Physical Aging Behavior of a Glassy Polyether
Abstract
:1. Introduction
2. Materials and Methods
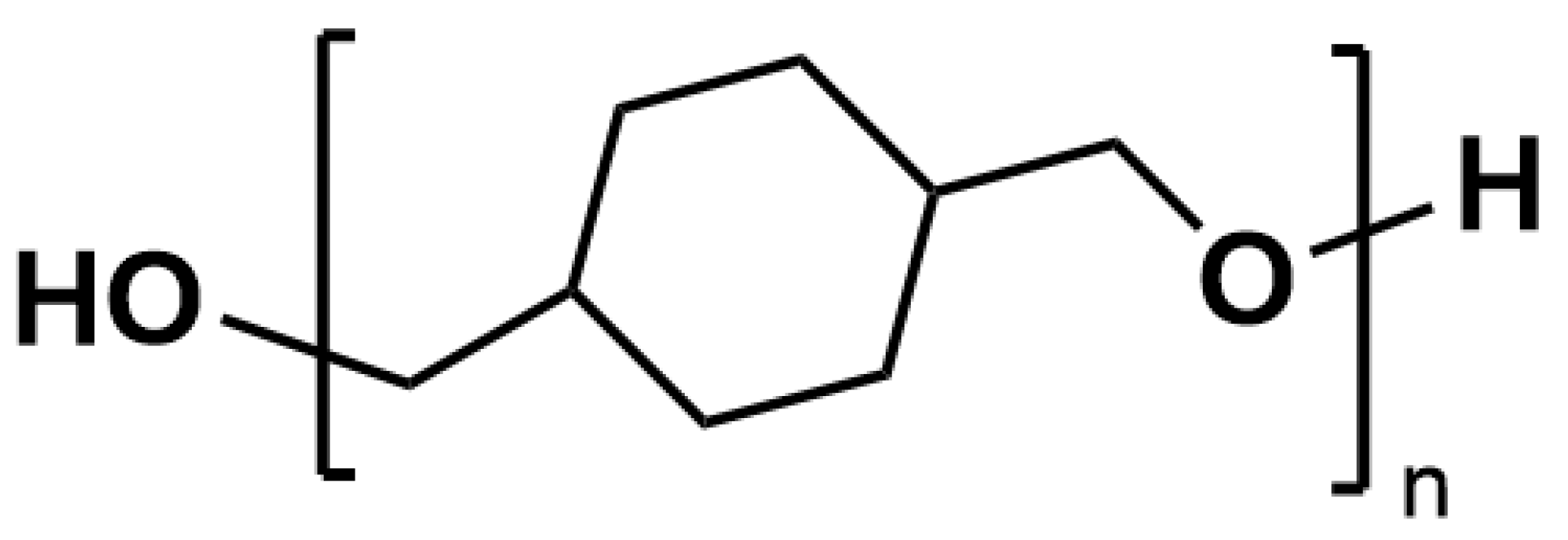
2.1. Materials
2.2. Methods
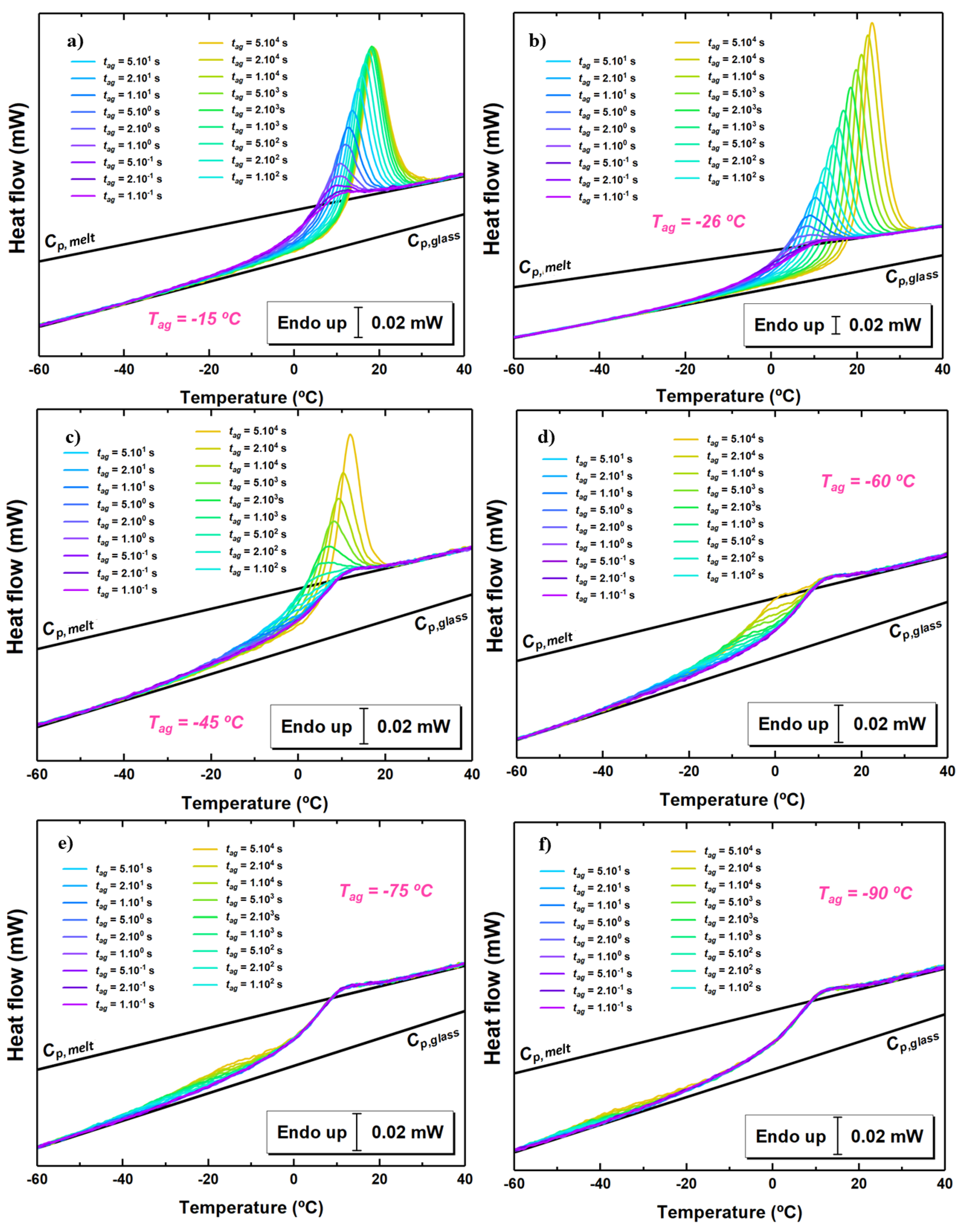
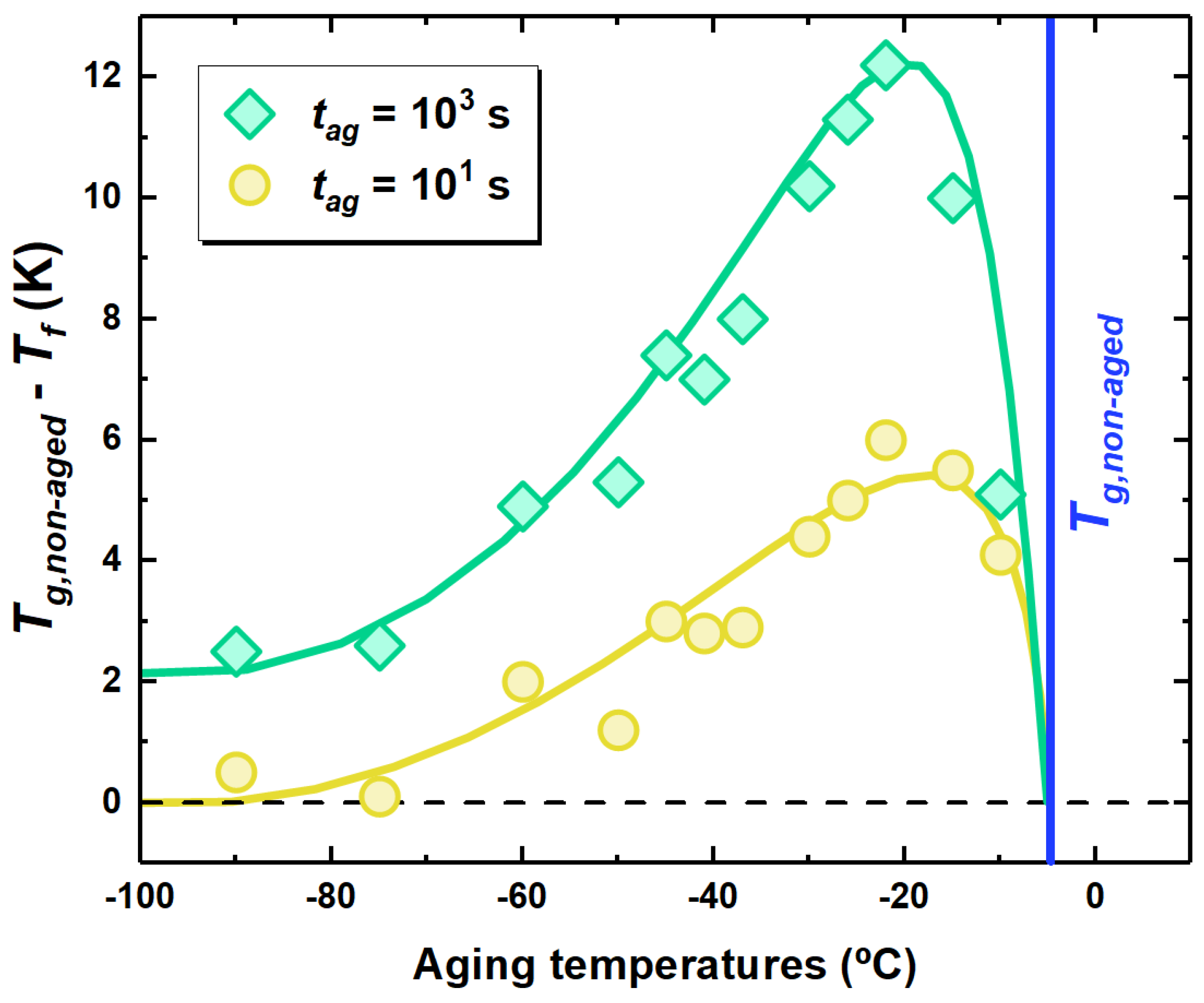
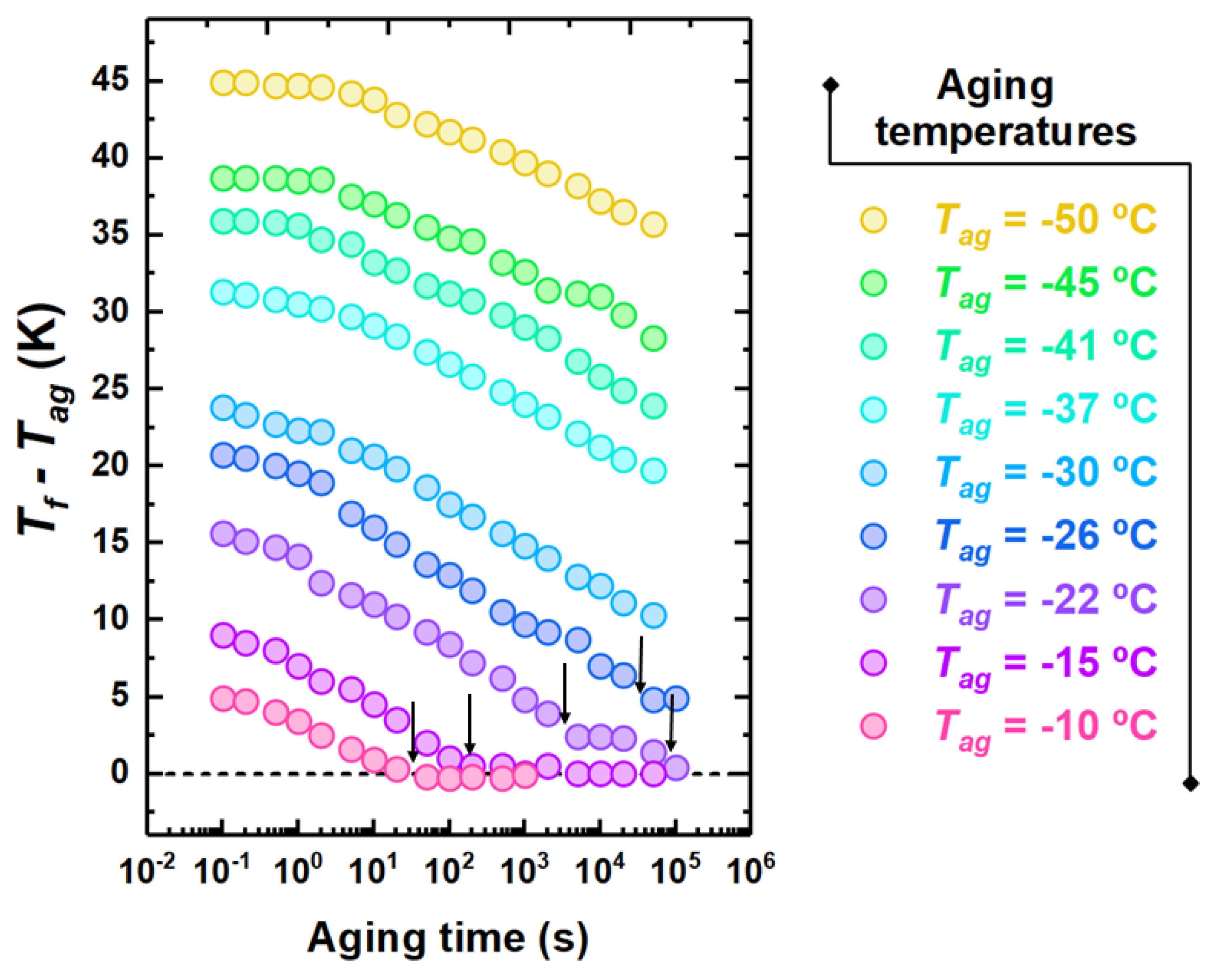
3. Results
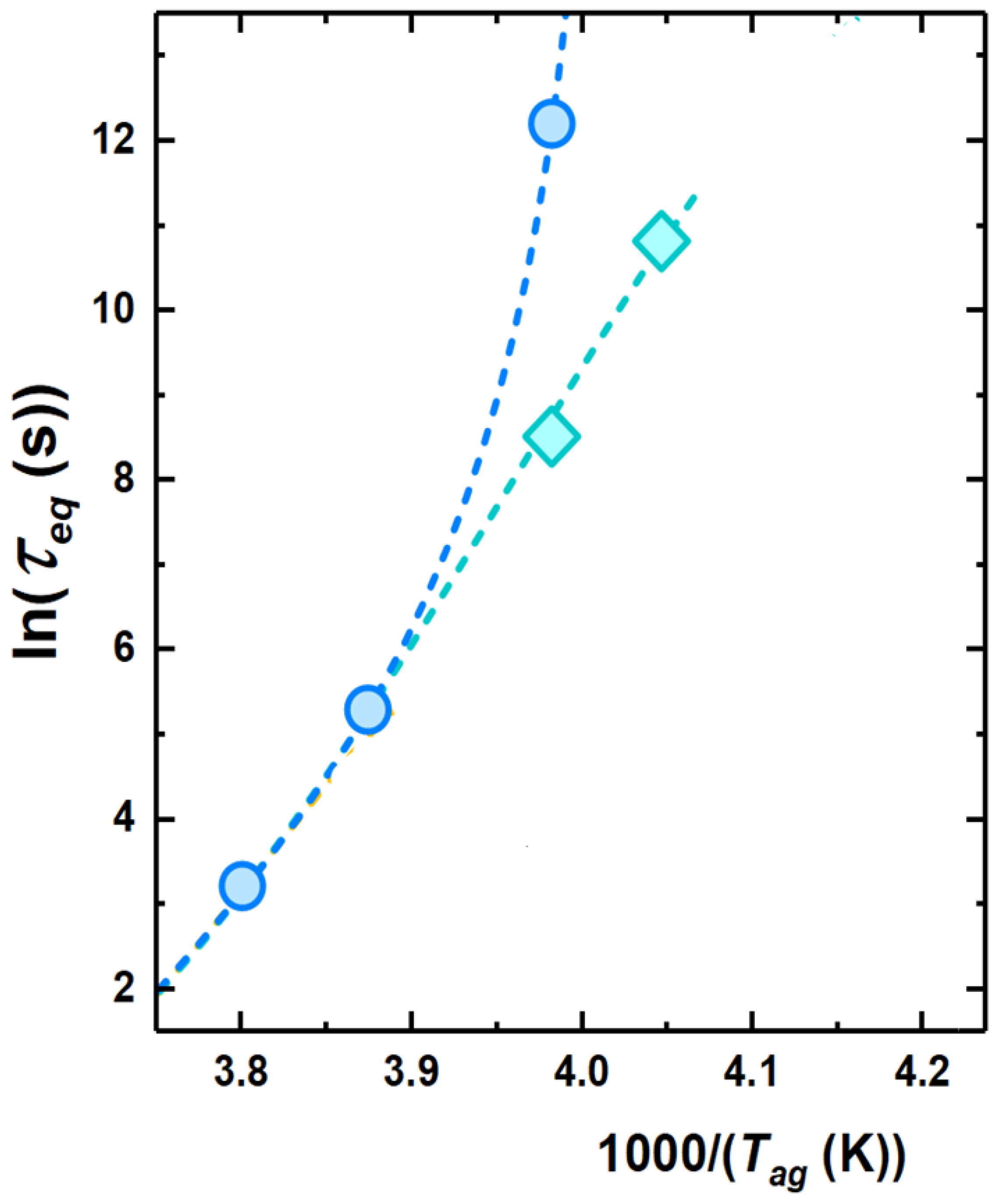
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmelzer, J.W.P.; Gutzow, I.S. Glasses and the Glass Transition; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Cangialosi, D.; Alegría, A.; Colmenero, J. Cooling Rate Dependent Glass Transition in Thin Polymer Films and in Bulk. In Fast Scanning Calorimetry; Schick, C., Mathot, V., Eds.; Springer: Cham, Switzerland, 2016; pp. 403–431. [Google Scholar]
- Tropin, T.V.; Schulz, G.; Schmelzer, J.W.; Schick, C. Heat capacity measurements and modeling of polystyrene glass transition in a wide range of cooling rates. J. Non-Cryst. Sol. 2015, 409, 63–75. [Google Scholar] [CrossRef]
- Kovacs, A.J. Glass transition in amorphous polymers: A phenomenological study. Fortsch. Hochpolym. Forsch. 1963, 3, 394–508. [Google Scholar]
- Struik, L.C.E. Physical Aging in Amorphous Polymers and Other Materials; Technische Hogeschool Delft: Delft, The Netherlands, 1977. [Google Scholar]
- Hutchinson, J.M. Physical aging of polymers. Progr. Pol. Sci. 1995, 20, 703–760. [Google Scholar] [CrossRef]
- Cangialosi, D.; Boucher, V.M.; Alegria, A.; Colmenero, J. Physical aging in polymers and polymer nanocomposites: Recent results and open questions. Soft Matter 2013, 9, 8619–8630. [Google Scholar] [CrossRef]
- Cangialosi, D. Dynamics and thermodynamics of polymer glasses. J. Phys. Cond. Matt. 2014, 26, 153101. [Google Scholar] [CrossRef]
- Blanco, I. Lifetime prediction of polymers: To bet, or not to bet—Is this the question? Materials 2018, 11, 1383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, A.W.; Hill, A.J. Vacancy Diffusion with Time-Dependent Length Scale: An Insightful New Model for Physical Aging in Polymers. Ind. Eng. Chem. Res. 2010, 49, 12119–12124. [Google Scholar] [CrossRef]
- Rowe, B.W.; Freeman, B.D.; Paul, D.R. Physical aging of ultrathin glassy polymer films tracked by gas permeability. Polymer 2009, 50, 5565–5575. [Google Scholar] [CrossRef]
- Rowe, B.W.; Pas, S.J.; Hill, A.J.; Suzuki, R.; Freeman, B.D.; Paul, D.R. A variable energy positron annihilation lifetime spectroscopy study of physical aging in thin glassy polymer films. Polymer 2009, 50, 6149–6156. [Google Scholar] [CrossRef]
- Merrick, M.M.; Sujanani, R.; Freeman, B.D. Glassy polymers: Historical findings, membrane applications, and unresolved questions regarding physical aging. Polymer 2020, 211, 123176. [Google Scholar] [CrossRef]
- Longo, M.; De Santo, M.P.; Esposito, E.; Fuoco, A.; Monteleone, M.; Giorno, L.; Comesaña-Gándara, B.; Chen, J.; Bezzu, C.G.; Carta, M.; et al. Correlating gas permeability and Young’s modulus during the physical aging of polymers of intrinsic microporosity using atomic force microscopy. Ind. Eng. Chem. Res. 2019, 59, 5381–5391. [Google Scholar] [CrossRef]
- Murphy, T.M.; Langhe, D.; Ponting, M.; Baer, E.; Freeman, B.; Paul, D. Enthalpy recovery and structural relaxation in layered glassy polymer films. Polymer 2012, 53, 4002–4009. [Google Scholar] [CrossRef]
- Cangialosi, D.; Alegria, A.; Colmenero, J. Effect of nanostructure on the thermal glass transition and physical aging in polymer materials. Prog. Pol. Sci. 2016, 54–55, 128–147. [Google Scholar] [CrossRef]
- Royall, C.P.; Turci, F.; Tatsumi, S.; Russo, J.; Robinson, J. The race to the bottom: Approaching the ideal glass? J. Phys. Cond. Matt. 2018, 30, 363001. [Google Scholar] [CrossRef] [Green Version]
- Cangialosi, D.; Boucher, V.M.; Alegría, A.; Colmenero, J. Direct Evidence of Two Equilibration Mechanisms in Glassy Polymers. Phys. Rev. Lett. 2013, 111, 095701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Golovchak, R.; Kozdras, A.; Balitska, V.; Shpotyuk, O. Step-wise kinetics of natural physical ageing in arsenic selenide glasses. J. Phys. Cond. Matt. 2012, 24, 505106. [Google Scholar] [CrossRef]
- Miller, R.S.; MacPhail, R.A. Ultraslow nonequilibrium dynamics in supercooled glycerol by stimulated Brillouin gain spectroscopy. J. Chem. Phys. 1997, 106, 3393–3401. [Google Scholar] [CrossRef]
- Gallino, I.; Cangialosi, D.; Evenson, Z.; Schmitt, L.; Hechler, S.; Stolpe, M.; Ruta, B. Hierarchical aging pathways and reversible fragile-to-strong transition upon annealing of a metallic glass former. Acta Mat. 2018, 144, 400–410. [Google Scholar] [CrossRef]
- Song, L.; Xu, W.; Huo, J.; Wang, J.Q.; Wang, X.; Li, R. Two-step relaxations in metallic glasses during isothermal annealing. Intermetallics 2018, 93, 101–105. [Google Scholar] [CrossRef]
- Buquet, C.L.; Hamonic, F.; Saiter, A.; Dargent, E.; Langevin, D.; Nguyen, Q. Physical ageing and molecular mobilities of sulfonated polysulfone for proton exchange membranes. Thermochim. Acta 2010, 509, 18–23. [Google Scholar] [CrossRef]
- Wisitsorasak, A.; Wolynes, P.G. Dynamical Heterogeneity of the Glassy State. J. Phys. Chem. B 2014, 118, 7835–7847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ngai, K.; Capaccioli, S.; Wang, L.M. Segmental α-relaxation for the first step and sub-rouse modes for the second step in enthalpy recovery in the glassy state of polystyrene. Macromolecules 2019, 52, 1440–1446. [Google Scholar] [CrossRef]
- Liu, G.; Zuo, Y.; Zhao, D.; Zhang, M. Study on enthalpy relaxation of polystyrene by assuming the existence of an intermediate aging plateau. J. Non-Cryst. Sol. 2014, 402, 160–165. [Google Scholar] [CrossRef]
- Perez-De Eulate, N.G.; Cangialosi, D. The very long-term physical aging of glassy polymers. Phys. Chem. Chem. Phys. 2018, 20, 12356–12361. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Cooper, E.I.; Angell, C.A. Glasses with Strong Calorimetric β-Glass Transitions and the Relation to the Protein Glass Transition Problem. J. Phys. Chem. 1994, 98, 9345–9349. [Google Scholar] [CrossRef]
- Aji, D.P.B.; Johari, G.P. Kinetic-freezing and unfreezing of local-region fluctuations in a glass structure observed by heat capacity hysteresis. J. Chem. Phys. 2015, 142, 214501. [Google Scholar] [CrossRef] [PubMed]
