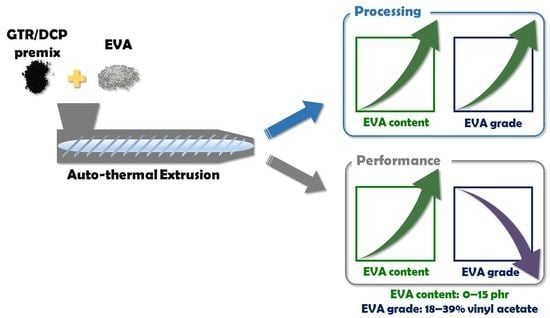
Processing, Performance Properties, and Storage Stability of Ground Tire Rubber Modified by Dicumyl Peroxide and Ethylene-Vinyl Acetate Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.2.1. GTR Modification and Curing
2.2.2. Preparation of Rubber/Modified GTR Blends
2.3. Measurements
3. Results and Discussion
3.1. GTR Modification
3.2. Curing Characteristics of Modified GTR
3.3. Physico-Mechanical Properties of Modified GTR
3.4. VOCs’ Emission Profiles Determined for Modified GTR
3.5. Storage Stability Assessment of Modified GTR
3.6. Characterization of SBR/Modified GTR and NR/Modified GTR Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bockstal, L.; Berchem, T.; Schmetz, Q.; Richel, A. Devulcanisation and reclaiming of tires and rubber by physical and chemical processes: A review. J. Clean. Prod. 2019, 236, 117574. [Google Scholar] [CrossRef]
- Saputra, R.; Walvekar, R.; Khalid, M.; Mubarak, N.M.; Sillanpää, M. Current progress in waste tire rubber devulcanization. Chemosphere 2021, 265, 129033. [Google Scholar] [CrossRef] [PubMed]
- Markl, E.; Lackner, M. Devulcanization technologies for recycling of tire-derived rubber: A review. Materials 2020, 13, 1246. [Google Scholar] [CrossRef] [Green Version]
- Schaefer, R.; Isringhaus, R.A. Chapter 18: Reclaimed rubber. In Rubber Technology; Morton, M., Ed.; Springer: Dordrecht, The Netherlands, 1999; pp. 505–517. [Google Scholar]
- Zedler, Ł.; Klein, M.; Saeb, M.R.; Colom, X.; Cañavate, J.; Formela, K. Synergistic effects of bitumen plasticization and microwave treatment on short-term devulcanization of ground tire rubber. Polymers 2018, 10, 1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seghar, S.; Asaro, L.; Aït Hocine, N. Experimental validation of the Horikx theory to be used in the rubber devulcanization analysis. J. Polym. Environ. 2019, 27, 2318–2323. [Google Scholar] [CrossRef]
- Dierkes, W.K.; Dijkhuis, K.; Hoek, H.V.; Noordermeer, J.W.M.; Reuvekamp, L.A.E.M.; Saiwari, S.; Blume, A. Designing of cradle-to-cradle loops for elastomer products. Plast. Rubber Compos. 2019, 48, 3–13. [Google Scholar] [CrossRef]
- Formela, K. Sustainable development of waste tires recycling technologies—Recent advances, challenges and future trends. Adv. Ind. Eng. Polym. Res. 2021, 4, 209–222. [Google Scholar] [CrossRef]
- Yazdani, H.; Karrabi, M.; Ghasmi, I.; Azizi, H.; Bakhshandeh, G.R. Devulcanization of waste tires using a twin-screw extruder: The effects of processing conditions and as a consequence the interfacial interactions between matrix and reclaimed rubbers. J. Vinyl Addit. Technol. 2011, 17, 64–69. [Google Scholar] [CrossRef]
- Tao, G.; He, Q.; Xia, Y.; Jia, G.; Yang, H.; Ma, W. The effect of devulcanization level on mechanical properties of reclaimed rubber by thermal-mechanical shearing devulcanization. J. Appl. Polym. Sci. 2013, 129, 2598–2605. [Google Scholar] [CrossRef]
- Meysami, M.; Tzoganakis, C.; Mutyala, P.; Zhu, S.H.; Bulsari, M. Devulcanization of scrap tire rubber with supercritical CO2: A study of the effects of process parameters on the properties of devulcanized rubber. Int. Polym. Process. 2017, 32, 183–193. [Google Scholar] [CrossRef]
- Mangili, I.; Lasagni, M.; Anzano, M.; Collina, E.; Tatangelo, V.; Franzetti, A.; Caracino, P.; Isayev, A.I. Mechanical and rheological properties of natural rubber com-pounds containing devulcanized ground tire rubber from several methods. Polym. Degrad. Stab. 2015, 121, 369–377. [Google Scholar] [CrossRef]
