Preparation and Characterization of Chitosan-Coated Manganese-Ferrite Nanoparticles Conjugated with Laccase for Environmental Bioremediation
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Materials
2.2. Magnetic Nanoparticle Synthesis
2.3. MNP Coating with Chitosan
2.4. Characterization of MNPs before and after Chitosan Coating
2.4.1. Elemental Analysis
2.4.2. Fourier-Transform Infrared Spectroscopy—Attenuated Total Reflectance (FTIR–ATR) Analysis
2.4.3. Morphological Analysis
2.5. Laccase Immobilization onto Chitosan-Coated MNPs
2.6. Laccase Immobilization Yield
2.7. Free and Immobilized Laccase Activity
2.8. pH, Temperature, and Storage Stability of the Free and Immobilized Laccase
2.9. Reusability of Immobilized Laccase
2.10. Removal of a Model Drug
3. Results and Discussion
3.1. Nanoparticle Characterization
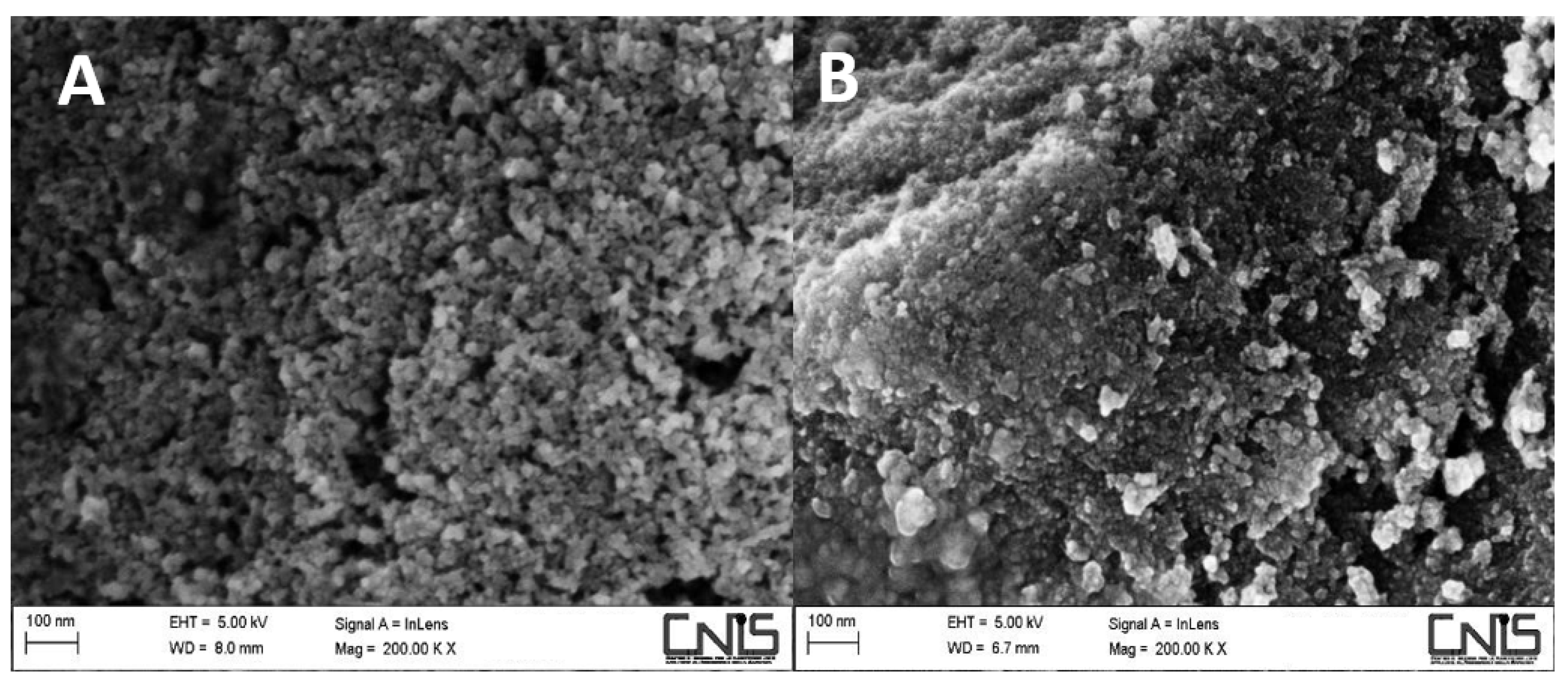
3.1.1. Morphological Analysis
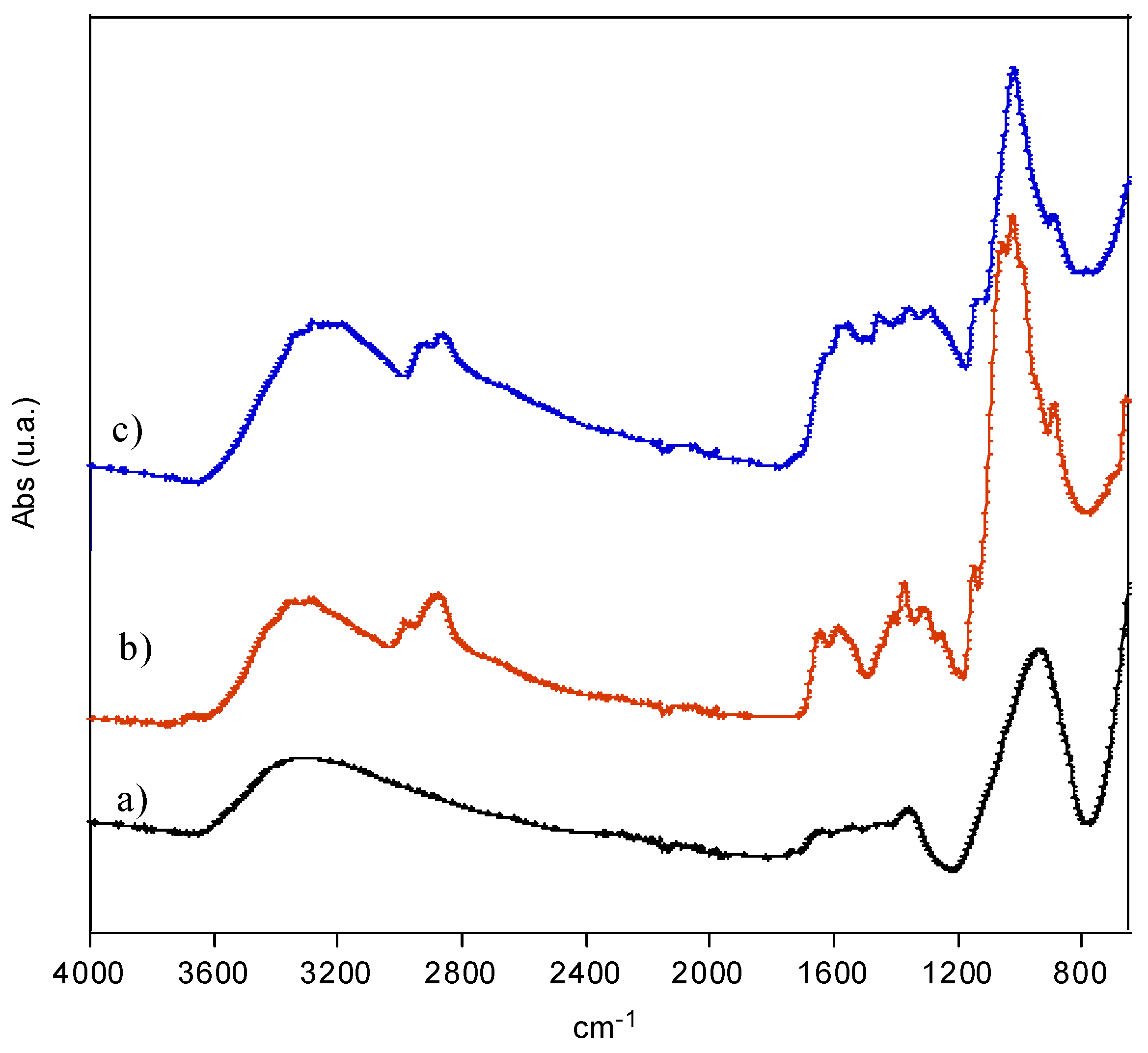
3.1.2. FTIR Spectroscopy Analysis
3.1.3. Elemental Analysis
3.2. Analysis and Optimization of Laccase-Immobilized Systems
3.3. Bioreactor Characterization
3.4. Diclofenac Degradation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Homaei, A.A.; Sariri, R.; Vianello, F.; Stevanato, R. Enzyme immobilization: An update. J. Chem. Biol. 2013, 6, 185–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zucca, P.; Sanjust, E. Inorganic materials as supports for covalent enzyme immobilization: Methods and mechanisms. Molecules 2014, 19, 14139–14194. [Google Scholar] [CrossRef]
- Thurston, C.F. The structure and function of fungal laccases. Microbiology 1994, 140, 19–26. [Google Scholar] [CrossRef] [Green Version]
- Desai, S.S.; Nityanand, C. Microbial laccases and their applications: A review. Asian J. Biotechnol. 2011, 3, 98–124. [Google Scholar] [CrossRef] [Green Version]
