3635. https://doi.org/10.3390/polym13213635
Lin S, Tao B, Zhao X, Chen G, Wang D-Y. Surface Functionalization of Black Phosphorus via Amine Compounds and Its Impacts on the Flame Retardancy and Thermal Decomposition Behaviors of Epoxy Resin. Polymers. 2021; 13(21):3635. https://doi.org/10.3390/polym13213635
Chicago/Turabian StyleLin, Shaoling, Boqing Tao, Xiaomin Zhao, Guohua Chen, and De-Yi Wang. 2021. "Surface Functionalization of Black Phosphorus via Amine Compounds and Its Impacts on the Flame Retardancy and Thermal Decomposition Behaviors of Epoxy Resin" Polymers 13, no. 21: 3635. https://doi.org/10.3390/polym13213635






