Gas Separation by Mixed Matrix Membranes with Porous Organic Polymer Inclusions within o-Hydroxypolyamides Containing m-Terphenyl Moieties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Monomers Synthesis
2.2.1. Synthesis of 4-Carboxy-Phenylboronic Acid
2.2.2. Synthesis of (5′-Terbuthyl-m-terphenyl-4,4′′-dicarboxylic acid (p))
2.2.3. Synthesis of tBTpCl Dichloride
2.3. Polymers Synthesis
2.4. Casting Of Polymer Films
2.4.1. Films of Polymer Matrixes
2.4.2. Preparation of Mixed Matrix Membranes
2.5. Thermal Rearrangement
2.6. Characterization
2.6.1. Polymer Characterization
2.6.2. Thermogravimetric Analysis
2.6.3. DSC
2.6.4. Fourier Transform Infrared Spectroscopy
2.6.5. Density Measurements
2.6.6. WAXS
2.6.7. Mechanical Properties
2.6.8. Gas Transport: Permeability and Selectivity
3. Results and Discussion
3.1. Chemical Properties
3.1.1. Characterization of Polymer Matrixes
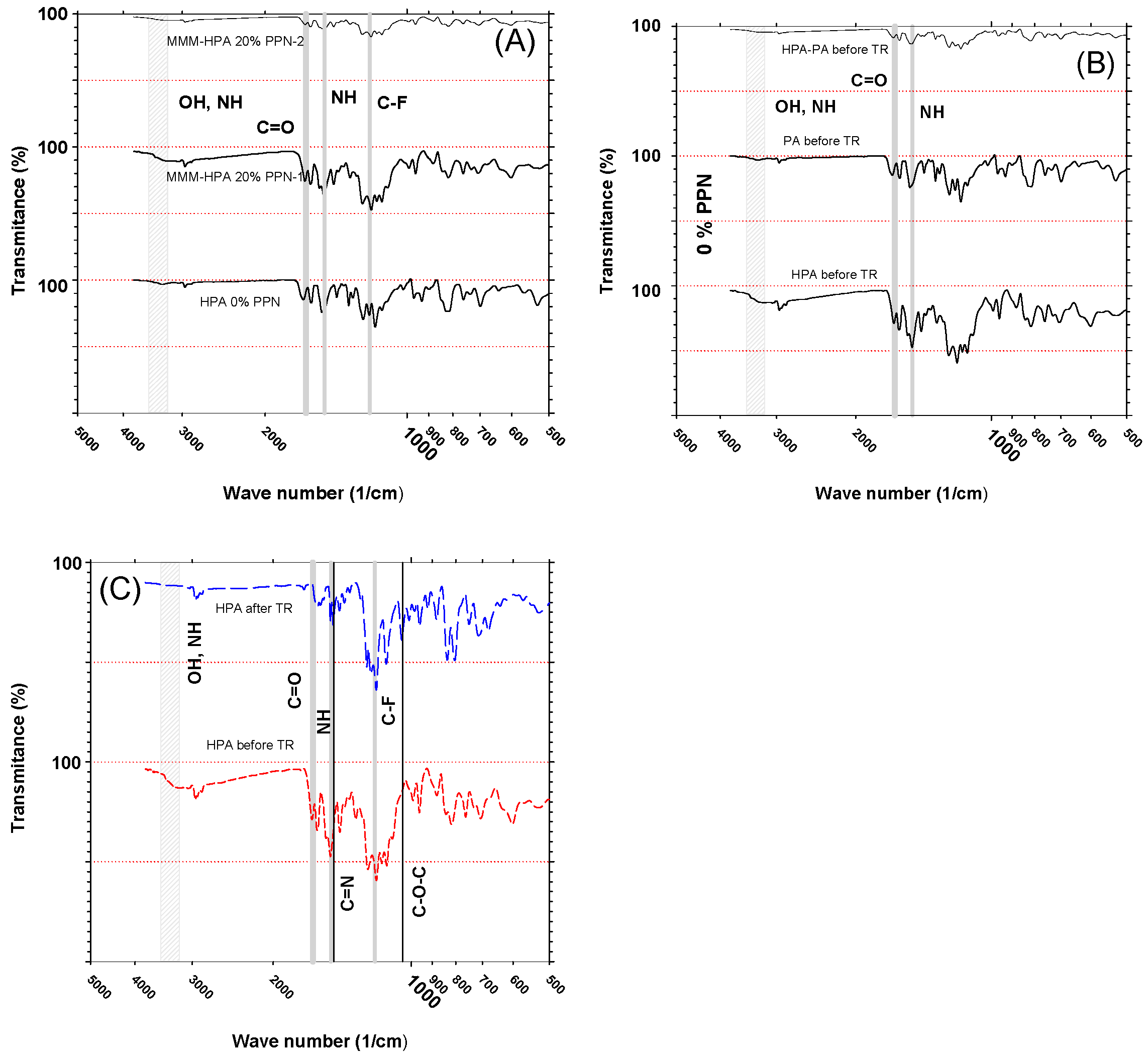
3.1.2. Infrared Spectroscopy (FTIR) Measurements
3.2. Thermal Properties
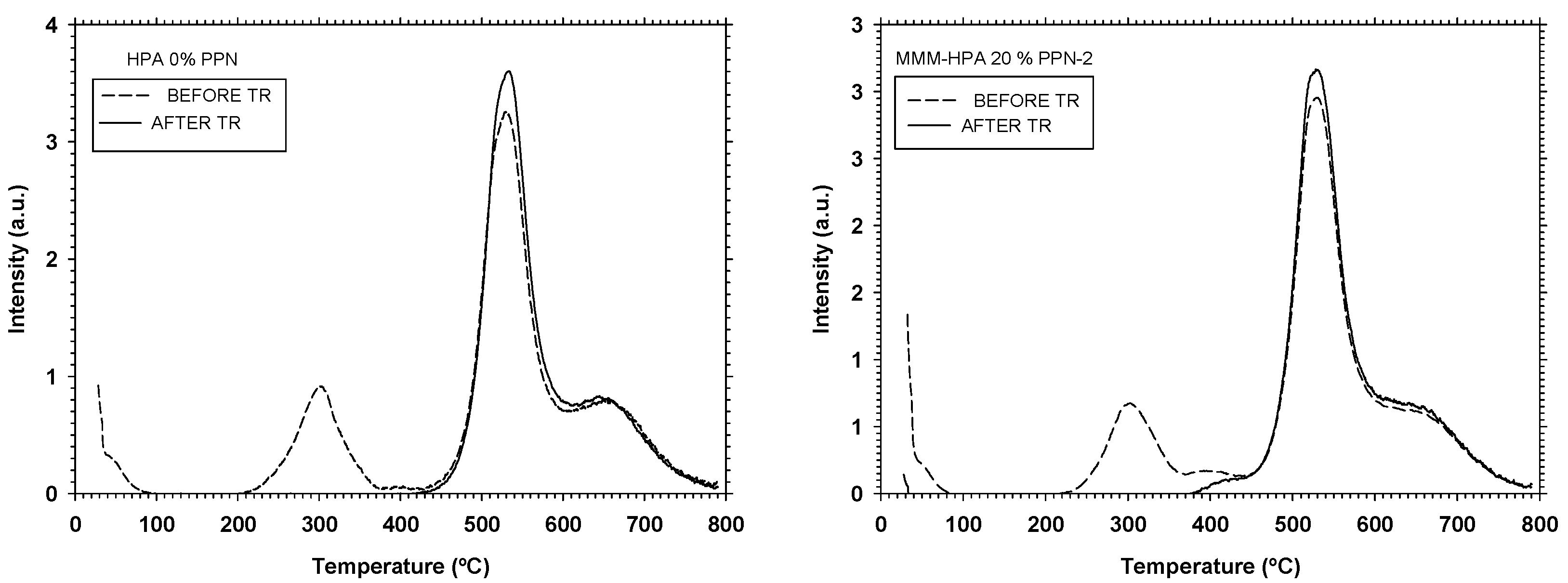
3.2.1. Thermogravimetric Analysis
3.2.2. DSC for Glass Transition Temperatures
3.3. Mechanical Properties
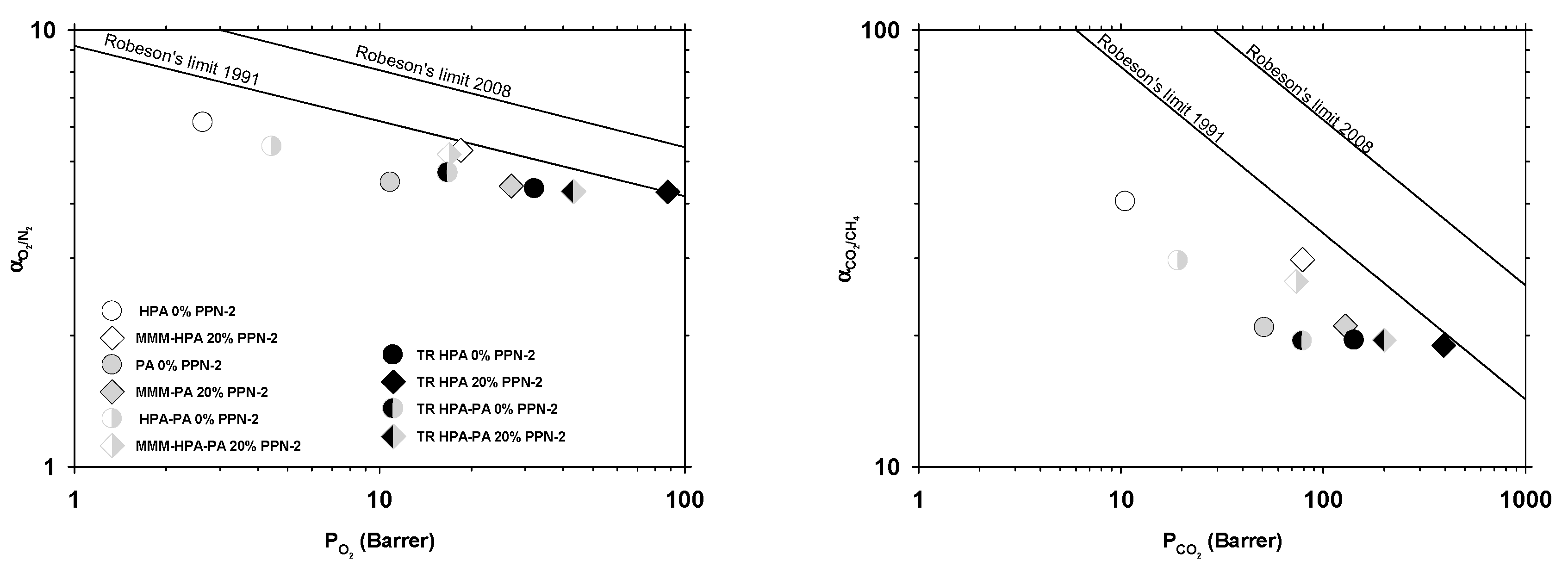
3.4. Gas Separation Properties
3.5. Morphology of the MMMs
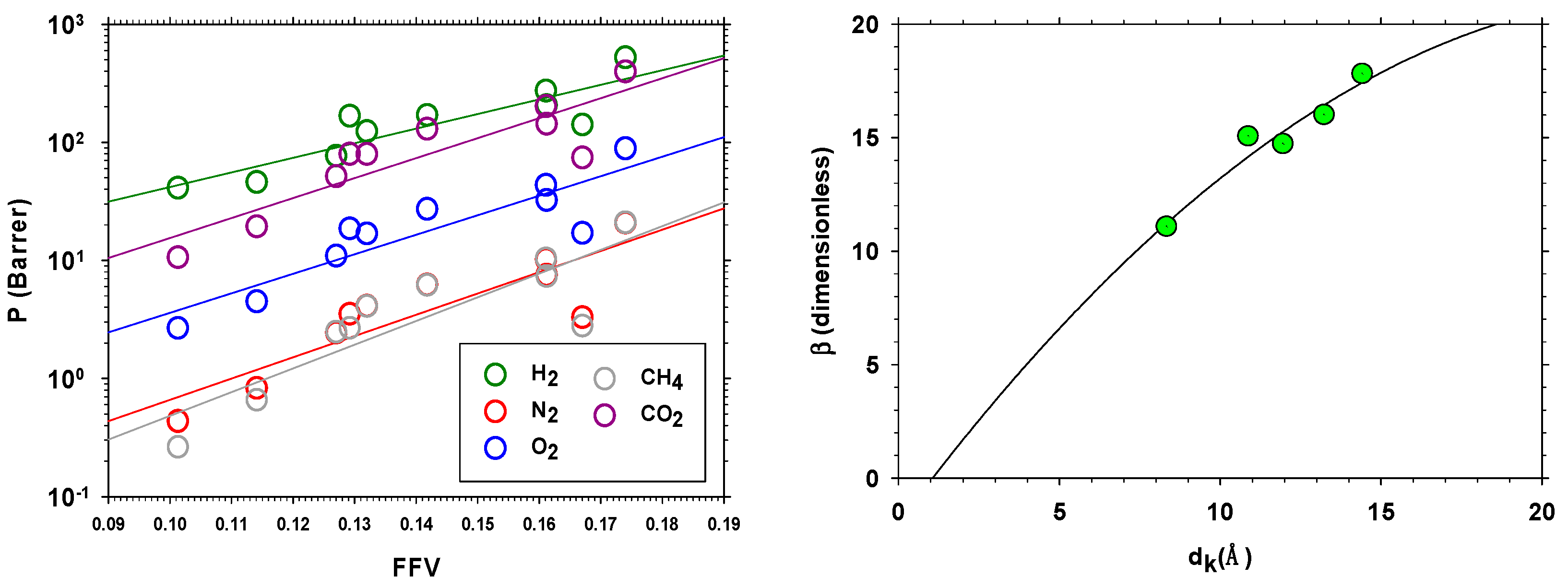
3.5.1. Density and Fractional Free Volume (FFV)
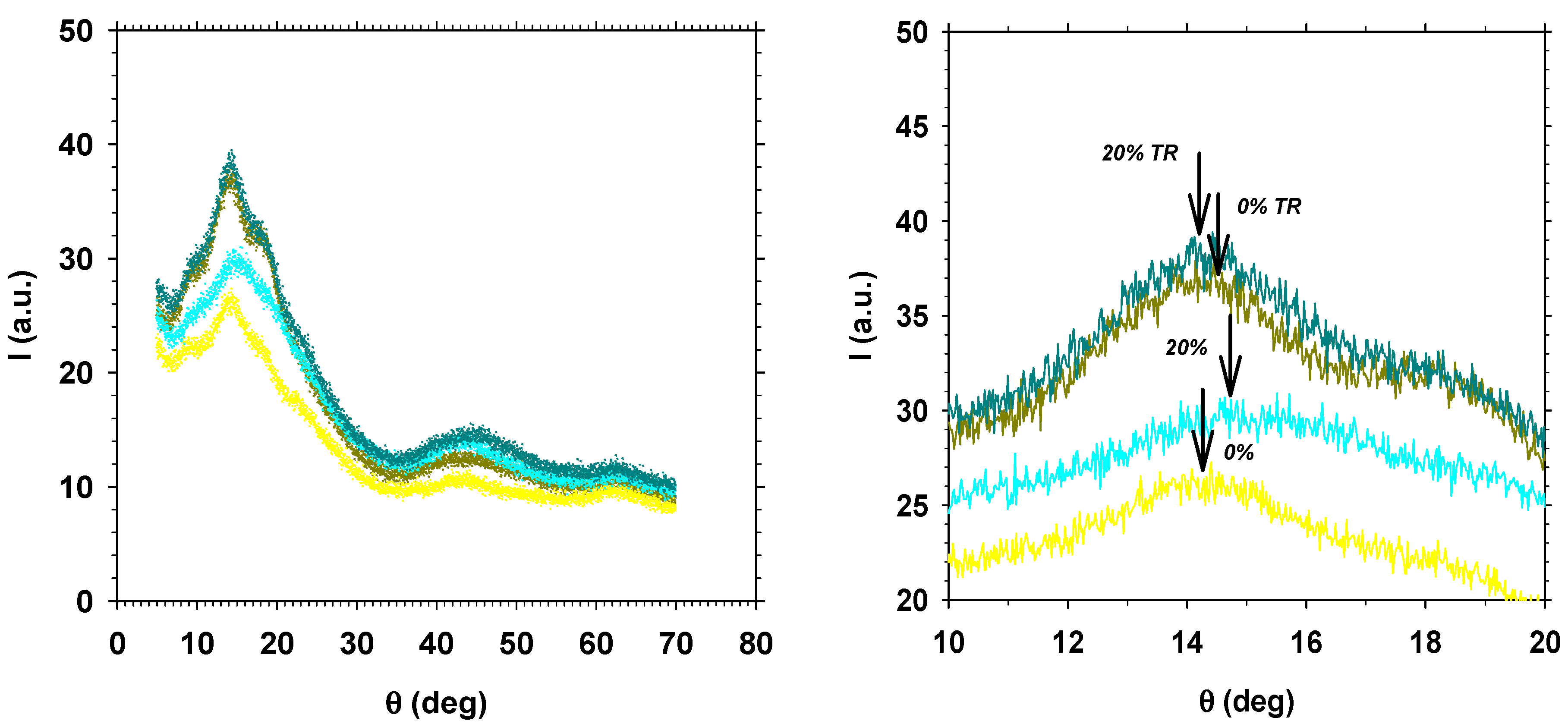
3.5.2. WAXD Intersegmental Distance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kim, J.S.; Moon, S.J.; Wang, H.H.; Kim, S.; Lee, Y.M. Mixed matrix membranes with a thermally rearranged polymer and ZIF-8 for hydrogen separation. J. Membr. Sci. 2019, 582, 381–390. [Google Scholar] [CrossRef]
- Al-Mufachi, N.; Rees, N.; Steinberger-Wilkens, R. Hydrogen selective membranes: A review of palladium-based dense metal membranes. Renew. Sustain. Energy Rev. 2015, 47, 540–551. [Google Scholar] [CrossRef]
- IEA. The Future of Hydrogen. Paris. Available online: https://www.iea.org/reports/the-future-of-hydrogen (accessed on 11 February 2020).
- Glenk, G.; Reichelstein, S. Economics of converting renewable power to hydrogen. Nat. Energy 2019, 4, 216–222. [Google Scholar] [CrossRef]
- Kim, S.; Shamsaei, E.; Lin, X.; Hu, Y.; Simon, G.P.; Seong, J.G.; Kim, J.S.; Lee, W.H.; Lee, Y.M.; Wang, H. The enhanced hydrogen separation performance of mixed matrix membranes by incorporation of two-dimensional ZIF-L into polyimide containing hydroxyl group. J. Membr. Sci. 2018, 549, 260–266. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, Q.; Zhu, L.; Lin, H.; Kazanowska, B.A.; Doherty, C.M.; Hill, A.J.; Gao, P.; Guo, R. Highly Selective and Permeable Microporous Polymer Membranes for Hydrogen Purification and CO2 Removal from Natural Gas. Chem. Mater. 2018, 30, 5322–5332. [Google Scholar] [CrossRef]
- Bakonyi, P.; Kumar, G.; Nemestóthy, N.; Lin, C.; Bélafi-Bakó, K. Biohydrogen purification using a commercial polyimide membrane module: Studying the effects of some process variables. Int. J. Hydrogen Energy 2013, 38, 15092–15099. [Google Scholar] [CrossRef] [Green Version]
- Kumar, G.; Bakonyi, P.; Kobayashi, T.; Xu, K.-Q.; Sivagurunathan, P.; Kim, S.-H.; Buitrón, G.; Nemestóthy, N.; Bélafi-Bakó, K. Enhancement of biofuel production via microbial augmentation: The case of dark fermentative hydrogen. Renew. Sustain. Energy Rev. 2016, 57, 879–891. [Google Scholar] [CrossRef]
- Schorer, L.; Schmitz, S.; Weber, A. Membrane based purification of hydrogen system (MEMPHYS). Int. J. Hydrog. Energy 2019, 44, 12708–12714. [Google Scholar] [CrossRef]
- Li, P.; Wang, Z.; Qiao, Z.; Liu, Y.; Cao, X.; Li, W.; Wang, J.; Wang, S. Recent developments in membranes for efficient hydrogen purification. J. Membr. Sci. 2015, 495, 130–168. [Google Scholar] [CrossRef]
- Dechnik, J.; Gascon, J.; Doonan, C.J.; Janiak, C.; Sumby, C.J. Mixed-Matrix Membranes. Angew. Chem. Int. Ed. 2017, 56, 9292–9310. [Google Scholar] [CrossRef]
- Vinoba, M.; Bhagiyalakshmi, M.; Alqaheem, Y.; Alomair, A.A.; Pérez, A.; Rana, M.S. Recent progress of fillers in mixed matrix membranes for CO2 separation: A review. Sep. Purif. Technol. 2017, 188, 431–450. [Google Scholar] [CrossRef]
- Chung, T.-S.; Jiang, L.Y.; Li, Y.; Kulprathipanja, S. Mixed matrix membranes (MMMs) comprising organic polymers with dispersed inorganic fillers for gas separation. Prog. Polym. Sci. 2007, 32, 483–507. [Google Scholar] [CrossRef]
- Etxeberria-Benavides, M.; David, O.; Johnson, T.; Łozińska, M.M.; Orsi, A.; Wright, P.A.; Mastel, S.; Hillenbrand, R.; Kapteijn, F.; Gascon, J. High performance mixed matrix membranes (MMMs) composed of ZIF-94 filler and 6FDA-DAM polymer. J. Membr. Sci. 2018, 550, 198–207. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Jiang, Z.; Wu, Y.; Zhang, H.; Cheng, Y.; Guo, R.; Wu, H. High-performance composite membranes incorporated with carboxylic acid nanogels for CO2 separation. J. Membr. Sci. 2015, 495, 72–80. [Google Scholar] [CrossRef]
- Smith, S.J.; Hou, R.; Lau, C.H.; Konstas, K.; Kitchin, M.; Dong, G.; Lee, J.; Lee, W.H.; Seong, J.G.; Lee, Y.M.; et al. Highly permeable Thermally Rearranged Mixed Matrix Membranes (TR-MMM). J. Membr. Sci. 2019, 585, 260–270. [Google Scholar] [CrossRef]
- Soto, C.; Lugo, C.A.; Rodríguez, S.; Palacio, L.; Lozano, Á.E.; Prádanos, P.; Hernandez, A. Enhancement of CO2/CH4 permselectivity via thermal rearrangement of mixed matrix membranes made from an o-hydroxy polyamide with an optimal load of a porous polymer network. Sep. Purif. Technol. 2020, 247, 116895. [Google Scholar] [CrossRef]
- Han, S.H.; Kwon, H.J.; Kim, K.Y.; Seong, J.G.; Park, C.H.; Kim, S.; Doherty, C.M.; Thornton, A.W.; Hill, A.J.; Lozano, Á.E.; et al. Tuning microcavities in thermally rearranged polymer membranes for CO2 capture. Phys. Chem. Chem. Phys. 2012, 14, 4365–4373. [Google Scholar] [CrossRef]
- Cheng, Y.; Ying, Y.; Japip, S.; Jiang, S.-D.; Chung, T.-S.; Zhang, S.; Zhao, D. Advanced Porous Materials in Mixed Matrix Membranes. Adv. Mater. 2018, 30, 1802401. [Google Scholar] [CrossRef]
- Lin, R.; Hernandez, B.V.; Ge, L.; Zhu, Z. Metal organic framework based mixed matrix membranes: An overview on filler/polymer interfaces. J. Mater. Chem. A 2018, 6, 293–312. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted Synthesis of a Porous Aromatic Framework with High Stability and Exceptionally High Surface Area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef] [PubMed]