- Lante, A.; Crapisi, A.; Pasini, G.; Zamorani, A.; Spettoli, P. Characteristics of Laccase Immobilized on Different Supports for Wine-Making Technology. Adv. Mol. Cell Biol. 1996, 15, 229–236. [Google Scholar] [CrossRef]
- Couto, S.R.; Toca Herrera, J.L. Industrial and biotechnological applications of laccases: A review. Biotechnol. Adv. 2006, 24, 500–513. [Google Scholar] [CrossRef]
- Shraddha; Shekher, R.; Sehgal, S.; Kamthania, M.; Kumar, A. Laccase: Microbial sources, production, purification, and potential biotechnological applications. Enzyme Res. 2011, 2011, 217861. [Google Scholar] [CrossRef] [Green Version]
- Durán, N.; Rosa, M.A.; D’annibale, A.; Gianfreda, L. Enzyme and Microbial Technology 31 (2002) 907-931 Review Applications of laccases and tyrosinases (phenoloxidases) immobilized on different supports: A review. Enzyme Microb. Technol. 2002, 31, 907–931. [Google Scholar] [CrossRef]
- Hou, J.; Dong, G.; Ye, Y.; Chen, V. Laccase immobilization on titania nanoparticles and titania-functionalized membranes. J. Memb. Sci. 2014, 452, 229–240. [Google Scholar] [CrossRef]
- Plagemann, R.; Jonas, L.; Kragl, U. Ceramic honeycomb as support for covalent immobilization of laccase from Trametes versicolor and transformation of nuclear fast red. Appl. Microbiol. Biotechnol. 2011, 90, 313–320. [Google Scholar] [CrossRef]
- Bilal, M.; Zhao, Y.; Rasheed, T.; Iqbal, H.M.N. Magnetic nanoparticles as versatile carriers for enzymes immobilization: A review. Int. J. Biol. Macromol. 2018, 120, 2530–2544. [Google Scholar] [CrossRef] [PubMed]
- Sharma, B.; Dangi, A.K.; Shukla, P. Contemporary enzyme based technologies for bioremediation: A review. J. Environ. Manage. 2018, 210, 10–22. [Google Scholar] [CrossRef]
- Tyumina, E.A.; Bazhutin, G.A.; Cartagena Gómez, A.d.P.; Ivshina, I.B. Nonsteroidal Anti-inflammatory Drugs as Emerging Contaminants. Microbiology 2020, 89, 148–163. [Google Scholar] [CrossRef]
- Bonnefille, B.; Gomez, E.; Courant, F.; Escande, A.; Fenet, H. Diclofenac in the marine environment: A review of its occurrence and effects. Mar. Pollut. Bull. 2018, 131, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Angosto, J.M.; Roca, M.J.; Fernández-López, J.A. Removal of diclofenac in wastewater using biosorption and advanced oxidation techniques: Comparative results. Water 2020, 12, 3567. [Google Scholar] [CrossRef]
- Lei, M.; Zhang, L.; Lei, J.; Zong, L.; Li, J.; Wu, Z.; Wang, Z. Overview of emerging contaminants and associated human health effects. Biomed Res. Int. 2015, 2015, 404796. [Google Scholar] [CrossRef] [Green Version]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef]
- Díaz-Hernández, A.; Gracida, J.; García-Almendárez, B.E.; Regalado, C.; Núñez, R.; Amaro-Reyes, A. Characterization of Magnetic Nanoparticles Coated with Chitosan: A Potential Approach for Enzyme Immobilization. J. Nanomater. 2018, 2018, 9468574. [Google Scholar] [CrossRef]
- Bellusci, M.; Canepari, S.; Ennas, G.; La Barbera, A.; Padella, F.; Santini, A.; Scano, A.; Seralessandri, L.; Varsano, F. Phase evolution in synthesis of manganese ferrite nanoparticles. J. Am. Ceram. Soc. 2007, 90, 3977–3983. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Pelt, S. Enzyme immobilisation in biocatalysis: Why, what and how. Chem. Soc. Rev. 2013, 42, 6223–6235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kafshgari, L.A.; Ghorbani, M.; Azizi, A. Synthesis and characterization of manganese ferrite nanostructure by co-precipitation, sol-gel, and hydrothermal methods. Part. Sci. Technol. 2019, 37, 900–906. [Google Scholar] [CrossRef]
- