Construction of KB@ZIF-8/PP Composite Separator for Lithium–Sulfur Batteries with Enhanced Electrochemical Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of ZIF-8
2.2. Preparation of KB@ZIF-8/PP Separator
2.3. Electrode and Cell Preparation
2.4. Material Characterization and Electrochemical Measurements
3. Results and Discussion
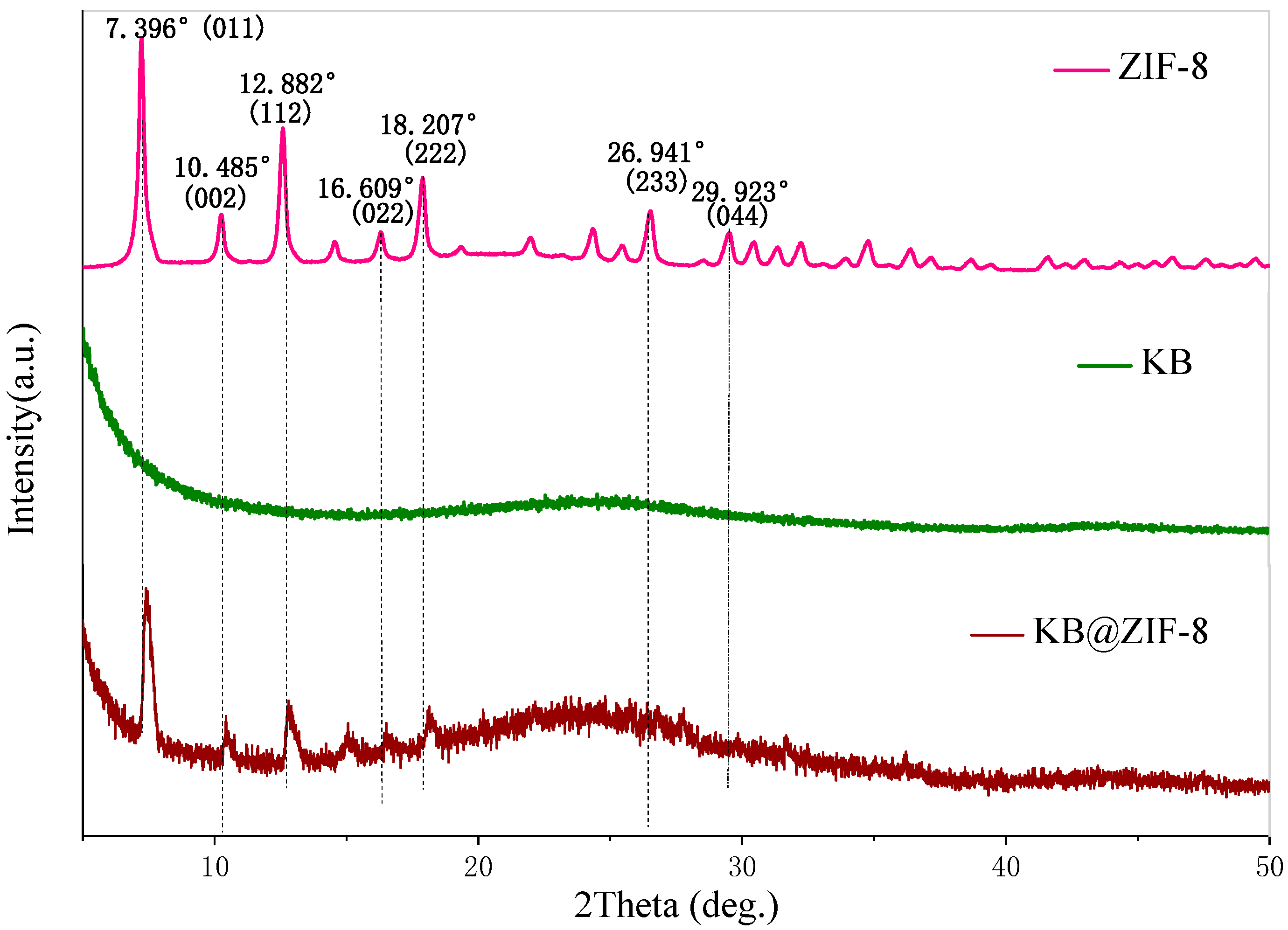
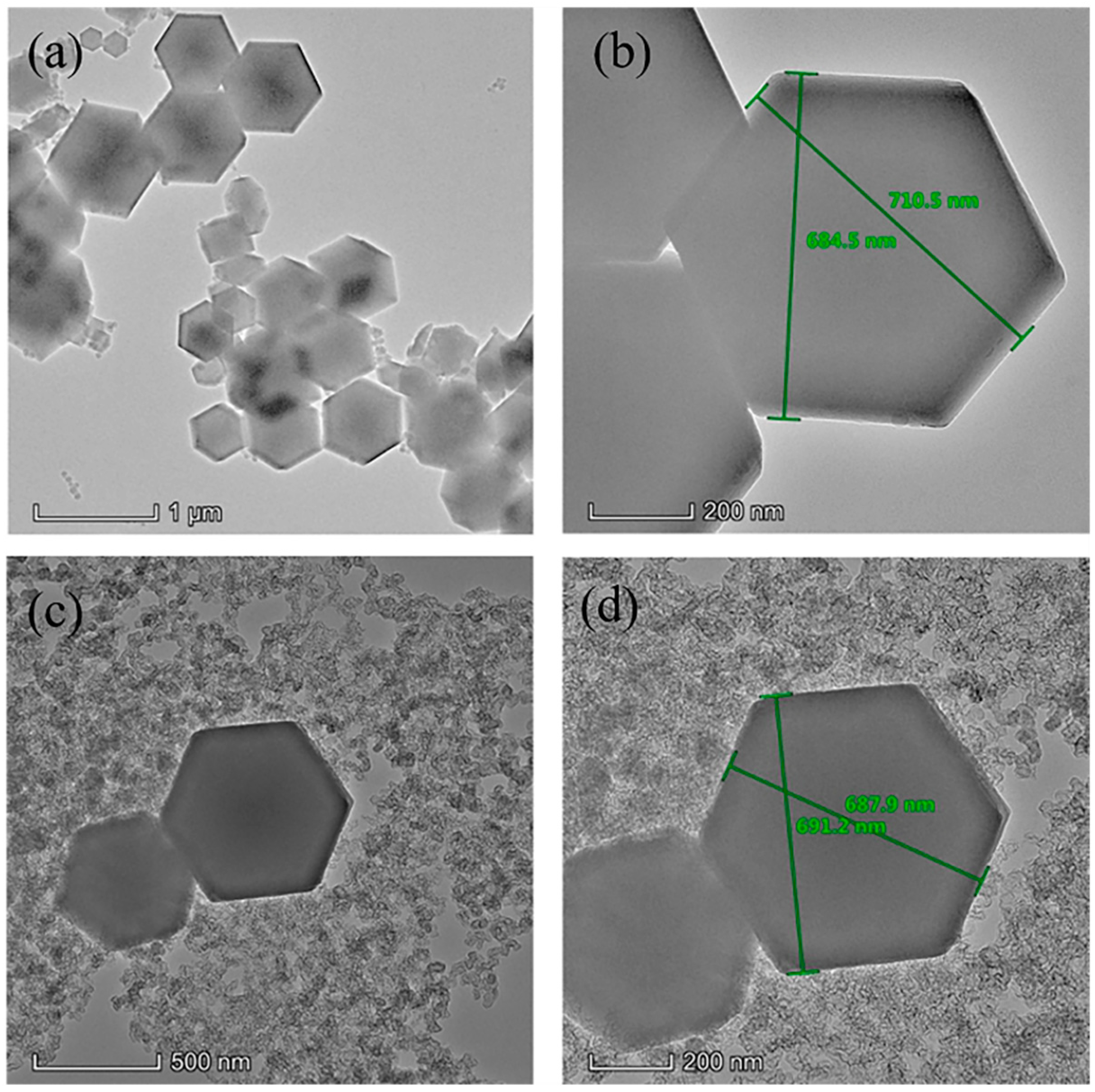
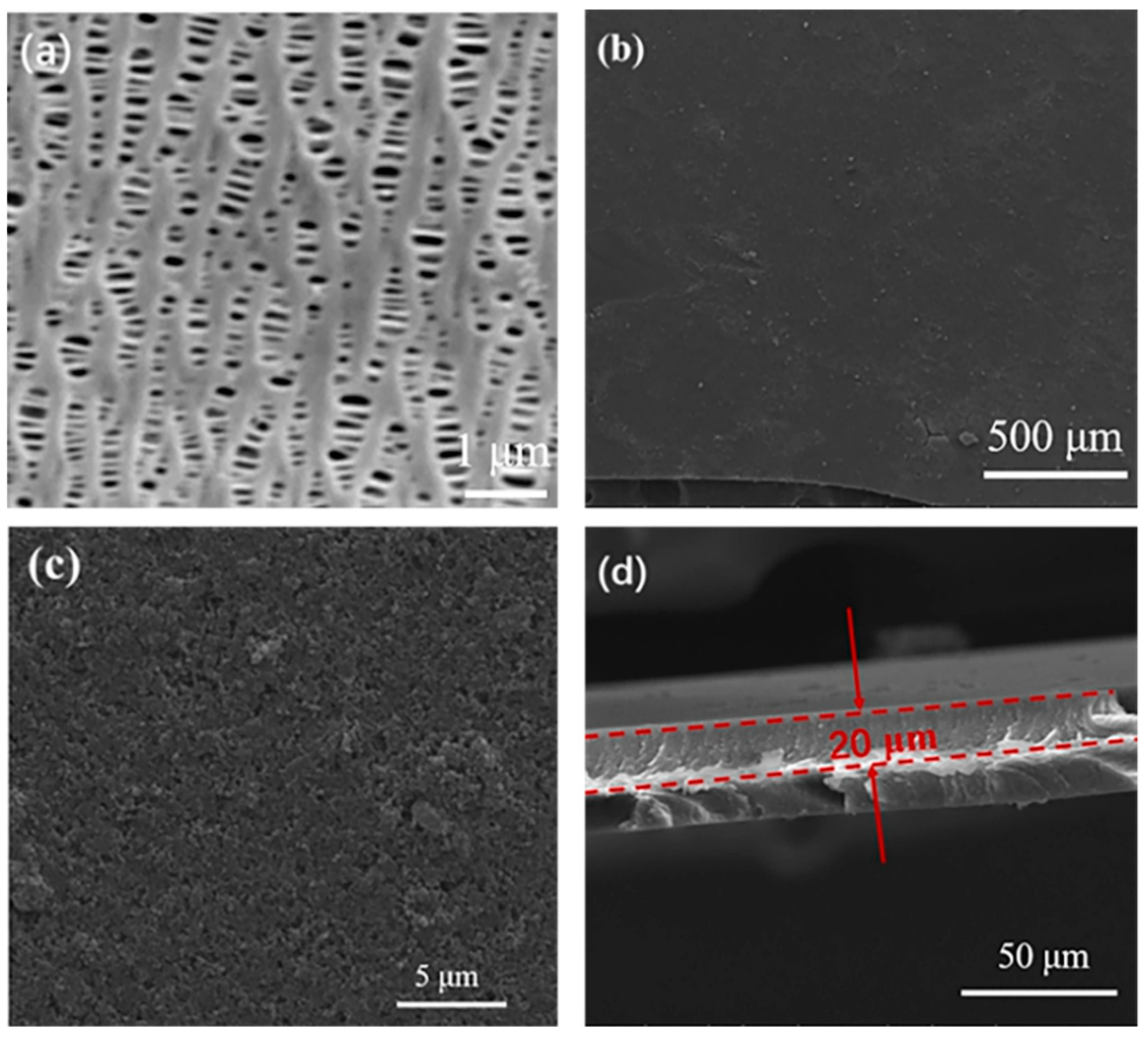
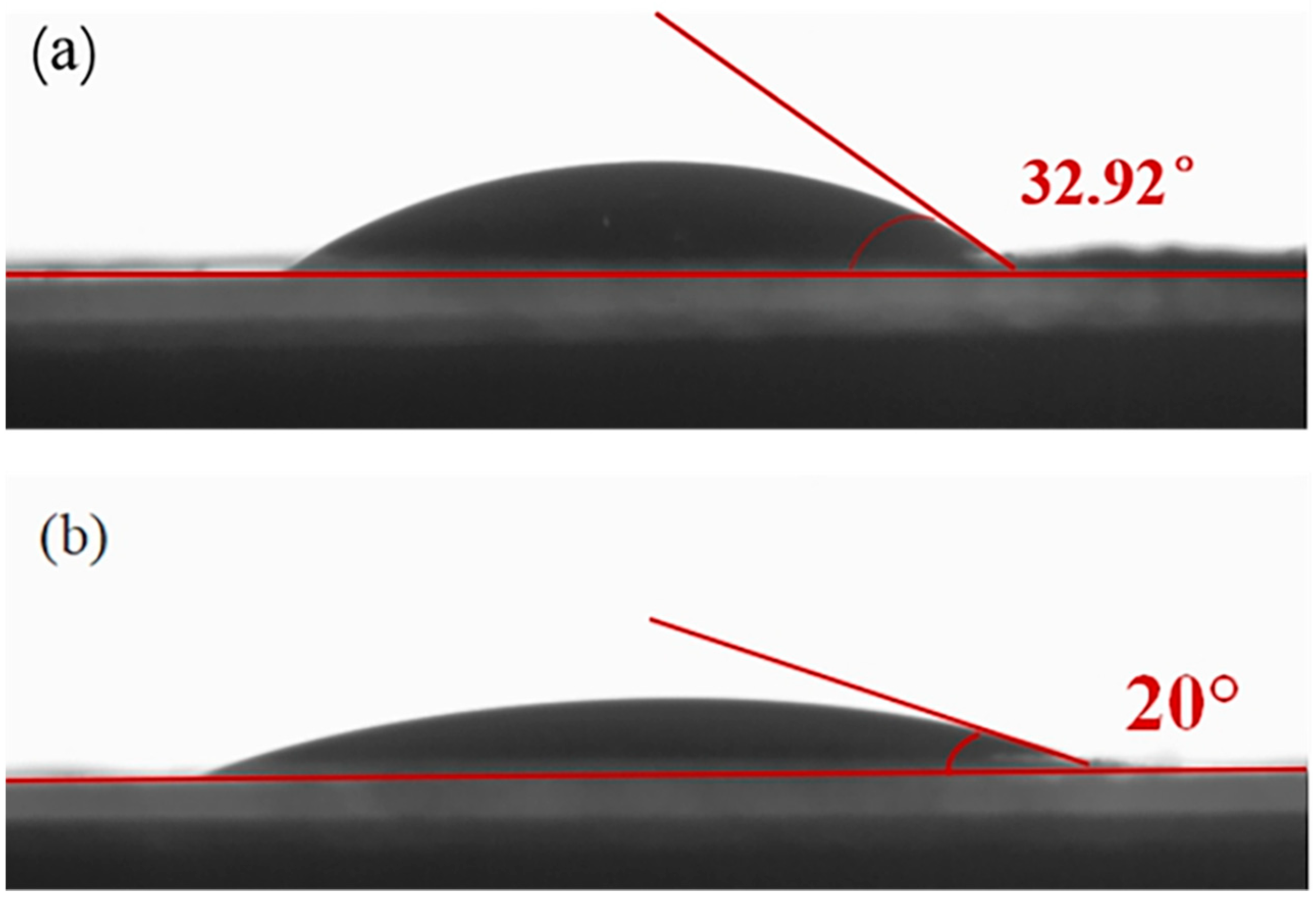
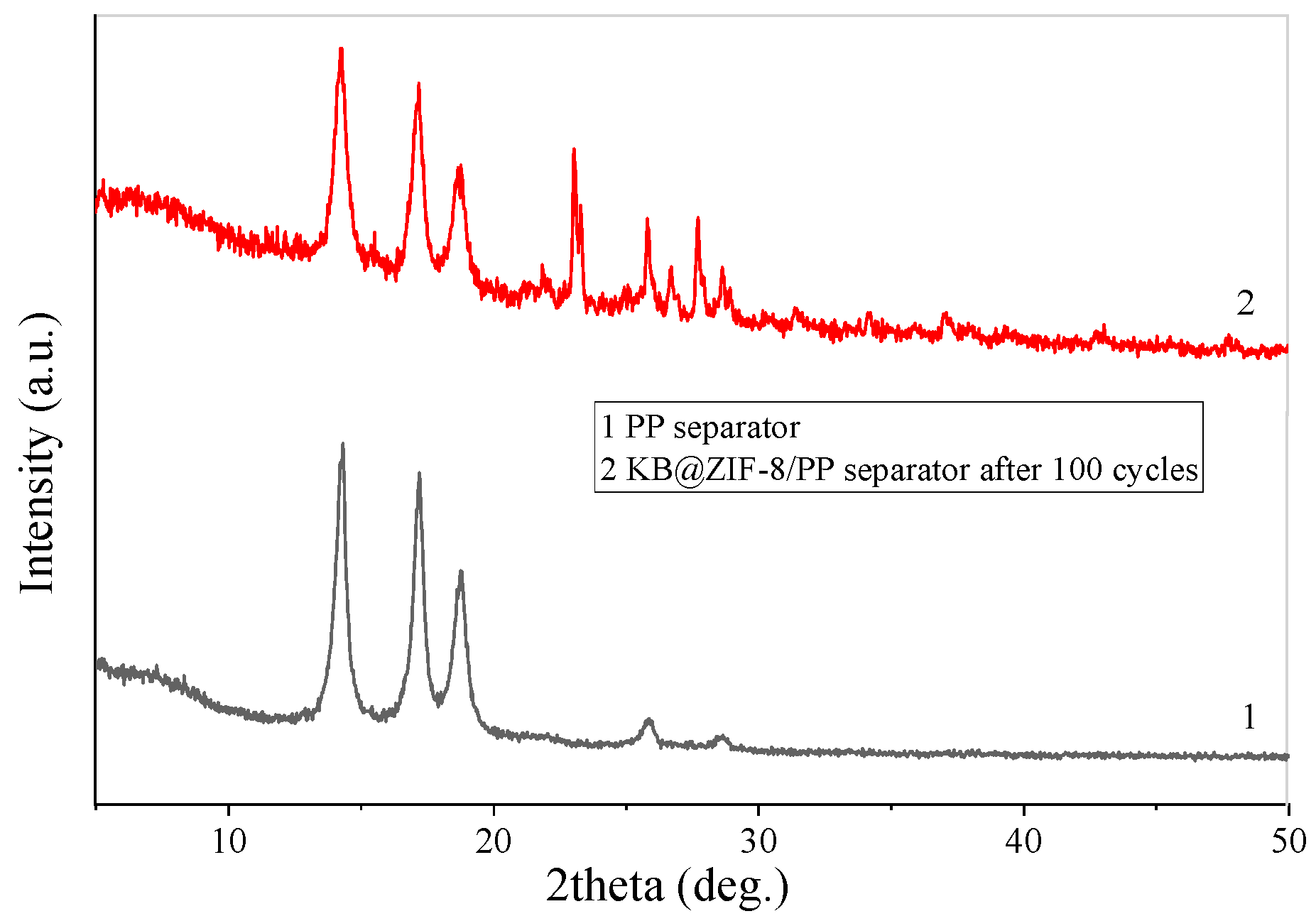
3.1. Characterization
3.2. Electrochemical Performance
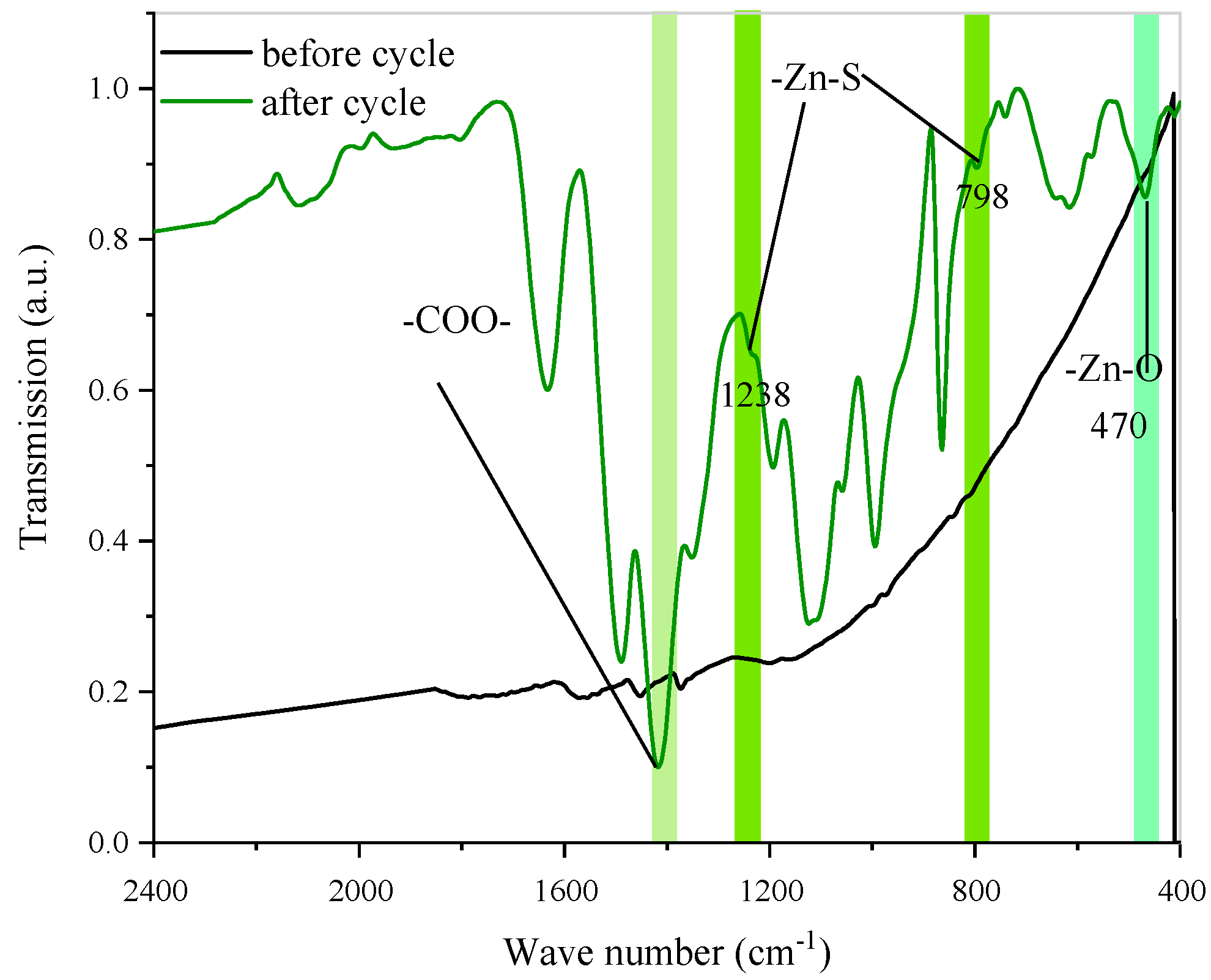
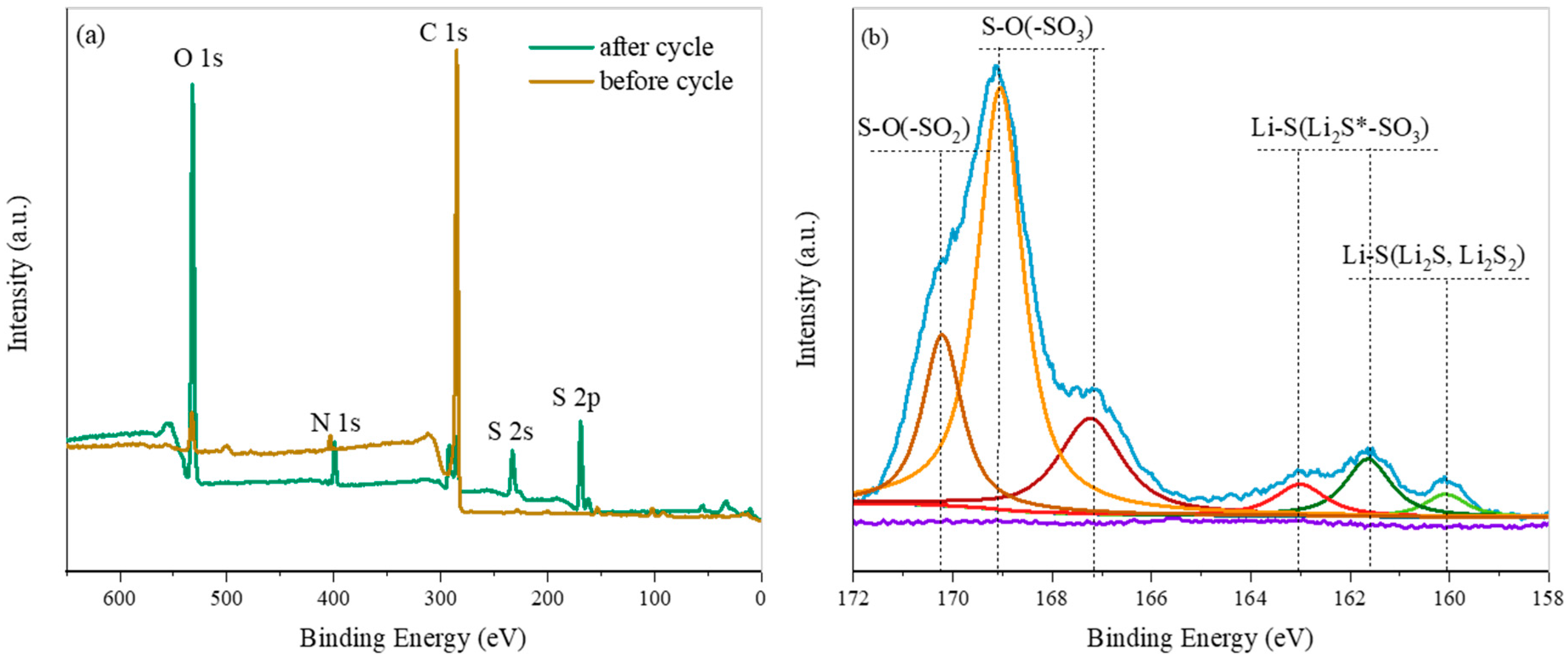
3.3. About Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yin, Y.X.; Xin, S.; Guo, Y.G.; Wan, L.J. Lithium-Sulfur Batteries: Electrochemistry, Materials, and Prospects. Angew. Chem.-Int. Ed. 2013, 52, 13186–13200. [Google Scholar] [CrossRef]
- Dunn, B.; Kamath, H.; Tarascon, J.M. Electrical Energy Storage for the Grid: A Battery of Choices. Science 2011, 334, 928–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ji, X.L.; Nazar, L.F. Advances in Li-S batteries. J. Mater. Chem. 2010, 20, 9821–9826. [Google Scholar] [CrossRef]
- Bruce, P.G.; Freunberger, S.A.; Hardwick, L.J.; Tarascon, J.M. Li-O-2 and Li-S batteries with high energy storage. Nat. Mater. 2012, 11, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Dong, Y.F.; Li, H.J.; Zhao, Z.B.; Wu, H.B.; Hao, C.; Liu, S.H.; Qiu, J.S.; Lou, X.W. Enhancing lithium-sulphur battery performance by strongly binding the discharge products on amino-functionalized reduced graphene oxide. Nat. Commun. 2014, 5, 5002. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Peng, H.J.; Hou, T.Z.; Huang, J.Q.; Chen, C.M.; Wang, D.W.; Cheng, X.B.; Wei, F.; Zhang, Q. Powering Lithium-Sulfur Battery Performance by Propelling Polysulfide Redox at Sulfiphilic Hosts. Nano Lett. 2016, 16, 519–527. [Google Scholar] [CrossRef]
- Ji, X.; Lee, K.T.; Nazar, L.F. A highly ordered nanostructured carbon-sulphur cathode for lithium-sulphur batteries. Nat. Mater. 2009, 8, 500–506. [Google Scholar] [CrossRef]
- Tarascon, J.M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Wang, Y.; Sahadeo, E.; Rubloff, G.; Lin, C.F.; Lee, S.B. High-capacity lithium sulfur battery and beyond: A review of metal anode protection layers and perspective of solid-state electrolytes. J. Mater. Sci. 2019, 54, 3671–3693. [Google Scholar] [CrossRef]
- Park, K.; Cho, J.H.; Jang, J.-H.; Yu, B.-C.; De la Hoz, A.T.; Miller, K.M.; Ellison, C.J.; Goodenough, J.B. Trapping lithium polysulfides of a Li-S battery by forming lithium bonds in a polymer matrix. Energy Environ. Sci. 2015, 8, 2389–2395. [Google Scholar] [CrossRef]
- Zhuang, T.Z.; Huang, J.Q.; Peng, H.J.; He, L.Y.; Cheng, X.B.; Chen, C.M.; Zhang, Q. Rational Integration of Polypropylene/Graphene Oxide/Nafion as Ternary-Layered Separator to Retard the Shuttle of Polysulfi des for Lithium-Sulfur Batteries. Small 2016, 12, 381–389. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.S.; Kim, T.Y.; Heo, S.B.; Hong, J.-A.; Park, Y.; Kang, S.J. Improving the performance of quantum-dot light-emitting diodes via an organic-inorganic hybrid hole injection layer. RSC Adv. 2021, 11, 4168–4172. [Google Scholar] [CrossRef]
- Chiu, L.-L.; Chung, S.-H. A Poly(ethylene oxide)/Lithium bis(trifluoromethanesulfonyl)imide-Coated Polypropylene Membrane for a High-Loading Lithium-Sulfur Battery. Polymers 2021, 13, 535. [Google Scholar] [CrossRef]
- Arora, P.; Zhang, Z.M. Battery separators. Chem. Rev. 2004, 104, 4419–4462. [Google Scholar] [CrossRef]
- Rana, M.; Li, M.; Huang, X.; Luo, B.; Gentle, I.; Knibbe, R. Recent advances in separators to mitigate technical challenges associated with re-chargeable lithium sulfur batteries. J. Mater. Chem. A 2019, 7, 6596–6615. [Google Scholar] [CrossRef]
- Weng, W.; Pol, V.G.; Amine, K. Ultrasound Assisted Design of Sulfur/Carbon Cathodes with Partially Fluorinated Ether Electrolytes for Highly Efficient Li/S Batteries. Adv. Mater. 2013, 25, 1608–1615. [Google Scholar] [CrossRef]
- Zhou, G.; Paek, E.; Hwang, G.S.; Manthiram, A. Long-life Li/polysulphide batteries with high sulphur loading enabled by lightweight three-dimensional nitrogen/sulphur-codoped graphene sponge. Nat. Commun. 2015, 6, 7760. [Google Scholar] [CrossRef] [PubMed]
- Pang, Q.; Kundu, D.; Cuisinier, M.; Nazar, L.F. Surface-enhanced redox chemistry of polysulphides on a metallic and polar host for lithium-sulphur batteries. Nat. Commun. 2014, 5, 4759. [Google Scholar] [CrossRef]
- Chen, S.; Wei, Z.; Qi, X.; Dong, L.; Guo, Y.-G.; Wan, L.; Shao, Z.; Li, L. Nanostructured Polyaniline-Decorated Pt/C@PANI Core-Shell Catalyst with Enhanced Durability and Activity. J. Am. Chem. Soc. 2012, 134, 13252–13255. [Google Scholar] [CrossRef]
- Yu, X.; Joseph, J.; Manthiram, A. Polymer lithium-sulfur batteries with a Nafion membrane and an advanced sulfur electrode. J. Mater. Chem. A 2015, 3, 15683–15691. [Google Scholar] [CrossRef]
- Hong, X.J.; Song, C.L.; Yang, Y.; Tan, H.C.; Li, G.H.; Cai, Y.P.; Wang, H.X. Cerium Based Metal-Organic Frameworks as an Efficient Separator Coating Catalyzing the Conversion of Polysulfides for High Performance Lithium-Sulfur Batteries. ACS Nano 2019, 13, 1923–1931. [Google Scholar] [CrossRef]
- Shekhah, O.; Liu, J.; Fischer, R.A.; Woll, C. MOF thin films: Existing and future applications. Chem. Soc. Rev. 2011, 40, 1081–1106. [Google Scholar] [CrossRef] [PubMed]
- Wu, F.; Zhao, S.Y.; Chen, L.; Lu, Y.; Su, Y.F.; Jia, Y.N.; Bao, L.Y.; Wang, J.; Chen, S.; Chen, R.J. Metal-organic frameworks composites threaded on the CNT knitted separator for suppressing the shuttle effect of lithium sulfur batteries. Energy Storage Mater. 2018, 14, 383–391. [Google Scholar] [CrossRef]