- Lau, C.H.; Mulet, X.; Konstas, K.; Doherty, C.M.; Sani, M.-A.; Separovic, F.; Hill, M.R.; Wood, C.D. Hypercrosslinked Additives for Ageless Gas-Separation Membranes. Angew. Chem. Int. Ed. 2016, 55, 1998–2001. [Google Scholar] [CrossRef]
- Aguilar-Lugo, C.; Suárez-García, F.; Hernández, A.; Miguel, J.A.; Lozano, Á.E.; de la Campa, J.G.; Álvarez, C. New Materials for Gas Separation Applications: Mixed Matrix Membranes Made from Linear Polyimides and Porous Polymer Networks Having Lactam Groups. Ind. Eng. Chem. Res. 2019, 58, 9585–9595. [Google Scholar] [CrossRef]
- Lopez-Iglesias, B.; Suárez-García, F.; Aguilar-Lugo, C.; Ortega, A.G.; Bartolomé, C.; Martínez-Ilarduya, J.M.; de la Campa, J.G.; Lozano, Á.E.; Álvarez, C. Microporous Polymer Networks for Carbon Capture Applications. ACS Appl. Mater. Interfaces 2018, 10, 26195–26205. [Google Scholar] [CrossRef] [PubMed]
- García, C.; Lozano, A.E.; de la Campa, J.G.; de Abajo, J. Soluble Polyimides from a New Dianhydride: 5′-tert-Butyl-m-terphenyl-3,4,3′′,4′′-tetracarboxylic Acid Dianhydride. Macromol. Rapid Commun. 2003, 24, 686–691. [Google Scholar] [CrossRef]
- García, C.; Tiemblo, P.; Lozano, A.E.; de Abajo, J.; de la Campa, J.G. Gas separation properties of new poly(aryl ether ketone)s with pendant groups. J. Membr. Sci. 2002, 205, 73–81. [Google Scholar] [CrossRef]
- Bermejo, L.A.; Alvarez, C.; Maya, E.M.; García, C.; de la Campa, J.G.; Lozano, A.E. Synthesis, characterization and gas separation properties of novel polyimides containing cardo and tert-butyl-m-terphenyl moieties. Express Polym. Lett. 2018, 12, 479–489. [Google Scholar] [CrossRef]
- de Abajo, J.; de la Campa, J.G.; Lozano, A.E. Designing aromatic polyamides and polyimides for gas separation membranes. Macromol. Symp. 2003, 199, 293–306. [Google Scholar] [CrossRef]
- Klotz, E.J.F.; Claridge, T.D.W.; Anderson, H.L. Homo- and Hetero-[3]Rotaxanes with Two π-Systems Clasped in a Single Macrocycle. J. Am. Chem. Soc. 2006, 128, 15374–15375. [Google Scholar] [CrossRef]
- Smith, Z.P.; Czenkusch, K.; Wi, S.; Gleason, K.L.; Hernández, G.; Doherty, C.M.; Konstas, K.; Bastow, T.J.; Álvarez, C.; Hill, A.J.; et al. Investigation of the chemical and morphological structure of thermally rearranged polymers. Polymer 2014, 55, 6649–6657. [Google Scholar] [CrossRef] [Green Version]
- Miyaura, N.; Suzuki, A. Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds. Chem. Rev. 1995, 95, 2457–2483. [Google Scholar] [CrossRef] [Green Version]
- Liao, D.C.; Hsieh, K.H. Synthesis and characterization of bismaleimides derived from polyurethanes. J. Polym. Sci. Part A Polym. Chem. 1994, 32, 1665–1672. [Google Scholar] [CrossRef]
- Smith, Z.P.; Sanders, D.F.; Ribeiro, C.P.; Guo, R.; Freeman, B.D.; Paul, D.R.; McGrath, J.E.; Swinnea, S. Gas sorption and characterization of thermally rearranged polyimides based on 3,3′-dihydroxy-4,4′-diamino-biphenyl (HAB) and 2,2′-bis-(3,4-dicarboxyphenyl) hexafluoropropane dianhydride (6FDA). J. Membr. Sci. 2012, 415–416, 558–567. [Google Scholar] [CrossRef]
- Muñoz, D.M.; Calle, M.; de la Campa, J.G.; de Abajo, J.; Lozano, A.E. An Improved Method for Preparing Very High Molecular Weight Polyimides. Macromolecules 2009, 42, 5892–5894. [Google Scholar] [CrossRef]
- Muñoz, D.M.; de la Campa, J.G.; de Abajo, J.; Lozano, A.E. Experimental and Theoretical Study of an Improved Activated Polycondensation Method for Aromatic Polyimides. Macromolecules 2007, 40, 8225–8232. [Google Scholar] [CrossRef]
- Lozano, A.E.; de Abajo, J.; de la Campa, J.G. Synthesis of Aromatic Polyisophthalamides by in Situ Silylation of Aromatic Diamines. Macromolecules 1997, 30, 2507–2508. [Google Scholar] [CrossRef]
- Lozano, A.E.; de Abajo, J.; De la Campa, J.G. Quantum semiempirical study on the reactivity of silylated diamines in the synthesis of aromatic polyamides. Macromol. Theory Simul. 1998, 7, 41–48. [Google Scholar] [CrossRef]
- Comesaña-Gándara, B.; Calle, M.; Jo, H.J.; Hernández, A.; de la Campa, J.G.; de Abajo, J.; Lozano, A.E.; Lee, Y.M. Thermally rearranged polybenzoxazoles membranes with biphenyl moieties: Monomer isomeric effect. J. Membr. Sci. 2014, 450, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Sanders, D.F.; Smith, Z.P.; Ribeiro, C.P.; Guo, R.; McGrath, J.E.; Paul, D.R.; Freeman, B.D. Gas permeability, diffusivity, and free volume of thermally rearranged polymers based on 3,3′-dihydroxy-4,4′-diamino-biphenyl (HAB) and 2,2′-bis-(3,4-dicarboxyphenyl) hexafluoropropane dianhydride (6FDA). J. Membr. Sci. 2012, 409–410, 232–241. [Google Scholar] [CrossRef]
- Wiederhorn, S.; Fields, R.; Low, S.; Bahng, G.-W.; Wehrstedt, A.; Hahn, J.; Tomota, Y.; Miyata, T.; Lin, H.; Freeman, B.; et al. Mechanical Properties. In Handbook of Materials Measurement Methods; Czichos, H., Saito, T., Smith, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Smith, Z.P.; Hernández, G.; Gleason, K.L.; Anand, A.; Doherty, C.M.; Konstas, K.; Alvarez, C.; Hill, A.J.; Lozano, A.E.; Paul, D.R.; et al. Effect of polymer structure on gas transport properties of selected aromatic polyimides, polyamides and TR polymers. J. Membr. Sci. 2015, 493, 766–781. [Google Scholar] [CrossRef] [Green Version]
- Díez, B.; Cuadrado, P.; Marcos-Fernández, Á.; de la Campa, J.G.; Tena, A.; Prádanos, P.; Palacio, L.; Lee, Y.M.; Alvarez, C.; Lozano, Á.E.; et al. Thermally rearranged polybenzoxazoles made from poly(ortho-hydroxyamide)s. Characterization and evaluation as gas separation membranes. React. Funct. Polym. 2018, 127, 38–47. [Google Scholar] [CrossRef]
- Wang, H.; Chung, T.-S.; Paul, D. Thickness dependent thermal rearrangement of an ortho-functional polyimide. J. Membr. Sci. 2014, 450, 308–312. [Google Scholar] [CrossRef]
- Park, H.B.; Jung, C.H.; Lee, Y.M.; Hill, A.J.; Pas, S.J.; Mudie, S.T.; Van Wagner, E.; Freeman, B.D.; Cookson, D.J. Polymers with Cavities Tuned for Fast Selective Transport of Small Molecules and Ions. Science 2007, 318, 254. [Google Scholar] [CrossRef]
- Galizia, M.; Chi, W.S.; Smith, Z.P.; Merkel, T.C.; Baker, R.W.; Freeman, B.D. 50th Anniversary Perspective: Polymers and Mixed Matrix Membranes for Gas and Vapor Separation: A Review and Prospective Opportunities. Macromolecules 2017, 50, 7809–7843. [Google Scholar] [CrossRef]
- Park, H.B.; Han, S.H.; Jung, C.H.; Lee, Y.M.; Hill, A.J. Thermally rearranged (TR) polymer membranes for CO2 separation. J. Membr. Sci. 2010, 359, 11–24. [Google Scholar] [CrossRef]
