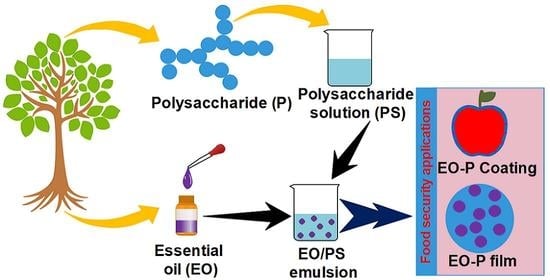
Essential Oil-Containing Polysaccharide-Based Edible Films and Coatings for Food Security Applications
Abstract
:1. Introduction
2. Polysaccharides
2.1. Carrageenan
2.2. Chitosan
2.3. Alginate
2.4. Cellulose Derivatives
2.5. Agar
2.6. Tamarind Kernel Powder and Tamarind Starch and Polysaccharide
2.7. Pectin
2.8. Soluble Soybean Polysaccharide
2.9. Mushroom Polysaccharides
2.10. Konjac Glucomannan
2.11. Chickpea Hull Polysaccharides
2.12. Okra Mucilage Polysaccharide
2.13. Cashew Gum
3. Essential Oils
4. EO-Based Active Packaging Systems
4.1. Synthesis Methods
4.2. Characterization Methods
4.2.1. Thickness of the Films
4.2.2. Moisture Content Analysis
4.2.3. Water Solubility
4.2.4. Optical Properties
4.2.5. Film Microstructure
4.2.6. Water Vapor Transmission Rate (WVTR)
4.2.7. Fourier-Transform Infrared Spectroscopy (FTIR)
4.2.8. X-ray Diffraction (XRD) Analysis
4.2.9. Mechanical Test
4.2.10. Antimicrobial Test
5. New Trends and Technologies
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bahrami, A.; Delshadi, R.; Assadpour, E.; Jafari, S.M.; Williams, L. Antimicrobial-loaded nanocarriers for food packaging applications. Adv. Colloid Int. Sci. 2020, 278, 102140. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, H.; Licciardello, F.; Fava, P.; Siesler, H.W.; Pulvirenti, A. Recent advances on chitosan-based films for sustainable food packaging applications. Food Packag. Shelf Life 2020, 26, 100551. [Google Scholar] [CrossRef]
- Cazón, P.; Velazquez, G.; Ramírez, J.A.; Vázquez, M. Polysaccharide-based films and coatings for food packaging: A review. Food Hydrocoll. 2017, 68, 136–148. [Google Scholar] [CrossRef]
- Benbettaïeb, N.; Gay, J.-P.; Karbowiak, T.; Debeaufort, F. Tuning the Functional Properties of Polysaccharide-Protein Bio-Based Edible Films by Chemical, Enzymatic, and Physical Cross-Linking. Compr. Rev. Food Sci. Food Saf. 2016, 15, 739–752. [Google Scholar] [CrossRef] [Green Version]
- Ju, J.; Chen, X.; Xie, Y.; Yu, H.; Guo, Y.; Cheng, Y.; Qian, H.; Yao, W. Application of essential oil as a sustained release preparation in food packaging. Trends Food Sci. Technol. 2019, 92, 22–32. [Google Scholar] [CrossRef]
- Lacroix, M.; Le Tien, C. 20-Edible films and coatings from nonstarch polysaccharides. In Innovations in Food Packaging; Han, J.H., Ed.; Academic Press: London, UK, 2005; pp. 338–361. [Google Scholar] [CrossRef]
- Sandhu, K.S.; Sharma, L.; Kaur, M.; Kaur, R. Physical, structural and thermal properties of composite edible films prepared from pearl millet starch and carrageenan gum: Process optimization using response surface methodology. Int. J. Biol. Macromol. 2020, 143, 704–713. [Google Scholar] [CrossRef]
- Farhan, A.; Hani, N.M. Active edible films based on semi-refined κ-carrageenan: Antioxidant and color properties and application in chicken breast packaging. Food Packag. Shelf Life 2020, 24, 100476. [Google Scholar] [CrossRef]
- Xu, Y.; Chu, Y.; Feng, X.; Gao, C.; Wu, D.; Cheng, W.; Meng, L.; Zhang, Y.; Tang, X. Effects of zein stabilized clove essential oil Pickering emulsion on the structure and properties of chitosan-based edible films. Int. J. Biol. Macromol. 2020, 156, 111–119. [Google Scholar] [CrossRef] [PubMed]
- da Mata Cunha, O.; Lima, A.M.F.; Assis, O.B.G.; Tiera, M.J.; de Oliveira Tiera, V.A. Amphiphilic diethylaminoethyl chitosan of high molecular weight as an edible film. Int. J. Biol. Macromol. 2020, 164, 3411–3420. [Google Scholar] [CrossRef]
- Zareie, Z.; Tabatabaei Yazdi, F.; Mortazavi, S.A. Development and characterization of antioxidant and antimicrobial edible films based on chitosan and gamma-aminobutyric acid-rich fermented soy protein. Carbohydr. Polym. 2020, 244, 116491. [Google Scholar] [CrossRef]
- Nair, M.S.; Tomar, M.; Punia, S.; Kukula-Koch, W.; Kumar, M. Enhancing the functionality of chitosan- and alginate-based active edible coatings/films for the preservation of fruits and vegetables: A review. Int. J. Biol. Macromol. 2020, 164, 304–320. [Google Scholar] [CrossRef]
- Park, J.; Nam, J.; Yun, H.; Jin, H.-J.; Kwak, H.W. Aquatic Polymer-Based Edible Films of Fish Gelatin Crosslinked with Alginate Dialdehyde having Enhanced Physicochemical Properties. Carbohydr. Polym. 2021, 254, 117317. [Google Scholar] [CrossRef]
- Lin, D.; Zheng, Y.; Wang, X.; Huang, Y.; Ni, L.; Chen, X.; Wu, Z.; Huang, C.; Yi, Q.; Li, J. Study on physicochemical properties, antioxidant and antimicrobial activity of okara soluble dietary fiber/sodium carboxymethyl cellulose/thyme essential oil active edible composite films incorporated with pectin. Int. J. Biol. Macromol. 2020, 165, 1241–1249. [Google Scholar] [CrossRef]
- Lan, W.; Zhang, R.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Dai, J.; He, L.; Liu, Y. Improving nisin production by encapsulated Lactococcus lactis with starch/carboxymethyl cellulose edible films. Carbohydr. Polym. 2021, 251, 117062. [Google Scholar] [CrossRef]
- Salama, H.E.; Abdel Aziz, M.S.; Sabaa, M.W. Development of antibacterial carboxymethyl cellulose/chitosan biguanidine hydrochloride edible films activated with frankincense essential oil. Int. J. Biol. Macromol. 2019, 139, 1162–1167. [Google Scholar] [CrossRef] [PubMed]
- Indumathi, M.P.; Saral Sarojini, K.; Rajarajeswari, G.R. Antimicrobial and biodegradable chitosan/cellulose acetate phthalate/ZnO nano composite films with optimal oxygen permeability and hydrophobicity for extending the shelf life of black grape fruits. Int. J. Biolog. Macromol. 2019, 132, 1112–1120. [Google Scholar] [CrossRef] [PubMed]
- Decroix, C.; Chalamet, Y.; Sudre, G.; Caroll, V. Thermo-mechanical properties and blend behaviour of cellulose acetate/lactates and acid systems: Natural-based plasticizers. Carbohydr. Polym. 2020, 237, 116072. [Google Scholar] [CrossRef]
- Herniou-Julien, C.; Mendieta, J.R.; Gutiérrez, T.J. Characterization of biodegradable/non-compostable films made from cellulose acetate/corn starch blends processed under reactive extrusion conditions. Food Hydrocoll. 2019, 89, 67–79. [Google Scholar] [CrossRef]
- El Fawal, G.; Hong, H.; Song, X.; Wu, J.; Sun, M.; He, C.; Mo, X.; Jiang, Y.; Wang, H. Fabrication of antimicrobial films based on hydroxyethylcellulose and ZnO for food packaging application. Food Packag. Shelf Life 2020, 23, 100462. [Google Scholar] [CrossRef]
- Villalobos, R.; Hernández-Muñoz, P.; Chiralt, A. Effect of surfactants on water sorption and barrier properties of hydroxypropyl methylcellulose films. Food Hydrocoll. 2006, 20, 502–509. [Google Scholar] [CrossRef]
- Mostafavi, F.S.; Zaeim, D. Agar-based edible films for food packaging applications—A review. Int. J. Biolog. Macromol. 2020, 159, 1165–1176. [Google Scholar] [CrossRef]
- Wongphan, P.; Harnkarnsujarit, N. Characterization of starch, agar and maltodextrin blends for controlled dissolution of edible films. Int. J. Biolog. Macromol. 2020, 156, 80–93. [Google Scholar] [CrossRef]
- Agarwal, S.; Hoque, M.; Bandara, N.; Pal, K.; Sarkar, P. Synthesis and characterization of tamarind kernel powder-based antimicrobial edible films loaded with geraniol. Food Packag. Shelf Life 2020, 26, 100562. [Google Scholar] [CrossRef]
- Chandra mohan, C.; Rakhavan, K.R.; Sudharsan, K.; Radha krishnan, K.; Babuskin, S.; Sukumar, M. Design and characterization of spice fused tamarind starch edible packaging films. Lebensm. Wiss. Technol. Food Sci. Technol. 2016, 68, 642–652. [Google Scholar] [CrossRef]
- Jahromi, M.; Niakousari, M.; Golmakani, M.T.; Mohammadifar, M.A. Physicochemical and structural characterization of sodium caseinate based film-forming solutions and edible films as affected by high methoxyl pectin. Int. J. Biolog. Macromol. 2020, 165, 1949–1959. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Xu, L.; Wang, Y.; Chen, Z.; Zhang, M.; Chen, H. Characterization and functional properties of a pectin/tara gum based edible film with ellagitannins from the unripe fruits of Rubus chingii Hu. Food Chem. 2020, 325, 126964. [Google Scholar] [CrossRef] [PubMed]
- Shafie, M.H.; Yusof, R.; Samsudin, D.; Gan, C.-Y. Averrhoa bilimbi pectin-based edible films: Effects of the linearity and branching of the pectin on the physicochemical, mechanical, and barrier properties of the films. Int. J. Biolog. Macromol. 2020, 163, 1276–1282. [Google Scholar] [CrossRef] [PubMed]
- Ghani, S.; Barzegar, H.; Noshad, M.; Hojjati, M. The preparation, characterization and in vitro application evaluation of soluble soybean polysaccharide films incorporated with cinnamon essential oil nanoemulsions. Int. J. Biolog. Macromol. 2018, 112, 197–202. [Google Scholar] [CrossRef]
- Liu, C.; Huang, J.; Zheng, X.; Liu, S.; Lu, K.; Tang, K.; Liu, J. Heat sealable soluble soybean polysaccharide/gelatin blend edible films for food packaging applications. Food Packag. Shelf Life 2020, 24, 100485. [Google Scholar] [CrossRef]