- Talin, A.A.; Centrone, A.; Ford, A.C.; Foster, M.E.; Stavila, V.; Haney, P.; Kinney, R.A.; Szalai, V.; El Gabaly, F.; Yoon, H.P.; et al. Tunable Electrical Conductivity in Metal-Organic Framework Thin-Film Devices. Science 2014, 343, 66–69. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.-Q.; Zhang, Q.; Peng, H.-J.; Liu, X.-Y.; Qian, W.-Z.; Wei, F. Ionic shield for polysulfides towards highly-stable lithium-sulfur batteries. Energy Environ. Sci. 2014, 7, 347–353. [Google Scholar] [CrossRef]
- Jiang, G.Y.; Zheng, N.; Chen, X.; Ding, G.Y.; Li, Y.H.; Sun, F.G.; Li, Y.S. In-situ decoration of MOF-derived carbon on nitrogen-doped ultrathin MXene nanosheets to multifunctionalize separators for stable Li-S batteries. Chem. Eng. J. 2019, 373, 1309–1318. [Google Scholar] [CrossRef]
- Chen, H.H.; Xiao, Y.W.; Chen, C.; Yang, J.Y.; Gao, C.; Chen, Y.S.; Wu, J.S.; Shen, Y.; Zhang, W.N.; Li, S.; et al. Conductive MOF-Modified Separator for Mitigating the Shuttle Effect of Lithium-Sulfur Battery through a Filtration Method. ACS Appl. Mater. Interfaces 2019, 11, 11459–11465. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Li, Y.; Ban, Y.; Jin, H.; Jiao, W.; Liu, X.; Yang, W. Metal-organic framework nanosheets as building blocks for molecular sieving membranes. Science 2014, 346, 1356–1359. [Google Scholar] [CrossRef]
- Su, Y.-S.; Manthiram, A. Lithium-sulphur batteries with a microporous carbon paper as a bifunctional interlayer. Nat. Commun. 2012, 3, 1166. [Google Scholar] [CrossRef] [Green Version]
- He, J.R.; Chen, Y.F.; Manthiram, A. Vertical Co9S8 hollow nanowall arrays grown on a Celgard separator as a multifunctional polysulfide barrier for high-performance Li-S batteries. Energy Environ. Sci. 2018, 11, 2560–2568. [Google Scholar] [CrossRef]
- Bai, S.Y.; Liu, X.Z.; Zhu, K.; Wu, S.C.; Zhou, H.S. Metal-organic framework-based separator for lithium-sulfur batteries. Nat. Energy 2016, 1, 16094. [Google Scholar] [CrossRef]
- Sun, X.X.; Li, M.C.; Ren, S.X.; Lei, T.Z.; Lee, S.Y.; Lee, S.Y.; Wu, Q.L. Zeolitic imidazolate framework-cellulose nanofiber hybrid membrane as Li-Ion battery separator: Basic membrane property and battery performance. J. Power Sources 2020, 454, 11. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, Y.-C.; An, B.; Huang, R.; Wang, C.; Zhou, Z.; Lin, W. Networking Pyrolyzed Zeolitic Imidazolate Frameworks by Carbon Nanotubes Improves Conductivity and Enhances Oxygen-Reduction Performance in Polymer-Electrolyte-Membrane Fuel Cells. Adv. Mater. 2017, 29, 1604556. [Google Scholar] [CrossRef]
- Li, C.; Li, Z.; Li, Q.; Zhang, Z.; Dong, S.; Yin, L. MOFs Derived Hierarchically Porous TiO2 as Effective Chemical and Physical Immobilizer for Sulfur Species as Cathodes for High-Performance Lithium-Sulfur Batteries. Electrochim. Acta 2016, 215, 689–698. [Google Scholar] [CrossRef]
- Duan, J.; Chen, S.; Zhao, C. Ultrathin metal-organic framework array for efficient electrocatalytic water splitting. Nat. Commun. 2017, 8, 1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghosh, A.; Kumar, A.; Dos, T.; Ghosh, A.; Chakrabory, S.; Kar, M.; MacFarlane, D.R.; Mitra, S. Lewis Acid-Base Interactions between Polysulfides and Boehmite Enables Stable Room-Temperature Sodium-Sulfur Batteries. Adv. Funct. Mater. 2020, 30, 2005669. [Google Scholar] [CrossRef]
- Qian, X.; Jin, L.; Zhao, D.; Yang, X.; Wang, S.; Shen, X.; Rao, D.; Yao, S.; Zhou, Y.; Xi, X. Ketjen Black-MnO Composite Coated Separator For High Performance Rechargeable Lithium-Sulfur Battery. Electrochim. Acta 2016, 192, 346–356. [Google Scholar] [CrossRef]
- Chung, S.H.; Manthiram, A. Bifunctional Separator with a Light-Weight Carbon-Coating for Dynamically and Statically Stable Lithium-Sulfur Batteries. Adv. Funct. Mater. 2014, 24, 5299–5306. [Google Scholar] [CrossRef]
- Schejn, A.; Balan, L.; Falk, V.; Aranda, L.; Medjahdi, G.; Schneider, R. Controlling ZIF-8 nano- and microcrystal formation and reactivity through zinc salt variations. Crystengcomm 2014, 16, 4493–4500. [Google Scholar] [CrossRef]
- Shi, X.; Yang, L.; Li, S.; Wang, Y.; Chen, X.; Wu, Z.; Zhong, Y.; Chen, Y.; Gao, S.; Wang, G.; et al. Promoting electrochemical kinetics of Li-S batteries with C@SnS2 modified separator via synergic effect between porous carbon matrix and polar SnS2. Electrochim. Acta 2021, 390, 138829. [Google Scholar] [CrossRef]
- Fan, Y.; Niu, Z.; Zhang, F.; Zhang, R.; Zhao, Y.; Lu, G. Suppressing the Shuttle Effect in Lithium-Sulfur Batteries by a UiO-66-Modified Polypropylene Separator. ACS Omega 2019, 4, 10328–10335. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Yang, Y.; Jia, W.; Xiao, R.; Wang, H. Defect-engineered bilayer MOFs separator for high stability lithium-sulfur batteries. J. Alloys Compd. 2021, 874, 159917. [Google Scholar] [CrossRef]
- Zhu, L.; Jiang, H.; Yang, Q.; Yao, S.; Shen, X.; Tu, F. An Effective Porous Activated Carbon Derived from Puffed Corn Employed as the Separator Coating in a Lithium-Sulfur Battery. Energy Technol. 2019, 7, 1900752. [Google Scholar] [CrossRef]
- Zhou, X.; Liao, Q.; Tang, J.; Bai, T.; Chen, F.; Yang, J. A high-level N-doped porous carbon nanowire modified separator for long-life lithium-sulfur batteries. J. Electroanal. Chem. 2016, 768, 55–61. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ma, B.; Zhang, X.; Deng, X.; Huang, S.; Xiao, M.; Wang, S.; Han, D.; Meng, Y. Construction of KB@ZIF-8/PP Composite Separator for Lithium–Sulfur Batteries with Enhanced Electrochemical Performance. Polymers 2021, 13, 4210. https://doi.org/10.3390/polym13234210
Ma B, Zhang X, Deng X, Huang S, Xiao M, Wang S, Han D, Meng Y. Construction of KB@ZIF-8/PP Composite Separator for Lithium–Sulfur Batteries with Enhanced Electrochemical Performance. Polymers. 2021; 13(23):4210. https://doi.org/10.3390/polym13234210
Chicago/Turabian StyleMa, Bingyi, Xin Zhang, Xiaoqian Deng, Sheng Huang, Min Xiao, Shuanjin Wang, Dongmei Han, and Yuezhong Meng. 2021. "Construction of KB@ZIF-8/PP Composite Separator for Lithium–Sulfur Batteries with Enhanced Electrochemical Performance" Polymers 13, no. 23: 4210. https://doi.org/10.3390/polym13234210