- Ali, M.A.M.; Raslan, H.A.; El-Nemr, K.F.; Hassan, M.M. Thermal and mechanical behavior of SBR/devulcanized waste tire rubber blends using mechano-chemical and microwave methods. J. Polym. Eng. 2020, 40, 815–822. [Google Scholar] [CrossRef]
- Klajn, K.; Gozdek, T.; Bieliński, D.M.; Siciński, M.; Zarzecka-Napierała, M.; Pędzich, Z. SBR vulcanizates filled with modified ground tire rubber. Materials 2021, 14, 3991. [Google Scholar] [CrossRef]
- Saiwari, S.; Dierkes, W.K.; Noordermeer, J.W.M. Comparative investigation of the devulcanization parameters of tire rubbers. Rubber Chem. Technol. 2014, 87, 31–42. [Google Scholar] [CrossRef] [Green Version]
- Shi, J.; Jiang, K.; Ren, D.; Zou, H.; Wang, Y.; Lv, X.; Zhang, L. Structure and performance of reclaimed rubber obtained by different methods. J. Appl. Polym. Sci. 2013, 129, 999–1007. [Google Scholar] [CrossRef]
- Guo, L.; Wang, C.; Lv, D.; Ren, D.; Zhai, T.; Sun, C.; Liu, H. Rubber reclamation with high bond-breaking selectivity using a low-temperature mechano-chemical devulcanization method. J. Clean. Prod. 2021, 279, 123266. [Google Scholar] [CrossRef]
- Guo, L.; Lv, D.; Ren, D.; Qu, L.; Wang, W.; Hao, K.; Guo, X.; Chen, T.; Sun, J.; Wang, C.; et al. Effectiveness of original additives in waste rubbers for revulcanization after reclamation with a low-temperature mechanochemical devulcanization method. J. Clean. Prod. 2021, 297, 126620. [Google Scholar] [CrossRef]
- Simon, D.A.; Bárány, T. Effective thermomechanical devulcanization of ground tire rubber with a co-rotating twin-screw extruder. Polym. Degrad. Stab. 2021, 190, 109626. [Google Scholar] [CrossRef]
- Gągol, M.; Boczkaj, B.; Haponiuk, J.; Formela, K. Investigation of volatile low molecular weight compounds formed during continuous reclaiming of ground tire rubber. Polym. Degrad. Stab. 2015, 119, 113–120. [Google Scholar] [CrossRef]
- Seghar, S.; Asaro, L.; Rolland-Monnet, M.; Hocine, N.A. Thermo-mechanical devulcanization and recycling of rubber industry waste. Resour. Conserv. Recy. 2019, 144, 180–186. [Google Scholar] [CrossRef]
- Diaz, R.; Colomines, G.; Peuvrel-Disdier, E.; Deterre, R. Thermo-mechanical recycling of rubber: Relationship between material properties and specific mechanical energy. J. Mater. Process. Technol. 2018, 252, 454–468. [Google Scholar] [CrossRef]
- Formela, K.; Cysewska, M.; Haponiuk, J. Thermomechanical reclaiming of ground tire rubber via extrusion at low temperature: Efficiency and limits. J. Vinyl Addit. Technol. 2016, 22, 213–221. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, S.T.; Xu, Z.; Chen, X.; Zhang, M. Process for Devulcanization of Rubber. U.S. Patent Application 2009/0082475A1, 26 March 2009. [Google Scholar]
- Wang, Y.-H.; Chen, Y.-K.; Rodrigue, D. Production of thermoplastic elastomers based on recycled PE and ground tire rubber: Morphology, mechanical properties and effect of compatibilizer addition. Int. Polym. Proc. 2018, 33, 525–534. [Google Scholar] [CrossRef]
- Zedler, Ł.; Kowalkowska-Zedler, D.; Colom, X.; Cañavate, J.; Saeb, M.R.; Formela, K. Reactive sintering of ground tire rubber (GTR) modified by a trans-polyoctenamer rubber and curing additives. Polymers 2020, 12, 3018. [Google Scholar] [CrossRef] [PubMed]