- Liu, Q.-R.; Wang, W.; Qi, J.; Huang, Q.; Xiao, J. Oregano essential oil loaded soybean polysaccharide films: Effect of Pickering type immobilization on physical and antimicrobial properties. Food Hydrocoll. 2019, 87, 165–172. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tafaghodi, M.; Bazzaz, B.S.F. Soluble soybean polysaccharide/TiO2 bionanocomposite film for food application. Carbohydr. Polym. 2018, 186, 384–393. [Google Scholar] [CrossRef]
- Salarbashi, D.; Noghabi, M.S.; Bazzaz, B.S.F.; Shahabi-Ghahfarrokhi, I.; Jafari, B.; Ahmadi, R. Eco-friendly soluble soybean polysaccharide/nanoclay Na+ bionanocomposite: Properties and characterization. Carbohydr. Polym. 2017, 169, 524–532. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.R.; Freitas, M.M.S.; Oliveira, L.C.; Martins, L.H.S.; Almada-Vilhena, A.O.; Oliveira, R.M.; Pieczarka, J.C.; Davi do Socorro, B.; Junior, R.N.C. Obtaining extracts rich in antioxidant polysaccharides from the edible mushroom Pleurotus ostreatus using binary system with hot water and supercritical CO2. Food Chem. 2020, 330, 127173. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, K.; Li, C.; Cheng, S.; Zhou, J.; Wu, Z. A novel biodegradable film from edible mushroom (F. velutipes) by product: Microstructure, mechanical and barrier properties associated with the fiber morphology. Innov. Food Sci. Emerg. Technol. 2018, 47, 153–160. [Google Scholar] [CrossRef]
- Mingyi, Y.; Belwal, T.; Devkota, H.P.; Li, L.; Luo, Z. Trends of utilizing mushroom polysaccharides (MPs) as potent nutraceutical components in food and medicine: A comprehensive review. Trends Food Sci. Technol. 2019, 92, 94–110. [Google Scholar] [CrossRef]
- Liu, Z.; Lin, D.; Lopez-Sanchez, P.; Yang, X. Characterizations of bacterial cellulose nanofibers reinforced edible films based on konjac glucomannan. Int. J. Biolog. Macromol. 2020, 145, 634–645. [Google Scholar] [CrossRef]
- Lei, Y.; Wu, H.; Jiao, C.; Jiang, Y.; Liu, R.; Xiao, D.; Lu, J.; Zhang, Z.; Shen, G.; Li, S. Investigation of the structural and physical properties, antioxidant and antimicrobial activity of pectin-konjac glucomannan composite edible films incorporated with tea polyphenol. Food Hydrocoll. 2019, 94, 128–135. [Google Scholar] [CrossRef]
- Wu, C.; Peng, S.; Wen, C.; Wang, X.; Fan, L.; Deng, R.; Pang, J. Structural characterization and properties of konjac glucomannan/curdlan blend films. Carbohydr. Polym. 2012, 89, 497–503. [Google Scholar] [CrossRef]
- Ye, Z.; Wang, W.; Yuan, Q.; Ye, H.; Sun, Y.; Zhang, H.; Zeng, X. Box-Behnken design for extraction optimization, characterization and in vitro antioxidant activity of Cicer arietinum L. hull polysaccharides. Carbohydr. Polym. 2016, 147, 354–364. [Google Scholar] [CrossRef]
- Akhtar, H.M.S.; Riaz, A.; Hamed, Y.S.; Abdin, M.; Chen, G.; Wan, P.; Zeng, X. Production and characterization of CMC-based antioxidant and antimicrobial films enriched with chickpea hull polysaccharides. Int. J. Biolog. Macromol. 2018, 118, 469–477. [Google Scholar] [CrossRef] [PubMed]
- Araújo, A.; Galvão, A.; Filho, C.S.; Mendes, F.; Oliveira, M.; Barbosa, F.; Filho, M.S.; Bastos, M. Okra mucilage and corn starch bio-based film to be applied in food. Polym. Test. 2018, 71, 352–361. [Google Scholar] [CrossRef]
- Varghese, S.A.; Siengchin, S.; Parameswaranpillai, J. Essential oils as antimicrobial agents in biopolymer-based food packaging-A comprehensive review. Food Biosci. 2020, 38, 100785. [Google Scholar] [CrossRef]
- Forato, L.A.; de Britto, D.; de Rizzo, J.S.; Gastaldi, T.A.; Assis, O.B.G. Effect of cashew gum-carboxymethylcellulose edible coatings in extending the shelf-life of fresh and cut guavas. Food Packag. Shelf Life 2015, 5, 68–74. [Google Scholar] [CrossRef]
- Miranda, J.A.L.d.; Barreto, J.E.F.; Martins, D.S.; Pimentel, P.V.d.S.; Costa, D.V.d.S.; Silva, R.R.e.; Souza, L.K.M.d.; Lima, C.N.d.C.; Rocha, J.A.; Freitas, A.P.F.d.; et al. Protective Effect of Cashew Gum (Anacardium occidentale L.) on 5-Fluorouracil-Induced Intestinal Mucositis. Pharmaceuticals 2019, 12, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, M.A.; Furtado, R.F.; Bastos, M.S.R.; Leitão, R.C.; Benevides, S.D.; Muniz, C.R.; Cheng, H.N.; Biswas, A. Performance evaluation of cashew gum and gelatin blend for food packaging. Food Packag. Shelf Life 2018, 17, 57–64. [Google Scholar] [CrossRef]
- Bhavaniramya, S.; Vishnupriya, S.; Al-Aboody, M.S.; Vijayakumar, R.; Baskaran, D. Role of essential oils in food safety: Antimicrobial and antioxidant applications. Grain Oil Sci. Technol. 2019, 2, 49–55. [Google Scholar] [CrossRef]
- Białoń, M.; Krzyśko-Łupicka, T.; Nowakowska-Bogdan, E.; Wieczorek, P.P. Chemical Composition of Two Different Lavender Essential Oils and Their Effect on Facial Skin Microbiota. Molecules 2019, 24, 3270. [Google Scholar] [CrossRef] [Green Version]
- Muráriková, A.; Ťažký, A.; Neugebauerová, J.; Planková, A.; Jampílek, J.; Mučaji, P.; Mikuš, P. Characterization of essential oil composition in different basil species and pot cultures by a GC-MS method. Molecules 2017, 22, 1221. [Google Scholar] [CrossRef] [Green Version]
- Sebei, K.; Sakouhi, F.; Herchi, W.; Khouja, M.L.; Boukhchina, S. Chemical composition and antibacterial activities of seven Eucalyptus species essential oils leaves. Biol. Res. 2015, 48, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurdjannah, N.; Bermawie, N. 11-Cloves. In Handbook of Herbs and Spices, 2nd ed.; Peter, K.V., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 197–215. [Google Scholar] [CrossRef]
- Zeng, W.C.; Zhang, Z.; Gao, H.; Jia, L.R.; He, Q. Chemical composition, antioxidant, and antimicrobial activities of essential oil from pine needle (Cedrus deodara). J. Food Sci. 2012, 77, C824–C829. [Google Scholar] [CrossRef]
- Mohamed Hanaa, A.R.; Sallam, Y.I.; El-Leithy, A.S.; Aly, S.E. Lemongrass (Cymbopogon citratus) essential oil as affected by drying methods. Ann. Agric. Sci. 2012, 57, 113–116. [Google Scholar] [CrossRef] [Green Version]
- Abdelwahab, S.I.; Mariod, A.A.; Taha, M.M.E.; Zaman, F.Q.; Abdelmageed, A.H.A.; Khamis, S.; Sivasothy, Y.; Awang, K. Chemical composition and antioxidant properties of the essential oil of Cinnamomum altissimum Kosterm. (Lauraceae). Arab. J. Chem. 2017, 10, 131–135. [Google Scholar] [CrossRef] [Green Version]
- Verma, R.S.; Padalia, R.C.; Chauhan, A.; Upadhyay, R.K.; Singh, V.R. Productivity and essential oil composition of rosemary (Rosmarinus officinalis L.) harvested at different growth stages under the subtropical region of north India. J. Essent. Oil Res. 2020, 32, 144–149. [Google Scholar] [CrossRef]
- Dosoky, N.S.; Satyal, P.; Barata, L.M.; da Silva, J.K.R.; Setzer, W.N. Volatiles of Black Pepper Fruits (Piper nigrum L.). Molecules 2019, 24, 4244. [Google Scholar] [CrossRef] [Green Version]
- Wei, Z.; Yang, Y.; Yang, R.; Wang, C. Alkaline lignin extracted from furfural residues for pH-responsive Pickering emulsions and their recyclable polymerization. Green Chem. 2012, 14, 3230–3236. [Google Scholar] [CrossRef]
- Zhang, Y.; Cui, L.; Che, X.; Zhang, H.; Shi, N.; Li, C.; Chen, Y.; Kong, W. Zein-based films and their usage for controlled delivery: Origin, classes and current landscape. J. Controll. Release 2015, 206, 206–219. [Google Scholar] [CrossRef] [PubMed]
- Cai, J.; Xiao, J.; Chen, X.; Liu, H. Essential oil loaded edible films prepared by continuous casting method: Effects of casting cycle and loading position on the release properties. Food Packag. Shelf Life 2020, 26, 100555. [Google Scholar] [CrossRef]
- Umaraw, P.; Munekata, P.E.S.; Verma, A.K.; Barba, F.J.; Singh, V.P.; Kumar, P.; Lorenzo, J.M. Edible films/coating with tailored properties for active packaging of meat, fish and derived products. Trends Food Sci. Technol. 2020, 98, 10–24. [Google Scholar] [CrossRef]
- Acevedo-Fani, A.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Edible films from essential-oil-loaded nanoemulsions: Physicochemical characterization and antimicrobial properties. Food Hydrocoll. 2015, 47, 168–177. [Google Scholar] [CrossRef] [Green Version]
- Lian, H.; Shi, J.; Zhang, X.; Peng, Y. Effect of the added polysaccharide on the release of thyme essential oil and structure properties of chitosan based film. Food Packag. Shelf Life 2020, 23, 100467. [Google Scholar] [CrossRef]
- Gul, O.; Saricaoglu, F.T.; Besir, A.; Atalar, I.; Yazici, F. Effect of ultrasound treatment on the properties of nano-emulsion films obtained from hazelnut meal protein and clove essential oil. Ultrason. Sonochem. 2018, 41, 466–474. [Google Scholar] [CrossRef]
- Gavrilov, A.S.; Gusel’nikova, E.V.; Koneeva, L.A.; Bakharev, V.P.; Petrov, A.Y. Methods of Polymer Film Formation and Testing for Obtaining Optimum Tablet Coatings. Pharm. Chem. J. 2003, 37, 333–335. [Google Scholar] [CrossRef]
- Garcia, R. Essential Oils in Plastic Film. Available online: https://patents.google.com/patent/US20040034149A1/en (accessed on 5 November 2020).
- Dini, H.; Fallah, A.A.; Bonyadian, M.; Abbasvali, M.; Soleimani, M. Effect of edible composite film based on chitosan and cumin essential oil-loaded nanoemulsion combined with low-dose gamma irradiation on microbiological safety and quality of beef loins during refrigerated storage. Int. J. Biolog. Macromol. 2020, 164, 1501–1509. [Google Scholar] [CrossRef] [PubMed]
- Esmaeili, H.; Cheraghi, N.; Khanjari, A.; Rezaeigolestani, M.; Basti, A.A.; Kamkar, A.; Aghaee, E.M. Incorporation of nanoencapsulated garlic essential oil into edible films: A novel approach for extending shelf life of vacuum-packed sausages. Meat Sci. 2020, 166, 108135. [Google Scholar] [CrossRef] [PubMed]
- Xu, T.; Gao, C.; Feng, X.; Yang, Y.; Shen, X.; Tang, X. Structure, physical and antioxidant properties of chitosan-gum arabic edible films incorporated with cinnamon essential oil. Int. J. Biolog. Macromol. 2019, 134, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Alizadeh Behbahani, B.; Noshad, M.; Jooyandeh, H. Improving oxidative and microbial stability of beef using Shahri Balangu seed mucilage loaded with Cumin essential oil as a bioactive edible coating. Biocatal. Agricul. Biotechnol. 2020, 24, 101563. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, C.; Liu, S.; Gao, J.; Cui, S.W.; Xia, W. Coating white shrimp (Litopenaeus vannamei) with edible fully deacetylated chitosan incorporated with clove essential oil and kojic acid improves preservation during cold storage. Int. J. Biolog. Macromol. 2020, 162, 1276–1282. [Google Scholar] [CrossRef]
- Hashemi, S.M.B.; Mousavi Khaneghah, A. Characterization of novel basil-seed gum active edible films and coatings containing oregano essential oil. Prog. Org. Coat. 2017, 110, 35–41. [Google Scholar] [CrossRef]
- Yeddes, W.; Djebali, K.; Aidi Wannes, W.; Horchani-Naifer, K.; Hammami, M.; Younes, I.; Saidani Tounsi, M. Gelatin-chitosan-pectin films incorporated with rosemary essential oil: Optimized formulation using mixture design and response surface methodology. Int. J. Biolog. Macromol. 2020, 154, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Graü, M.A.; Avena-Bustillos, R.J.; Olsen, C.; Friedman, M.; Henika, P.R.; Martín-Belloso, O.; Pan, Z.; McHugh, T.H. Effects of plant essential oils and oil compounds on mechanical, barrier and antimicrobial properties of alginate—Apple puree edible films. J. Food Eng. 2007, 81, 634–641. [Google Scholar] [CrossRef]
- Abreu, F.O.M.S.; Oliveira, E.F.; Paula, H.C.B.; de Paula, R.C.M. Chitosan/cashew gum nanogels for essential oil encapsulation. Carbohydr. Polym. 2012, 89, 1277–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Espitia, P.J.P.; Soares, N.D.F.F.; Botti, L.C.M.; Silva, W.A. Effect of Essential Oils in the Properties of Cellulosic Active Packaging. Macromol. Symp. 2011, 299–300, 199–205. [Google Scholar] [CrossRef]
- Dong, F.; Wang, X. Effects of carboxymethyl cellulose incorporated with garlic essential oil composite coatings for improving quality of strawberries. Int. J. Biolog. Macromol. 2017, 104, 821–826. [Google Scholar] [CrossRef] [PubMed]
- Ranjbar, M.; Azizi, M.; Hashtjin, A.M. Evaluation of Physico-Mechanical and Antimicrobial Properties of Gelatin- Carboxymethyl Cellulose Film Containing Essential Oil of Bane (Pistacia atlantica). Nutr. Food Sci. Res. 2017, 4, 11–17. [Google Scholar] [CrossRef]
- Biswas, A.; Furtado, R.F.; Bastos, M.S.R.; Benevides, S.D.; Oliveira, M.d.A.; Boddu, V.; Cheng, H. Preparation and Characterization of Carboxymethyl Cellulose Films with Embedded Essential Oils. J. Mater. Sci. Res. 2018, 7, 16. [Google Scholar] [CrossRef]
- Correa-Pacheco, Z.N.; Corona-Rangel, M.L.; Bautista-Baños, S.; Ventura-Aguilar, R.I. Application of natural-based nanocoatings for extending the shelf life of green bell pepper fruit. J. Food Sci. 2021, 86, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Arezoo, E.; Mohammadreza, E.; Maryam, M.; Abdorreza, M.N. The synergistic effects of cinnamon essential oil and nano TiO2 on antimicrobial and functional properties of sago starch films. Int. J. Biolog. Macromol. 2020, 157, 743–751. [Google Scholar] [CrossRef]
- de Oliveira Filho, J.G.; de Deus, I.P.B.; Valadares, A.C.F.; Fernandes, C.C.; Estevam, E.B.B.; Egea, M.B. Chitosan Film with Citrus limonia Essential Oil: Physical and Morphological Properties and Antibacterial Activity. Colloid. Int. 2020, 4, 18. [Google Scholar] [CrossRef]
- Cui, H.; Zhang, C.; Li, C.; Lin, L. Preparation and antibacterial activity of Litsea cubeba essential oil/dandelion polysaccharide nanofiber. Ind. Crop. Prod. 2019, 140, 111739. [Google Scholar] [CrossRef]
- Salarbashi, D.; Tajik, S.; Shojaee-Aliabadi, S.; Ghasemlou, M.; Moayyed, H.; Khaksar, R.; Noghabi, M.S. Development of new active packaging film made from a soluble soybean polysaccharide incorporated Zataria multiflora Boiss and Mentha pulegium essential oils. Food Chem. 2014, 146, 614–622. [Google Scholar] [CrossRef]
- Choi, W.S.; Singh, S.; Lee, Y.S. Characterization of edible film containing essential oils in hydroxypropyl methylcellulose and its effect on quality attributes of ‘Formosa’ plum (Prunus salicina L.). Lebensm. Wiss. Technol. 2016, 70, 213–222. [Google Scholar] [CrossRef]
- Pranoto, Y.; Salokhe, V.M.; Rakshit, S.K. Physical and antibacte rial properties of alginate-based edible film incorporated with garlic oil. Food Res. Int. 2005, 38, 267–272. [Google Scholar] [CrossRef]
- Iamareerat, B.; Singh, M.; Sadiq, M.B.; Anal, A.K. Reinforced cassava starch based edible film incorporated with essential oil and sodium bentonite nanoclay as food packaging material. J. Food Sci. Technol. 2018, 55, 1953–1959. [Google Scholar] [CrossRef]
- Al-Hashimi, A.G.; Ammar, A.B.; Cacciola, F.; Lakhssassi, N. Development of a Millet Starch Edible Film Containing Clove Essential Oil. Foods 2020, 9, 184. [Google Scholar] [CrossRef] [Green Version]
- Venkatachalam, K.; Lekjing, S. A chitosan-based edible film with clove essential oil and nisin for improving the quality and shelf life of pork patties in cold storage. RSC Adv. 2020, 10, 17777–17786. [Google Scholar] [CrossRef]
- Souza, V.G.L.; Pires, J.R.A.; Vieira, É.T.; Coelhoso, I.M.; Duarte, M.P.; Fernando, A.L. Shelf Life Assessment of Fresh Poultry Meat Packaged in Novel Bionanocomposite of Chitosan/Montmorillonite Incorporated with Ginger Essential Oil. Coatings 2018, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Maleki, G.; Sedaghat, N.; Woltering, E.J.; Farhoodi, M.; Mohebbi, M. Chitosan-limonene coating in combination with modified atmosphere packaging preserve postharvest quality of cucumber during storage. J. Food Meas. Charact. 2018, 12, 1610–1621. [Google Scholar] [CrossRef]
- Shahbazi, Y. Application of carboxymethyl cellulose and chitosan coatings containing Mentha spicata essential oil in fresh strawberries. Int. J. Biolog. Macromol. 2018, 112, 264–272. [Google Scholar] [CrossRef]
- Rezaei, F.; Shahbazi, Y. Shelf-life extension and quality attributes of sauced silver carp fillet: A comparison among direct addition, edible coating and biodegradable film. Lebensm. Wiss. Technol. 2018, 87, 122–133. [Google Scholar] [CrossRef]
- Yang, S.-Y.; Cao, L.; Kim, H.; Beak, S.-E.; Song, K.B. Utilization of Foxtail Millet Starch Film Incorporated with Clove Leaf Oil for the Packaging of Queso Blanco Cheese as a Model Food. Starch-Stärke 2018, 70, 1700171. [Google Scholar] [CrossRef]
- Orsuwan, A.; Sothornvit, R. Active Banana Flour Nanocomposite Films Incorporated with Garlic Essential Oil as Multifunctional Packaging Material for Food Application. Food Bioprocess Technol. 2018, 11, 1199–1210. [Google Scholar] [CrossRef]
- Dashipour, A.; Razavilar, V.; Hosseini, H.; Shojaee-Aliabadi, S.; German, J.B.; Ghanati, K.; Khakpour, M.; Khaksar, R. Antioxidant and antimicrobial carboxymethyl cellulose films containing Zataria multiflora essential oil. Int. J. Biolog. Macromol. 2015, 72, 606–613. [Google Scholar] [CrossRef] [PubMed]
- Hasheminya, S.-M.; Mokarram, R.R.; Ghanbarzadeh, B.; Hamishekar, H.; Kafil, H.S.; Dehghannya, J. Development and characterization of biocomposite films made from kefiran, carboxymethyl cellulose and Satureja Khuzestanica essential oil. Food Chem. 2019, 289, 443–452. [Google Scholar] [CrossRef]
- Xue, F.; Gu, Y.; Wang, Y.; Li, C.; Adhikari, B. Encapsulation of essential oil in emulsion based edible films prepared by soy protein isolate-gum acacia conjugates. Food Hydrocolloids 2019, 96, 178–189. [Google Scholar] [CrossRef]
- Nilsjohan. The Principle of the CIELAB Colour Space. Available online: https://upload.wikimedia.org/wikipedia/commons/c/c6/The_principle_of_the_CIELAB_colour_space.svg (accessed on 5 November 2020).
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of lipid self-association on the microstructure and physical properties of hydroxypropyl-methylcellulose edible films containing fatty acids. Carbohydr. Polym. 2010, 82, 585–593. [Google Scholar] [CrossRef]
- Jiménez, A.; Fabra, M.J.; Talens, P.; Chiralt, A. Effect of re-crystallization on tensile, optical and water vapour barrier properties of corn starch films containing fatty acids. Food Hydrocoll. 2012, 26, 302–310. [Google Scholar] [CrossRef]
- Fabra, M.J.; Talens, P.; Chiralt, A. Microstructure and optical properties of sodium caseinate films containing oleic acid-beeswax mixtures. Food Hydrocoll. 2009, 23, 676–683. [Google Scholar] [CrossRef]
- Dhumal, C.V.; Ahmed, J.; Bandara, N.; Sarkar, P. Improvement of antimicrobial activity of sago starch/guar gum bi-phasic edible films by incorporating carvacrol and citral. Food Packag. Shelf Life 2019, 21, 100380. [Google Scholar] [CrossRef]
- Vilas Dhumal, C.; Pal, K.; Sarkar, P. Synthesis, characterization, and antimicrobial efficacy of composite films from guar gum/sago starch/whey protein isolate loaded with carvacrol, citral and carvacrol-citral mixture. J. Mater. Sci. Mater. Med. 2019, 30, 117. [Google Scholar] [CrossRef] [PubMed]
- Alpizar-Reyes, E.; Román-Guerrero, A.; Gallardo-Rivera, R.; Varela-Guerrero, V.; Cruz-Olivares, J.; Pérez-Alonso, C. Rheological properties of tamarind (Tamarindus indica L.) seed mucilage obtained by spray-drying as a novel source of hydrocolloid. Int. J. Biolog. Macromol. 2018, 107, 817–824. [Google Scholar] [CrossRef]
- Ghasemlou, M.; Aliheidari, N.; Fahmi, R.; Shojaee-Aliabadi, S.; Keshavarz, B.; Cran, M.J.; Khaksar, R. Physical, mechanical and barrier properties of corn starch films incorporated with plant essential oils. Carbohydr. Polym. 2013, 98, 1117–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, M.; Singh, S. Physicochemical, Morphological, Pasting, and Rheological Properties of Tamarind (Tamarindus indica L.) Kernel Starch. Int. J. Food Prop. 2016, 19, 2432–2442. [Google Scholar] [CrossRef] [Green Version]
- Yadav, I.; Nayak, S.K.; Rathnam, V.S.S.; Banerjee, I.; Ray, S.S.; Anis, A.; Pal, K. Reinforcing effect of graphene oxide reinforcement on the properties of poly (vinyl alcohol) and carboxymethyl tamarind gum based phase-separated film. J. Mech. Behav. Biomedic. Mater. 2018, 81, 61–71. [Google Scholar] [CrossRef] [PubMed]
- Dhumal, C.V.; Pal, K.; Sarkar, P. Characterization of Tri-Phasic Edible Films from Chitosan, Guar Gum, and Whey Protein Isolate Loaded with Plant-Based Antimicrobial Compounds. Polym.-Plast. Technol. Mater. 2019, 58, 255–269. [Google Scholar] [CrossRef]
- Shahbazi, Y. The properties of chitosan and gelatin films incorporated with ethanolic red grape seed extract and Ziziphora clinopodioides essential oil as biodegradable materials for active food packaging. Int. J. Biolog. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef]
- Song, X.; Zuo, G.; Chen, F. Effect of essential oil and surfactant on the physical and antimicrobial properties of corn and wheat starch films. Int. J. Biolog. Macromol. 2018, 107, 1302–1309. [Google Scholar] [CrossRef]
- de Araújo, G.K.P.; de Souza, S.J.; da Silva, M.V.; Yamashita, F.; Gonçalves, O.H.; Leimann, F.V.; Shirai, M.A. Physical, antimicrobial and antioxidant properties of starch-based film containing ethanolic propolis extract. Int. J. Food Sci. Technol. 2015, 50, 2080–2087. [Google Scholar] [CrossRef]
- Atef, M.; Rezaei, M.; Behrooz, R. Characterization of physical, mechanical, and antibacterial properties of agar-cellulose bionanocomposite films incorporated with savory essential oil. Food Hydrocoll. 2015, 45, 150–157. [Google Scholar] [CrossRef]






















| Essential Oil | Source Plant/Herb | Part of the Plant | Major Components | References |
|---|---|---|---|---|
| Lavender essential oil | Lavandula officinalis, L. angustifolia, L. latifolia | Flowers | Linalool, linalyl acetate, lavandulol, geraniol, and bornyl acetate | [48] |
| Basil essential oil | Ocimum Lamiifolium | Leaves | Methyl chavicol, linalool, methyl eugenol, and methyl cinnamate | [49] |
| Eucalyptus essential oil | Eucalyptus maideni, E. astrengens, E. cinerea, E. leucoxylon | Leaves | 1,8-cineol and α-pinene | [50] |
| Clove bud essential oil | Eugenia caryophyllata | Clove bud | Eugenol, eugenol acetate, and β-caryophyllene | [51] |
| Pine needle essential oil | Cedrus deodara | Pine needle | α-terpineol, linalool, limonene, anethole, caryophyllene, and eugenol | [52] |
| Lemongrass essential oil | Cymbopogon citratus | Leaves | Geranial, neral, and myrcene | [53] |
| Cinnamon bark essential oil | Cinnamomum altissimum | Bark | Linalool, methyl eugenol, limonene, α-terpineol, and terpinen-4-ol | [54] |
| Rosemary essential oil | Rosmarinus officinalis | Leaves | α-pinene, 1,8-cineole, and camphor | [43,55] |
| Black pepper essential oil | Piper nigrum | Fruit | α-pinene, sabinene, β-pinene, δ-3-carene, limonene, and β-caryophyllene | [43,56] |
| Peppermint essential oil | Mentha piperita | Leaves | Menthol and menthone | [5] |
| Polysaccharide(s) Used | Essential Oil | Functional Activities of the Films | Food Product Analyzed | Reference |
|---|---|---|---|---|
| Okara soluble dietary fiber, pectin, sodium carboxymethyl cellulose | Thyme essential oil | Free radical scavenging, antibacterial activity (e.g., Escherichia coli and Staphylococcus aureus) | -- | [14] |
| Chitosan | Clove essential oil | Antimicrobial activity (e.g., Escherichia coli and Staphylococcus aureus) | -- | [11] |
| Chitosan | Cumin essential oil | Antimicrobial activity (e.g., Listeria monocytogene) | Beef loins | [66] |
| Chitosan | Garlic essential oil | Free radical scavenging, antibacterial activity (e.g., Staphylococcus aureus) | Sausages | [67] |
| Carboxymethyl cellulose, chitosan biguanidine hydrochloride | Frankincense essential oil | Antimicrobial activity (e.g., Streptococcus pneumoniae, Bacillus subtilis, Escherichia coli) | -- | [16] |
| Chitosan, gum arabic | Cinnamon essential oil | Free radical scavenging | -- | [68] |
| Shahri Balangu seed mucilage | Cumin essential oil | Antimicrobial activity (e.g., Escherichia coli and Staphylococcus aureus) | Beef slices | [69] |
| Fully deacetylated chitosan | Clove essential oil | Antimicrobial activity | White prawn shrimp | [70] |
| Basil-seed gum | Oregano essential oil | Free radical scavenging, antibacterial activity (e.g., Escherichia coli, Staphylococcus aureus,Salmonella typhimurium, Pseudomonas aeruginosa, Bacillus cereus) | -- | [71] |
| Chitosan, pectin | Rosemary essential oil | Antimicrobial activity (e.g., Bacillus subtilis, Staphylococcus aureus, Enterococcus aerogenes, Enterococcus faecalis, Escherichia coli) | -- | [72] |
| Alginate–apple puree edible film | Oregano oil, Cinnamon oil and Lemongrass oil | Antimicrobial activity (e.g., Escherichia coli) | -- | [73] |
| Chitosan and cashew gum | Lippia sidoides oil | Larvicide (e.g., Aedes aegypti) | -- | [74] |
| Cellulosic films | Cinnamon, Oregano and Lemongrass EO | Antifungal activity | -- | [75] |
| Carboxymethyl cellulose | Garlic essential oil | -- | Strawberries | [76] |
| Gelatin–Carboxymethyl Cellulose Film | Essential Oil of Bane | Antimicrobial activity (e.g., Escherichia. coli, Salmonella enterica, Staphylococcus aureus, and Clostridium sporogenes) | -- -- | [77] |
| Carboxymethyl cellulose | Essential oils from Coriander, Rosemary and Nutmeg | -- | -- | [78] |
| Chitosan | Thyme essential oil | Antimicrobial activity (e.g., Pectobacterium carotovorum) | Bell Pepper | [79] |
| Sago Starch | Cinnamon essential oil | Antimicrobial activity (e.g., Escherichia coli, Salmonella typhimurium, and Staphylococcus aureus) | -- | [80] |
| Chitosan | Citrus limonia essential oil | Antimicrobial activity (e.g., Staphylococcus aureus) | -- | [81] |
| Dandelion polysaccharide | Litsea cubeba essential oil | Antimicrobial activity (e.g., Staphylococcus aureus) | -- | [82] |
| Soybean polysaccharide | Ataria multiflora Boiss and Mentha pulegium essential oils | Free radical scavenging, antibacterial activity(e.g., Staphylococcus aureus, Bacillus cereus, Escherichia coli, Pseudomonas aeruginosa) | -- | [83] |
| Hydroxypropyl methylcellulose | Oregano and bergamot essential oils | Antimicrobial activity (e.g., Escherichia coli) | Fresh ‘Formosa’ Plum | [84] |
| Sodium alginate | Garlic oil | Antimicrobial activity (e.g., Escherichia coli, Salmonella typhimurium, Staphylococcus aureus and Bacillus cereus) | -- | [85] |
| Cassava starch | Cinnamon essential oil | Antimicrobial activity (e.g., Escherichia coli, Salmonella typhimurium, Staphylococcus aureus) | -- | [86] |
| Sodium alginate | Thyme, lemongrass and sage essential oil | Antimicrobial activity (e.g., Escherichia coli) | -- | [61] |
| Millet Starch | Clove Essential Oil | Antimicrobial activity (e.g., Escherichia coli, Pseudomonas aeruginosa, Enterobacter spp., Bacillus cereus, Staphylococcus aureus) | -- | [87] |
| Chitosan | Clove essential oil | Antimicrobial activity (e.g., Pseudomonas spp. Enterobacteriaceae) | Pork Patties | [88] |
| Chitosan | Ginger Essential Oil | Free radical scavenging, Antimicrobial activity (e.g., Bacillus cereus, Enterococcus faecalis, Listeria monocytogenes, Staphylococcus aureus, Escherichia coli, Pseudomonas aeruginosa, Salmonella enterica, Candida albicans) | [89] | |
| Chitosan | Limonene essential oil | Antimicrobial activity | Cucumber | [90] |
| Chitosan/CMC | Mentha spicata essential oil | Antibacterial activity (e.g., Listeria monocytogenes) | Strawberries | [91] |
| Alginate/CMC | Ziziphora clinopodioides essential oil | Antibacterial activity (e.g., Listeria monocytogenes) | Carp fillets | [92] |
| Starch | Clove leaf oil | Antioxidant activities and Antibacterial activity (e.g., Listeria monocytogenes) | Cheese | [93] |
| Banana flour (starch) | Garlic essential oil | Antioxidant activities and Antibacterial activity (e.g., Aspergillus flavus) | Peanuts (roasted) | [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Anis, A.; Pal, K.; Al-Zahrani, S.M. Essential Oil-Containing Polysaccharide-Based Edible Films and Coatings for Food Security Applications. Polymers 2021, 13, 575. https://doi.org/10.3390/polym13040575
Anis A, Pal K, Al-Zahrani SM. Essential Oil-Containing Polysaccharide-Based Edible Films and Coatings for Food Security Applications. Polymers. 2021; 13(4):575. https://doi.org/10.3390/polym13040575
Chicago/Turabian StyleAnis, Arfat, Kunal Pal, and Saeed M. Al-Zahrani. 2021. "Essential Oil-Containing Polysaccharide-Based Edible Films and Coatings for Food Security Applications" Polymers 13, no. 4: 575. https://doi.org/10.3390/polym13040575