- Hodge, I.M. Effects of annealing and prior history on enthalpy relaxation in glassy polymers. 4. Comparison of five polymers. Macromolecules 1983, 16, 898–902. [Google Scholar] [CrossRef]
- Pradipkanti, L.; Chowdhury, M.; Satapathy, D.K. Stratification and two glass-like thermal transitions in aged polymer films. Phys. Chem. Chem. Phys. 2017, 19, 29263–29270. [Google Scholar] [CrossRef]
- Yang, Q.; Peng, S.X.; Wang, Z.; Yu, H.B. Shadow glass transition as a thermodynamics signature of β relaxation in hyper-quenched metallic glasses. Nat. Sci. Rev. 2020. [Google Scholar] [CrossRef]
- Napolitano, S.; Rotella, C.; Wubbenhorst, M. Can Thickness and Interfacial Interactions Univocally Determine the Behavior of Polymers Confined at the Nanoscale? ACS Macro Lett. 2012, 1, 1189–1193. [Google Scholar] [CrossRef]
- Napolitano, S.; Glynos, E.; Tito, N.B. Glass transition of polymers in bulk, confined geometries, and near interfaces. Rep. Progr. Phys. 2017, 80, 036602. [Google Scholar] [CrossRef] [PubMed]
- Boucher, V.M.; Cangialosi, D.; Alegria, A.; Colmenero, J. Reaching the ideal glass transition by aging polymer films. Phys. Chem. Chem. Phys. 2017, 19, 961–965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boucher, V.M.; Cangialosi, D.; Alegria, A.; Colmenero, J. Complex nonequilibrium dynamics of stacked polystyrene films deep in the glassy state. J. Chem. Phys. 2017, 146, 203312. [Google Scholar] [CrossRef] [PubMed]
- Perez-De-Eulate, N.G.; Cangialosi, D. Double Mechanism for Structural Recovery of Polystyrene Nanospheres. Macromolecules 2018, 51, 3299–3307. [Google Scholar] [CrossRef]
- Ma, M.; Huang, Y.; Guo, Y. Enthalpy relaxation and morphology evolution in polystyrene-b-poly (methyl methacrylate) diblock copolymer. Macromolecules 2018, 51, 7368–7376. [Google Scholar] [CrossRef]
- Ma, M.; Guo, Y. Accelerated aging of PS blocks in PS-b-PMMA diblock copolymer under hard confinement. J. Phys. Chem. B 2019, 123, 2448–2453. [Google Scholar] [CrossRef] [PubMed]
- Schick, C.; Mathot, V. Fast Scanning Calorimetry; Springer: Berlin, Germany, 2016. [Google Scholar]