- Nunes, A.T.; dos Santos, R.E.; Pereira, J.S.; Barbosa, R.; Ambrósio, J.D. Characterization of waste tire rubber devulcanized in twin-screw extruder with thermoplastics. Prog. Rubber Plast. Recycl. Technol. 2018, 34, 143–157. [Google Scholar] [CrossRef]
- Barbosa, R.; Ambrósio, J.D. Devulcanization of natural rubber compounds by extrusion using thermoplastics and characterization of revulcanized compounds. J. Polym. Res. 2019, 26, 160. [Google Scholar] [CrossRef]
- Zedler, Ł.; Kowalkowska-Zedler, D.; Vahabi, H.; Saeb, M.R.; Colom, X.; Cañavate, J.; Wang, S.; Formela, K. Preliminary investigation on auto-thermal extrusion of ground tire rubber. Materials 2019, 12, 2090. [Google Scholar] [CrossRef] [Green Version]
- Zedler, Ł.; Burger, P.; Wang, S.; Formela, K. Ground tire rubber modified by ethylene-vinyl acetate copolymer: Processing, physico-mechanical properties, volatile organic compounds emission and recycling possibility. Materials 2020, 13, 4669. [Google Scholar] [CrossRef]
- Khang, T.H.; Ariff, Z.M. Vulcanization kinetics study of natural rubber compounds having different formulation variables. J. Therm. Anal. Calorim. 2012, 109, 1545–1553. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 1, 512–520. [Google Scholar] [CrossRef]
- Kraus, G.J. Swelling of filler-reinforced vulcanizates. J. Appl. Polym. Sci. 1963, 7, 861–871. [Google Scholar] [CrossRef]
- Marć, M.; Namieśnik, J.; Zabiegała, B. BTEX concentration levels in urban air in the area of the Tri-City agglomeration (Gdansk, Gdynia, Sopot), Poland. Air Qual. Atmos. Health 2014, 7, 489–504. [Google Scholar] [CrossRef]
- Marć, M.; Zabiegała, B.; Namieśnik, J. Application of passive sampling technique in monitoring research on quality of atmospheric air in the area of Tczew, Poland. Int. J. Environ. Anal. Chem. 2014, 94, 151–167. [Google Scholar] [CrossRef]
- Hejna, A.; Marć, M.; Korol, J. Modifcation of cellulosic fller with diisocyanates–volatile organic compounds emission assessment and stability of chemical structure over time. Nord. Pulp Paper Res. J. 2021, 36, 353–372. [Google Scholar] [CrossRef]
- Klewicz, K.; Marć, M.; Zabiegała, B. Emission profile of butan-2-one oxime from commercially available neutral silicone sealant. Microchem. J. 2020, 156, 104982. [Google Scholar] [CrossRef]
- Marć, M.; Tsakovski, S.; Tobiszewski, M. Emissions and toxic units of solvent, monomer and additive residues released to gaseous phase from latex balloons. Environ. Res. 2021, 195, 110700. [Google Scholar] [CrossRef] [PubMed]
- Śmiełowska, M.; Marć, M.; Zabiegała, B. Small polymeric toys placed in child-dedicated chocolate food products—Do they contain harmful chemicals? Examination of quality by example of selected VOCs and SVOCs. Expos. Health 2021. [Google Scholar] [CrossRef]
- Godavarti, S.; Karwe, M.V. Determination of specific mechanical energy distribution on a twin-screw extruder. J. Agric. Eng. Res. 1997, 67, 277–287. [Google Scholar] [CrossRef]
- Bianchi, O.; Fiorio, R.; Martins, J.N.; Zattera, A.J.; Scuracchio, C.H.; Canto, L.B. Crosslinking kinetics of blends of ethylene vinyl acetate and ground tire rubber. J. Elastom. Plast. 2009, 41, 175–189. [Google Scholar] [CrossRef]
- Thomas, D.K. Crosslinking efficiency of dicumyl peroxide in natural rubber. J. Appl. Polym. Sci. 1962, 6, 613–616. [Google Scholar] [CrossRef]
- Di Somma, I.; Marotta, R.; Andreozzi, R.; Caprio, V. Kinetic and chemical characterization of thermal decomposition of dicumylperoxide in cumene. J. Hazard. Mater. 2011, 187, 157–163. [Google Scholar] [CrossRef] [PubMed]
- Kamarulzaman, N.H.; Le-Minh, N.; Stuetz, R.M. Identification of VOCs from natural rubber by different headspace techniques coupled using GC-MS. Talanta 2019, 191, 535–544. [Google Scholar] [CrossRef]
- Morand, J.L. Chain scission in the oxidation of polyisoprene. Rubber Chem. Technol. 1977, 50, 373–396. [Google Scholar] [CrossRef]
- Ginsberg, G.; Toal, B.; Simcox, N.; Bracker, A.; Golembiewski, B.; Kurland, T.; Hedman, C. Human health risk assessment of synthetic turf fields based upon investigation of five fields in Connecticut. J. Toxicol. Environ. Health Part A 2011, 74, 1150–1174. [Google Scholar] [CrossRef] [PubMed]
- Hulse, G.E.; Kersting, R.J.; Warfel, D.R. Chemistry of dicumyl peroxide-induced crosslinking of linear polyethylene. J. Polym. Sci. 1981, 19, 655–667. [Google Scholar] [CrossRef]
- Lu, N.; Shen, M.; Liu, J.; Prakashan, K.; Xin, Z. Effects of posttreatments on the storage stability of reclaimed rubber. Adv. Polym. Technol. 2021, 2021, 6617666. [Google Scholar] [CrossRef]
- Sreeja, T.D.; Kutty, S.K.N. Styrene butadiene rubber/reclaimed rubber blends. Int. J. Polym. Mater. 2003, 52, 599–609. [Google Scholar] [CrossRef]
- Sreeja, T.D.; Kutty, S.K.N. Cure characteristics and mechanical properties of natural rubber/reclaimed rubber blends. Polym. Plast. Technol. Eng. 2000, 39, 501–512. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, H.; Zhang, D.; Zhang, Z.; Peng, S.; Sun, Y. Curing behaviors, mechanical properties, dynamic mechanical analysis and morphologies of natural rubber vulcanizates containing reclaimed rubber. e-Polymers 2019, 19, 482–488. [Google Scholar] [CrossRef]
- Toncheva, A.; Brison, L.; Dubois, P.; Laoutid, F. Recycled tire rubber in additive manufacturing: Selective laser sintering for polymer-ground rubber composites. Appl. Sci. 2021, 11, 8778. [Google Scholar] [CrossRef]
- Hernández Santana, M.; Huete, M.; Lameda, P.; Araujo, J.; Verdejo, R.; López-Manchado, M.A. Design of a new generation of sustainable SBR compounds with good trade-off between mechanical properties and self-healing ability. Eur. Polym. J. 2018, 106, 273–283. [Google Scholar] [CrossRef]
- Toczek, K.; Lipińska, M.; Pietrasik, J. Smart TPE materials based on recycled rubber shred. Materials 2021, 14, 6237. [Google Scholar] [CrossRef] [PubMed]







| Properties | Standard | Additive | ||
|---|---|---|---|---|
| FL00218 * | UL04533EH2 * | UL05540EH2 * | ||
| Abbreviation in this study | - | EVA-18% | EVA-33% | EVA-39% |
| Vinyl acetate content (wt.%) | Producer method | 18 | 33 | 39 |
| Density at 25 °C (g/cm3) | ASTM D1505 | 0.940 | 0.956 | 0.966 |
| MFI190 °C, 2.16 kg (g/10 min) | ASTM D1238 | 1.7 | 45 | 60 |
| Vicat softening temperature (°C) | ASTM D1525 | 62 | 33 | - |
| Melting temperature (°C) | Producer method | 87 | 61 | 48 |
| Methodology | Standard | Modified GTR | Rubber/Modified GTR Blends |
|---|---|---|---|
| Sample preparation | - | Twin-screw extruder + compression molding | Two-roll mills + compression molding |
| Processing assessment | |||
| Energy consumption + IR camera | - | X | - |
| Mooney viscosity | ISO 287 | - | X |
| Curing characteristics | ISO 3417 | X | X |
| Physico-mechanical properties | |||
| Density | ISO 2781 | X | X |
| Swelling behavior | - | X | X |
| Tensile tests | ISO 37 | X | X |
| Hardness | 7619-1 | X | X |
| Abrasion resistance | ISO 4649 | - | X |
| Additional methods | |||
| VOCs emission characteristics by GC-MS and GC-FID | - | X | - |
| Microstructure evaluation by SEM | - | - | X |
| Sample Code | EVA Grade | EVA Content (phr) | Temperature at Die (°C) | SME (kWh/kg) |
|---|---|---|---|---|
| GTRDCP | - | 0 | 160 ± 1 | 0.131 ± 0.003 |
| GTRDCP + 2.5 phr EVA-18% | EVA-18% | 2.5 | 164 ± 6 | 0.125 ± 0.003 |
| GTRDCP + 5 phr EVA-18% | 5 | 172 ± 3 | 0.171 ± 0.010 | |
| GTRDCP + 10 phr EVA-18% | 10 | 174 ± 1 | 0.177 ± 0.002 | |
| GTRDCP + 15 phr EVA-18% | 15 | 173 ± 2 | 0.171 ± 0.003 | |
| GTRDCP + 2.5 phr EVA-33% | EVA-33% | 2.5 | 165 ± 4 | 0.160 ± 0.012 |
| GTRDCP + 5 phr EVA-33% | 5 | 172 ± 5 | 0.168 ± 0.004 | |
| GTRDCP + 10 phr EVA-33% | 10 | 164 ± 3 | 0.154 ± 0.001 | |
| GTRDCP + 15 phr EVA-33% | 15 | 158 ± 2 | 0.146 ± 0.003 | |
| GTRDCP + 2.5 phr EVA-39% | EVA-39% | 2.5 | 167 ± 4 | 0.147 ± 0.007 |
| GTRDCP + 5 phr EVA-39% | 5 | 161 ± 4 | 0.149 ± 0.004 | |
| GTRDCP + 10 phr EVA-39% | 10 | 150 ± 2 | 0.129 ± 0.006 | |
| GTRDCP + 15 phr EVA-39% | 15 | 152 ± 3 | 0.120 ± 0.005 |
| Retention Time (min) | Identified Compound | Chemical Structure | Molecular Weight (g/mol) | Match Quality (%) | Source | References |
|---|---|---|---|---|---|---|
| 4.34 | Benzene |  | 78.11 | 94 | styrene-butadiene rubber present in GTR | [20] |
| 5.38 | Methyl Isobutyl Ketone |  | 100.16 | 90 | natural rubber and anti-aging agents present in GTR | [44,45] |
| 6.02 | Toluene |  | 92.14 | 95 | styrene-butadiene rubber present in GTR | [20,46] |
| 7.87 | Xylene |  | 106.17 | 97 | styrene-butadiene rubber present in GTR | [20,46] |
| 8.20 | Styrene |  | 104.15 | 97 | styrene-butadiene rubber present in GTR | [20,46] |
| 8.94 | Cumene |  | 120.19 | 91 | dicumyl peroxide decomposition | [41] |
| 10.29 | α-Methylstyrene |  | 118.18 | 93 | dicumyl peroxide decomposition | [47] |
| 11.78 | Acetophenone |  | 120.15 | 97 | dicumyl peroxide decomposition | [47] |
| 12.16 | α-Cumyl alcohol |  | 136.91 | 91 | dicumyl peroxide decomposition | [47] |
| 12.74 | Undecane |  | 156.31 | 94 | EVA copolymer andnatural rubber present in GTR | - |
| 14.25 | Dodecane |  | 168.32 | 95 | EVA copolymer andnatural rubber present in GTR | - |
| 14.41 | Benzothiazole |  | 135.19 | 94 | vulcanization accelerators present in GTR | [5,20,44] |
| 14.98 | Hexylbenzene |  | 162.27 | 95 | styrene-butadiene rubber present in GTR | - |
| 15.49 | Tridecane |  | 184.36 | 96 | EVA copolymer andnatural rubber present in GTR | - |
| 16.57 | Tetradecane |  | 198.39 | 98 | EVA copolymer andnatural rubber present in GTR | - |
| Sample Code | EVA Grade | EVA Content (phr) | TVOCs (μg/g) | Acetophenone (mg/kg) | ||
|---|---|---|---|---|---|---|
| Uncured | Cured | Uncured | Cured | |||
| GTRDCP | - | 0 | 15.0 | 30.6 | 4.3 | 112.6 |
| GTRDCP + 2.5 phr EVA-18% | EVA-18% | 2.5 | 23.3 | 48.9 | 3.9 | 23.4 |
| GTRDCP + 5 phr EVA-18% | 5 | 18.4 | 60.4 | 11.0 | 162.2 | |
| GTRDCP + 10 phr EVA-18% | 10 | 25.3 | 39.2 | 3.6 | 38.3 | |
| GTRDCP + 15 phr EVA-18% | 15 | 23.7 | 44.5 | - | 77.5 | |
| GTRDCP + 2.5 phr EVA-33% | EVA-33% | 2.5 | 29.2 | 49.6 | 6.8 | 40.1 |
| GTRDCP + 5 phr EVA-33% | 5 | 22.2 | 53.6 | 7.0 | 22.4 | |
| GTRDCP + 10 phr EVA-33% | 10 | 22.9 | 50.5 | 12.8 | 68.3 | |
| GTRDCP + 15 phr EVA-33% | 15 | 22.6 | 37.9 | 12.1 | 23.1 | |
| GTRDCP + 2.5 phr EVA-39% | EVA-39% | 2.5 | 25.0 | 64.6 | 3.9 | 64.6 |
| GTRDCP + 5 phr EVA-39% | 5 | 24.2 | 58.0 | 3.3 | 41.1 | |
| GTRDCP + 10 phr EVA-39% | 10 | 17.8 | 63.3 | 3.6 | 111.0 | |
| GTRDCP + 15 phr EVA-39% | 15 | 18.4 | 64.0 | 5.3 | 40.0 | |
| Technique | emission chamber-GC-FID | HS-GC-MS | ||||
| Conditioning temperature | 40 °C | 100 °C | ||||
| Conditioning time | 20 min | 20 min | ||||
| Carrier gas flow rate | 25 mL/min | 2 mL/min | ||||
| Oven temperature program | 40 °C for 1 min 10 °C/min up to 280 °C 20 °C/min up to 300 °C for 3 min | 40 °C for 4.5 min 10 °C/min up to 200 °C 30 °C/min up to 290 °C for 5 min | ||||
| Sample Code | Curing Parameters | Curing Characteristics as a Function of Storage Time | Average | |||
|---|---|---|---|---|---|---|
| Few Days | 1 Month | 2 Months | 3 Months | |||
| GTRDCP | ML (dNm) | 37.8 | 38.5 | 15.4 | 24.8 | 29.1 ± 11.1 |
| ΔM (MH-ML) (dNm) | 14.2 | 13.3 | 6.1 | 9.8 | 10.9 ± 3.7 | |
| Scorch time (min) | 1.4 | 1.5 | 1.9 | 2.0 | 1.7 ± 0.3 | |
| Optimal cure time (min) | 6.5 | 6.7 | 6.1 | 6.4 | 6.4 ± 0.3 | |
| GTRDCP + 2.5 phr EVA-18% | ML (dNm) | 35.5 | 37.9 | 35.3 | 35.6 | 36.1 ± 1.2 |
| ΔM (MH-ML) (dNm) | 12.9 | 12.0 | 9.9 | 11.3 | 11.5 ± 1.3 | |
| Scorch time (min) | 1.7 | 1.9 | 2.1 | 2.2 | 2.0 ± 0.2 | |
| Optimal cure time (min) | 6.6 | 6.6 | 7.0 | 7.5 | 6.9 ± 0.4 | |
| GTRDCP + 5 phr EVA-18% | ML (dNm) | 31.7 | 35.3 | 31.6 | 34.2 | 33.2 ± 1.8 |
| ΔM (MH-ML) (dNm) | 15.8 | 12.8 | 9.8 | 9.8 | 12.1 ± 2.9 | |
| Scorch time (min) | 1.7 | 2.0 | 2.2 | 2.5 | 2.1 ± 0.3 | |
| Optimal cure time (min) | 7.4 | 7.1 | 7.3 | 7.9 | 7.4 ± 0.3 | |
| GTRDCP + 10 phr EVA-18% | ML (dNm) | 29.5 | 35.2 | 29.8 | 32.2 | 31.7 ± 2.6 |
| ΔM (MH-ML) (dNm) | 11.2 | 10.3 | 7.7 | 7.4 | 9.2 ± 1.9 | |
| Scorch time (min) | 2.3 | 2.5 | 2.6 | 2.8 | 2.6 ± 0.2 | |
| Optimal cure time (min) | 8.4 | 7.4 | 7.6 | 7.9 | 7.8 ± 0.4 | |
| GTRDCP + 15 phr EVA-18% | ML (dNm) | 26.2 | 30.7 | 28.2 | 29.7 | 28.7 ± 2.0 |
| ΔM (MH-ML) (dNm) | 8.7 | 6.4 | 5.6 | 5.4 | 6.5 ± 1.5 | |
| Scorch time (min) | 2.6 | 3.0 | 3.4 | 3.7 | 3.2 ± 0.5 | |
| Optimal cure time (min) | 8.2 | 7.7 | 7.9 | 8.4 | 8.1 ± 0.3 | |
| GTRDCP + 2.5 phr EVA-33% | ML (dNm) | 29.8 | 33.0 | 31.0 | 31.5 | 31.3 ± 1.3 |
| ΔM (MH-ML) (dNm) | 14.0 | 11.0 | 8.5 | 9.7 | 10.8 ± 2.4 | |
| Scorch time (min) | 1.8 | 2.3 | 2.6 | 2.6 | 2.3 ± 0.4 | |
| Optimal cure time (min) | 7.0 | 7.3 | 7.5 | 7.4 | 7.3 ± 0.2 | |
| GTRDCP + 5 phr EVA-33% | ML (dNm) | 27.2 | 28.1 | 28.2 | 28.0 | 27.9 ± 0.5 |
| ΔM (MH-ML) (dNm) | 8.7 | 9.0 | 8.5 | 8.0 | 8.6 ± 0.4 | |
| Scorch time (min) | 2.4 | 2.7 | 3.0 | 3.0 | 2.8 ± 0.3 | |
| Optimal cure time (min) | 7.9 | 7.6 | 8.4 | 8.0 | 8.0 ± 0.3 | |
| GTRDCP + 10 phr EVA-33% | ML (dNm) | 21.0 | 22.7 | 22.6 | 22.4 | 22.2 ± 0.8 |
| ΔM (MH-ML) (dNm) | 11.1 | 9.4 | 9.5 | 10.2 | 10.1 ± 0.8 | |
| Scorch time (min) | 2.5 | 3.0 | 3.0 | 2.9 | 2.9 ± 0.2 | |
| Optimal cure time (min) | 8.4 | 8.4 | 8.8 | 8.3 | 8.5 ± 0.2 | |
| GTRDCP + 15 phr EVA-33% | ML (dNm) | 16.1 | 17.9 | 17.2 | 17.1 | 17.1 ± 0.7 |
| ΔM (MH-ML) (dNm) | 9.9 | 10.3 | 9.0 | 9.9 | 9.8 ± 0.6 | |
| Scorch time (min) | 2.8 | 2.9 | 3.3 | 2.9 | 3.0 ± 0.2 | |
| Optimal cure time (min) | 8.5 | 8.7 | 9.1 | 8.7 | 8.8 ± 0.3 | |
| GTRDCP + 2.5 phr EVA-39% | ML (dNm) | 33.6 | 32.2 | 35.0 | 32.9 | 33.4 ± 1.2 |
| ΔM (MH-ML) (dNm) | 10.9 | 10.5 | 10.9 | 12.2 | 11.1 ± 0.7 | |
| Scorch time (min) | 2.2 | 2.2 | 2.1 | 1.8 | 2.1 ± 0.2 | |
| Optimal cure time (min) | 7.0 | 7.0 | 7.6 | 7.3 | 7.2 ± 0.3 | |
| GTRDCP + 5 phr EVA-39% | ML (dNm) | 27.7 | 26.0 | 30.3 | 29.0 | 28.3 ± 1.8 |
| ΔM (MH-ML) (dNm) | 10.9 | 11.8 | 11.6 | 11.1 | 11.4 ± 0.4 | |
| Scorch time (min) | 2.6 | 2.5 | 2.5 | 2.1 | 2.4 ± 0.2 | |
| Optimal cure time (min) | 8.0 | 8.0 | 8.8 | 7.6 | 8.1 ± 0.5 | |
| GTRDCP + 10 phr EVA-39% | ML (dNm) | 19.7 | 20.4 | 21.8 | 20.2 | 20.5 ± 0.9 |
| ΔM (MH-ML) (dNm) | 12.0 | 12.8 | 12.2 | 12.5 | 12.4 ± 0.4 | |
| Scorch time (min) | 2.9 | 2.6 | 2.8 | 2.6 | 2.7 ± 0.2 | |
| Optimal cure time (min) | 8.6 | 8.9 | 8.9 | 8.8 | 8.8 ± 0.1 | |
| GTRDCP + 15 phr EVA-39% | ML (dNm) | 14.8 | 15.5 | 15.7 | 14.7 | 15.2 ± 0.5 |
| ΔM (MH-ML) (dNm) | 11.7 | 11.8 | 11.6 | 11.7 | 11.7 ± 0.1 | |
| Scorch time (min) | 3.2 | 3.1 | 3.2 | 3.0 | 3.1 ± 0.1 | |
| Optimal cure time (min) | 9.1 | 8.9 | 9.5 | 9.4 | 9.2 ± 0.3 | |
| Sample Code | Performance Properties | Performance Properties as a Function of Storage Time | Average | |||
|---|---|---|---|---|---|---|
| Few Days | 1 Month | 2 Months | 3 Months | |||
| GTRDCP | Tensile strength (MPa) | 3.5 ± 0.2 | 3.6 ± 0.3 | 3.4 ± 0.1 | 3.0 ± 0.1 | 3.4 ± 0.3 |
| Elongation at break (%) | 98 ± 3 | 92 ± 3 | 93 ± 5 | 90 ± 3 | 93 ± 3 | |
| Hardness (Shore A) | 65 ± 1 | 68 ± 1 | 67 ± 1 | 66 ± 1 | 67 ± 1 | |
| GTRDCP + 2.5 phr EVA-18% | Tensile strength (MPa) | 5.2 ± 0.2 | 5.1 ± 0.3 | 5.1 ± 0.2 | 4.9 ± 0.1 | 5.1 ± 0.1 |
| Elongation at break (%) | 132 ± 4 | 114 ± 7 | 120 ± 3 | 134 ± 3 | 125 ± 10 | |
| Hardness (Shore A) | 68 ± 1 | 70 ± 1 | 70 ± 1 | 68 ± 1 | 69 ± 1 | |
| GTRDCP + 5 phr EVA-18% | Tensile strength (MPa) | 6.4 ± 0.5 | 6.3 ± 0.3 | 6.5 ± 0.5 | 6.3 ± 0.2 | 6.4 ± 0.1 |
| Elongation at break (%) | 143 ± 10 | 138 ± 7 | 157 ± 8 | 151 ± 6 | 147 ± 8 | |
| Hardness (Shore A) | 70 ± 1 | 71 ± 1 | 71 ± 1 | 70 ± 1 | 71 ± 1 | |
| GTRDCP + 10 phr EVA-18% | Tensile strength (MPa) | 8.3 ± 0.3 | 8.0 ± 0.4 | 7.5 ± 0.2 | 8.2 ± 0.2 | 8.0 ± 0.4 |
| Elongation at break (%) | 174 ± 4 | 171 ± 10 | 168 ± 9 | 194 ± 9 | 177 ± 12 | |
| Hardness (Shore A) | 72 ± 1 | 73 ± 1 | 72 ± 1 | 72 ± 1 | 72 ± 1 | |
| GTRDCP + 15 phr EVA-18% | Tensile strength (MPa) | 9.3 ± 0.2 | 8.7 ± 0.4 | 8.6 ± 0.2 | 8.3 ± 0.4 | 8.7 ± 0.4 |
| Elongation at break (%) | 225 ± 7 | 220 ± 10 | 217 ± 4 | 224 ± 10 | 222 ± 4 | |
| Hardness (Shore A) | 71 ± 1 | 73 ± 1 | 72 ± 1 | 71 ± 1 | 72 ± 1 | |
| GTRDCP + 2.5 phr EVA-33% | Tensile strength (MPa) | 4.0 ± 0.3 | 3.9 ± 0.3 | 3.6 ± 0.4 | 4.1 ± 0.1 | 3.9 ± 0.2 |
| Elongation at break (%) | 93 ± 7 | 103 ± 7 | 93 ± 7 | 103 ± 2 | 98 ± 6 | |
| Hardness (Shore A) | 68 ± 1 | 68 ± 1 | 69 ± 1 | 68 ± 1 | 68 ± 1 | |
| GTRDCP + 5 phr EVA-33% | Tensile strength (MPa) | 5.7 ± 0.2 | 5.0 ± 0.2 | 4.1 ± 0.2 | 4.3 ± 0.6 | 4.8 ± 0.7 |
| Elongation at break (%) | 147 ± 7 | 135 ± 5 | 112 ± 6 | 124 ± 15 | 130 ± 15 | |
| Hardness (Shore A) | 67 ± 1 | 67 ± 1 | 67 ± 1 | 65 ± 1 | 67 ± 1 | |
| GTRDCP + 10 phr EVA-33% | Tensile strength (MPa) | 6.5 ± 0.1 | 7.1 ± 0.2 | 6.4 ± 0.2 | 6.8 ± 0.3 | 6.7 ± 0.3 |
| Elongation at break (%) | 167 ± 7 | 187 ± 5 | 174 ± 5 | 178 ± 9 | 177 ± 8 | |
| Hardness (Shore A) | 67 ± 1 | 68 ± 1 | 67 ± 1 | 67 ± 1 | 67 ± 1 | |
| GTRDCP + 15 phr EVA-33% | Tensile strength (MPa) | 6.3 ± 0.6 | 6.7 ± 0.5 | 6.3 ± 0.3 | 7.0 ± 0.1 | 6.6 ± 0.3 |
| Elongation at break (%) | 177 ± 17 | 185 ± 11 | 183 ± 6 | 203 ± 2 | 187 ± 11 | |
| Hardness (Shore A) | 66 ± 2 | 68 ± 1 | 68 ± 1 | 67 ± 1 | 67 ± 1 | |
| GTRDCP + 2.5 phr EVA-39% | Tensile strength (MPa) | 2.6 ± 0.7 | 4.1 ± 0.2 | 4.0 ± 0.3 | 2.6 ± 0.2 | 3.3 ± 0.8 |
| Elongation at break (%) | 78 ± 17 | 96 ± 4 | 99 ± 5 | 79 ± 7 | 88 ± 11 | |
| Hardness (Shore A) | 65 ± 1 | 69 ± 1 | 68 ± 1 | 66 ± 1 | 67 ± 2 | |
| GTRDCP + 5 phr EVA-39% | Tensile strength (MPa) | 4.4 ± 0.2 | 4.8 ± 0.4 | 3.5 ± 1.0 | 4.2 ± 0.3 | 4.2 ± 0.5 |
| Elongation at break (%) | 111 ± 2 | 120 ± 11 | 95 ± 23 | 115 ± 8 | 110 ± 11 | |
| Hardness (Shore A) | 67 ± 1 | 68 ± 1 | 68 ± 1 | 67 ± 1 | 68 ± 1 | |
| GTRDCP + 10 phr EVA-39% | Tensile strength (MPa) | 5.4 ± 0.4 | 5.8 ± 0.4 | 4.7 ± 0.8 | 5.2 ± 0.4 | 5.3 ± 0.5 |
| Elongation at break (%) | 143 ± 9 | 147 ± 11 | 131 ± 15 | 148 ± 15 | 142 ± 8 | |
| Hardness (Shore A) | 65 ± 1 | 66 ± 1 | 67 ± 1 | 66 ± 1 | 66 ± 1 | |
| GTRDCP + 15 phr EVA-39% | Tensile strength (MPa) | 4.1 ± 0.4 | 5.6 ± 0.7 | 4.9 ± 0.7 | 4.9 ± 0.4 | 4.9 ± 0.6 |
| Elongation at break (%) | 132 ± 12 | 171 ± 15 | 160 ± 19 | 163 ± 15 | 157 ± 17 | |
| Hardness (Shore A) | 64 ± 1 | 65 ± 1 | 64 ± 1 | 63 ± 1 | 64 ± 1 | |
| Property | SBR/Modified GTR Blends | NR/Modified GTR Blends | ||||
|---|---|---|---|---|---|---|
| 75/25 | 50/50 | 25/75 | 75/25 | 50/50 | 25/75 | |
| Processing properties | ||||||
| Mooney viscosity ML(1+4) 100 °C (MU) | 55.6 ± 0.6 | 81.8 ± 1.8 | 123.7 ± 2.0 | 26.4 ± 1.0 | 21.2 ± 1.0 | 27.6 ± 2.2 |
| Minimal torque (dNm) | 9.2 | 13.5 | 19.9 | 4.7 | 4.5 | 6.2 |
| Maximal torque (dNm) | 22.4 | 36.9 | 45.3 | 12.5 | 21.9 | 34.4 |
| ΔM (MH-ML) (dNm) | 13.2 | 23.4 | 25.4 | 7.8 | 17.4 | 28.2 |
| Scorch time (min) | 1.1 | 0.9 | 0.7 | 1.5 | 1.1 | 0.8 |
| Optimal cure time (min) | 4.8 | 3.9 | 3.6 | 5.4 | 4.4 | 3.7 |
| Cure rate index (min−1) | 27.1 | 32.4 | 35.0 | 25.4 | 30.7 | 35.0 |
| Thermal aging resistance (%) | 0.0 | 0.5 | 1.3 | 0.0 | 0.4 | 1.3 |
| Physico-mechanical properties | ||||||
| Tensile strength (MPa) | 1.7 ± 0.1 | 3.1 ± 0.2 | 5.8 ± 0.1 | 2.0 ± 0.1 | 3.3 ± 0.6 | 6.4 ± 0.5 |
| Modulus at 100% (MPa) | 0.7 ± 0.1 | 1.3 ± 0.1 | 2.0 ± 0.1 | 0.4 ± 0.1 | 0.8 ± 0.1 | 1.8 ± 0.1 |
| Modulus at 300% (MPa) | 1.3 ± 0.1 | - | - | 0.9 ± 0.0 | 2.8 ± 0.2 | - |
| Elongation at break (%) | 451 ± 51 | 258 ± 15 | 274 ± 13 | 513 ± 9 | 333 ± 41 | 273 ± 11 |
| Hardness (Shore A) | 38 ± 2 | 49 ± 1 | 58 ± 1 | 25 ± 1 | 40 ± 1 | 55 ± 1 |
| Density (g/cm3) | 0.985 ± 0.002 | 1.038 ± 0.001 | 1.091 ± 0.002 | 0.958 ± 0.001 | 1.017 ± 0.004 | 1.081 ± 0.002 |
| Abrasion resistance (mm3) | 470 ± 66 | 292 ± 12 | 219 ± 3 | - * | 844 ± 46 | 377 ± 26 |
| Swelling degree (%) | 625 ± 5 | 293 ± 2 | 210 ± 2 | 637 ± 8 | 331 ± 4 | 193 ± 1 |
| Sol fraction (%) | 10.0 ± 0.2 | 7.3 ± 0.2 | 8.4 ± 0.1 | 7.6 ± 0.2 | 7.4 ± 0.0 | 8.1 ± 0.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wiśniewska, P.; Zedler, Ł.; Formela, K. Processing, Performance Properties, and Storage Stability of Ground Tire Rubber Modified by Dicumyl Peroxide and Ethylene-Vinyl Acetate Copolymers. Polymers 2021, 13, 4014. https://doi.org/10.3390/polym13224014
Wiśniewska P, Zedler Ł, Formela K. Processing, Performance Properties, and Storage Stability of Ground Tire Rubber Modified by Dicumyl Peroxide and Ethylene-Vinyl Acetate Copolymers. Polymers. 2021; 13(22):4014. https://doi.org/10.3390/polym13224014
Chicago/Turabian StyleWiśniewska, Paulina, Łukasz Zedler, and Krzysztof Formela. 2021. "Processing, Performance Properties, and Storage Stability of Ground Tire Rubber Modified by Dicumyl Peroxide and Ethylene-Vinyl Acetate Copolymers" Polymers 13, no. 22: 4014. https://doi.org/10.3390/polym13224014