- Rezakazemi, M.; Amooghin, A.E.; Montazer-Rahmati, M.M.; Ismail, A.F.; Matsuura, T. State-of-the-art membrane based CO2 separation using mixed matrix membranes (MMMs): An overview on current status and future directions. Prog. Polym. Sci. 2014, 39, 817–861. [Google Scholar] [CrossRef]
- Robeson, L.M. Correlation of separation factor versus permeability for polymeric membranes. J. Membr. Sci. 1991, 62, 165–185. [Google Scholar] [CrossRef]
- Robeson, L.M. The upper bound revisited. J. Membr. Sci. 2008, 320, 390–400. [Google Scholar] [CrossRef]
- Robeson, L.; Burgoyne, W.; Langsam, M.; Savoca, A.; Tien, C. High performance polymers for membrane separation. Polymer 1994, 35, 4970–4978. [Google Scholar] [CrossRef]
- Brunetti, A.; Cersosimo, M.; Kim, J.S.; Dong, G.; Fontananova, E.; Lee, Y.M.; Drioli, E.; Barbieri, G. Thermally rearranged mixed matrix membranes for CO 2 separation: An aging study. Int. J. Greenh. Gas Control. 2017, 61, 16–26. [Google Scholar] [CrossRef]
- Thornton, A.W.; Nairn, K.M.; Hill, A.J.; Hill, J.M. New relation between diffusion and free volume: I. Predicting gas diffusion. J. Membr. Sci. 2009, 338, 29–37. [Google Scholar] [CrossRef]
- Matteucci, S.; Yampolskii, Y.; Freeman, B.D.; Pinnau, I. Transport of Gases and Vapors in Glassy and Rubbery Polymers. In Materials Science of Membranes for Gas and Vapor Separation; Yampolskii, Y., Pinnau, I., Freeman, B.D., Eds.; John Wiley & Sons: Berlin, Germany, 2006; pp. 1–47. [Google Scholar]
- Teplyakov, V.V.; Durgar’yan, S.G. Correlation analysis of the gas permeability parameters of polymers. Polym. Sci. USSR 1984, 26, 1678–1688. [Google Scholar] [CrossRef]
- Breck, D.W. Zeolite Molecular Sieves: Structure, Chemistry and Use; John Wiley & Sons: New York, NY, USA, 1974. [Google Scholar]
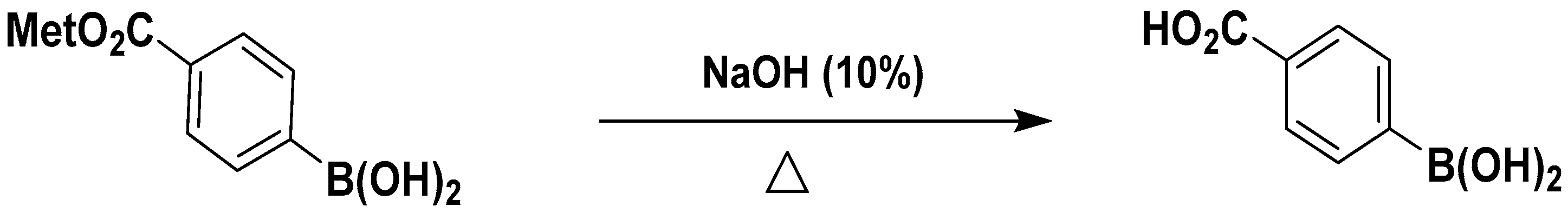
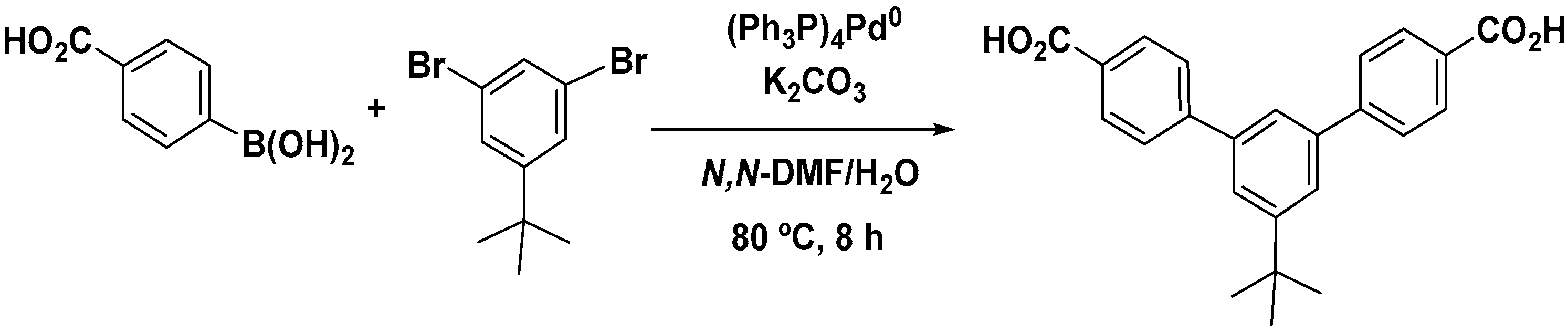






| Membrane | Maximum Stress (MPa) | Elongation at Break (%) | Young’s Modulus (GPa) |
|---|---|---|---|
| HPA | 103.3 ± 4.4 | 9.7 ± 0.4 | 2.9 ± 0.2 |
| TR-HPA | 79.2 ± 9.2 | 9.2 ± 0.5 | 1.8 ± 0.3 |
| MMM-HPA | 30.5 ± 2.0 | 9.2 ± 0.7 | 1.7 ± 0.1 |
| TR-MMM-HPA | 37.7 ± 9.9 | 9.0 ± 0.3 | 1.2 ± 0.1 |
| PA | 89.8 ± 3.8 | 10.1 ± 0.6 | 2.4 ± 0.09 |
| MMM-PA | 32.2 ± 10.5 | 7.6 ± 2.8 | 1.6 ± 0.097 |
| HPA-PA | 90.2 ± 14.2 | 9.974 ± 0.3 | 2.609 ± 0.3 |
| TR-HPA-PA | n.a. | n.a. | n.a. |
| MMM-HPA-PA | 37.4 ± 5.2 | 9.648 ± 0.4 | 1.907 ± 0.2 |
| TR-MMM-HPA-PA | 43.96 ± 8.00 | 9.778 ± 0.2 | 1.475 ± 0.1 |
| Membrane | Permeabilities (Barrer 1) | ||||
|---|---|---|---|---|---|
| H2 | N2 | O2 | CH4 | CO2 | |
| Non-TR materials | |||||
| HPA | 40.84 | 0.43 | 2.64 | 0.26 | 10.54 |
| MMM-HPA | 166.02 | 3.48 | 18.46 | 2.65 | 79.0 |
| PA | 76.14 | 2.42 | 10.85 | 2.46 | 51.23 |
| MMM-PA | 168.65 | 6.15 | 27.02 | 6.12 | 128.7 |
| HPA-PA | 45.65 | 0.82 | 4.44 | 0.65 | 19.18 |
| MMM-HPA-PA | 139.8 | 3.26 | 16.92 | 2.77 | 73.55 |
| TR materials | |||||
| TR-HPA | 122.9 | 4.07 | 16.71 | 4.06 | 78.8 |
| TR-MMM-HPA | 271.5 | 10.10 | 43.15 | 10.29 | 200.38 |
| TR-HPA-PA | 203.7 | 7.43 | 32.17 | 7.29 | 142.1 |
| TR-MMM-HPA-PA | 518.6 | 20.65 | 87.97 | 20.80 | 394.16 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soto, C.; Torres-Cuevas, E.S.; González-Ortega, A.; Palacio, L.; Lozano, Á.E.; Freeman, B.D.; Prádanos, P.; Hernández, A. Gas Separation by Mixed Matrix Membranes with Porous Organic Polymer Inclusions within o-Hydroxypolyamides Containing m-Terphenyl Moieties. Polymers 2021, 13, 931. https://doi.org/10.3390/polym13060931
Soto C, Torres-Cuevas ES, González-Ortega A, Palacio L, Lozano ÁE, Freeman BD, Prádanos P, Hernández A. Gas Separation by Mixed Matrix Membranes with Porous Organic Polymer Inclusions within o-Hydroxypolyamides Containing m-Terphenyl Moieties. Polymers. 2021; 13(6):931. https://doi.org/10.3390/polym13060931
Chicago/Turabian StyleSoto, Cenit, Edwin S. Torres-Cuevas, Alfonso González-Ortega, Laura Palacio, Ángel E. Lozano, Benny D. Freeman, Pedro Prádanos, and Antonio Hernández. 2021. "Gas Separation by Mixed Matrix Membranes with Porous Organic Polymer Inclusions within o-Hydroxypolyamides Containing m-Terphenyl Moieties" Polymers 13, no. 6: 931. https://doi.org/10.3390/polym13060931