- Vyazovkin, S. “Nothing can hide itself from thy heat”: Understanding polymers via unconventional applications of thermal analysis. Mac. Rap. Comm. 2019, 40, 1800334. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Chua, Y.Z.; Yang, B.; Schick, C.; Harrison, W.J.; Budd, P.M.; Boehning, M.; Schoenhals, A. First clear-cut experimental evidence of a glass transition in a polymer with intrinsic microporosity: Pim-1. J. Phys. Chem. Lett. 2018, 9, 2003–2008. [Google Scholar] [CrossRef] [PubMed]
- Monnier, X.; Maigret, J.E.; Lourdin, D.; Saiter, A. Glass transition of anhydrous starch by fast scanning calorimetry. Carbohydr. Polym. 2017, 173, 77–83. [Google Scholar] [CrossRef]
- Cebe, P.; Hu, X.; Kaplan, D.L.; Zhuravlev, E.; Wurm, A.; Arbeiter, D.; Schick, C. Beating the heat-fast scanning melts silk beta sheet crystals. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Monnier, X.; Napolitano, S.; Cangialosi, D. Direct observation of desorption of a melt of long polymer chains. Nat. Commun. 2020, 11, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Basterretxea, A.; Gabirondo, E.; Jehanno, C.; Zhu, H.; Flores, I.; Muller, A.J.; Etxeberria, A.; Mecerreyes, D.; Coulembier, O.; Sardon, H. Polyether Synthesis by Bulk Self-Condensation of Diols Catalyzed by Non-Eutectic Acid–Base Organocatalysts. ACS Sust. Chem. Eng. 2019, 7, 4103–4111. [Google Scholar] [CrossRef]
- Basterretxea, A.; Lopez de Pariza, X.; Gabirondo, E.; Marina, S.; Martin, J.; Etxeberria, A.; Mecerreyes, D.; Etxeberria, A.; Mecerreyes, D.; Sardon, H. Synthesis and Characterization of Fully Biobased Copolyether Polyols. Ind. Eng. Chem. Res 2020, 59, 10746–10753. [Google Scholar] [CrossRef]
- Monnier, X.; Cangialosi, D. Thermodynamic Ultrastability of a Polymer Glass Confined at the Micrometer Length Scale. Phys. Rev. Lett. 2018, 121, 137801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tool, A.Q. Relation between inelastic deformability and thermal expansion of glass in its annealing range. J. Am. Ceram. Soc. 1946, 29, 240–253. [Google Scholar] [CrossRef]
- Moynihan, C.T.; Macedo, P.B.; Montrose, C.J.; Gupta, P.K.; De Bolt, M.A.; Dill, J.F.; Dom, B.E.; Drake, P.W.; Eastel, A.J.; Elterman, P.B.; et al. Structural relaxation in vitreous materials. Ann. N. Y. Acad. Sci. 1976, 279, 15–35. [Google Scholar] [CrossRef]
- Gunawan, L.; Johari, G.; Shanker, R.M. Structural relaxation of acetaminophen glass. Pharmac. Res. 2006, 23, 967–979. [Google Scholar] [CrossRef]
- Righetti, M.C.; Gazzano, M.; Delpouve, N.; Saiter, A. Contribution of the rigid amorphous fraction to physical ageing of semi-crystalline PLLA. Polymer 2017, 125, 241–253. [Google Scholar] [CrossRef]
- Guo, J.; Xiao, R.; Tian, C.; Jiang, M. Optimizing physical aging in poly(ethylene terephthalate)-glycol (PETG). J. Non-Cryst. Sol. 2018, 502, 15–21. [Google Scholar] [CrossRef]
- Greiner, R.; Schwarzl, F.R. Thermal contraction and volume relaxation of amorphous polymers. Rheol. Acta 1984, 23, 378–395. [Google Scholar] [CrossRef]
- Boehm, L.; Ingram, M.; Angell, C. Test of a year-annealed glass for the cohen-grest percolation transition. J. Non-Cryst. Sol. 1981, 44, 305–313. [Google Scholar] [CrossRef]
- Frieberg, B.R.; Glynos, E.; Sakellariou, G.; Tyagi, M.; Green, P.F. Effect of Molecular Stiffness on the Physical Aging of Polymers. Macromolecules 2020, 53, 7684–7690. [Google Scholar] [CrossRef]
- Androsch, R.; Jariyavidyanont, K.; Schick, C. Enthalpy relaxation of polyamide 11 of different morphology far below the glass transition temperature. Entropy 2019, 21, 984. [Google Scholar] [CrossRef] [Green Version]
- McCaig, M.S.; Paul, D.R.; Barlow, J.W. Effect of film thickness on the changes in gas permeability of a glassy polyarylate due to physical agingPart II. Mathematical model. Polymer 2000, 41, 639–648. [Google Scholar] [CrossRef]
- Bauwens-Crowet, C.; Bauwens, J.C. Annealing of polycarbonate below the glass transition temperature up to equilibrium: A quantitative interpretation of enthalpy relaxation. Polymer 1986, 27, 709–713. [Google Scholar] [CrossRef]
- Andreozzi, L.; Faetti, M.; Giordano, M.; Palazzuoli, D. Enthalpy recovery in low molecular weight PMMA. J. Non-Cryst. Solids 2003, 332, 229–241. [Google Scholar] [CrossRef]
- McGonigle, E.A.; Cowie, J.M.G.; Arrighi, V.; Pethrick, R.A. Enthalpy relaxation and free volume changes in aged styrene copolymers containing a hydrogen bonding co-monomer. J. Mater. Sci. 2005, 40, 1869–1881. [Google Scholar] [CrossRef]
- Zhao, H.Y.; Koh, Y.P.; Pyda, M.; Sen, S.; Simon, S.L. The kinetics of the glass transition and physical aging in germanium selenide glasses. J. Non-Cryst. Solids 2013, 368, 63–70. [Google Scholar] [CrossRef]
- Boucher, V.M.; Cangialosi, D.; Alegría, A.; Colmenero, J. Enthalpy Recovery of Glassy Polymers: Dramatic Deviations from the Extrapolated Liquidlike Behavior. Macromolecules 2011, 44, 8333–8342. [Google Scholar] [CrossRef]
- Sasaki, K.; Takatsuka, M.; Kita, R.; Shinyashiki, N.; Yagihara, S. Enthalpy and Dielectric Relaxation of Poly (vinyl methyl ether). Macromolecules 2018, 51, 5806–5811. [Google Scholar] [CrossRef]
- Chen, H.S.; Inoue, A.; Masumoto, T. Two-stage enthalpy relaxation behaviour of (Fe0.5Ni0.5)83P17 and (Fe0.5Ni0.5)83B17 amorphous alloys upon annealing. J. Mat. Sci. 1985, 20, 2417–2438. [Google Scholar] [CrossRef]
- Yu, H.B.; Wang, W.H.; Bai, H.Y.; Samwer, K. The β-relaxation in metallic glasses. Nat. Sci. Rev. 2014, 1, 429–461. [Google Scholar] [CrossRef] [Green Version]
- Gao, M.; Perepezko, J. Separating β relaxation from α relaxation in fragile metallic glasses based on ultrafast flash differential scanning calorimetry. Phys. Rev. Mat. 2020, 4, 025602. [Google Scholar] [CrossRef]
- Peng, S.X.; Cheng, Y.; Pries, J.; Wei, S.; Yu, H.B.; Wuttig, M. Uncovering β-relaxations in amorphous phase-change materials. Sci. Adv. 2020, 6, eaay6726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakatsuji, W.; Konishi, T.; Miyamoto, Y. Origin of two maxima in specific heat in enthalpy relaxation under thermal history composed of cooling, annealing, and heating. Phys. Rev. E 2016, 94, 062501. [Google Scholar] [CrossRef]
- Ju, J.; Jang, D.; Nwankpa, A.; Atzmon, M. An atomically quantized hierarchy of shear transformation zones in a metallic glass. J. Appl. Phys. 2011, 109, 053522. [Google Scholar] [CrossRef] [Green Version]
- Charbonneau, P.; Kurchan, J.; Parisi, G.; Urbani, P.; Zamponi, F. Fractal free energy landscapes in structural glasses. Nat. Comm. 2014, 5, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, G.; Gibbs, J.H. On the Temperature Dependence of Cooperative Relaxation Properties in Glass-Forming Liquids. J. Chem. Phys. 1965, 43, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Hodge, I.M. Adam-Gibbs formulation of enthalpy relaxation near the glass transition. J. Res. Natl. Inst. Stand. Technol. 1997, 102, 195. [Google Scholar] [CrossRef]
- Chandran, S.; Baschnagel, J.; Cangialosi, D.; Fukao, K.; Glynos, E.; Janssen, L.M.; Mueller, M.; Muthukumar, M.; Steiner, U.; Xu, J.; et al. Processing pathways decide polymer properties at the molecular level. Macromolecules 2019, 52, 7146–7156. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Huang, W.M. On the origin of the Vogel–Fulcher–Tammann law in the thermo-responsive shape memory effect of amorphous polymers. Smart Mater. Struct. 2013, 22, 105021. [Google Scholar] [CrossRef]
- Simavilla, D.N.; Huang, W.; Vandestrick, P.; Ryckaert, J.P.; Sferrazza, M.; Napolitano, S. Mechanisms of polymer adsorption onto solid substrates. ACS Macro Lett. 2017, 6, 975–979. [Google Scholar] [CrossRef]
- Raegen, A.; Chowdhury, M.; Calers, C.; Schmatulla, A.; Steiner, U.; Reiter, G. Aging of Thin Polymer Films Cast from a Near-Theta Solvent. Phys. Rev. Lett. 2010, 105, 227801. [Google Scholar] [CrossRef] [PubMed]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Monnier, X.; Marina, S.; Lopez de Pariza, X.; Sardón, H.; Martin, J.; Cangialosi, D. Physical Aging Behavior of a Glassy Polyether. Polymers 2021, 13, 954. https://doi.org/10.3390/polym13060954
Monnier X, Marina S, Lopez de Pariza X, Sardón H, Martin J, Cangialosi D. Physical Aging Behavior of a Glassy Polyether. Polymers. 2021; 13(6):954. https://doi.org/10.3390/polym13060954
Chicago/Turabian StyleMonnier, Xavier, Sara Marina, Xabier Lopez de Pariza, Haritz Sardón, Jaime Martin, and Daniele Cangialosi. 2021. "Physical Aging Behavior of a Glassy Polyether" Polymers 13, no. 6: 954. https://doi.org/10.3390/polym13060954







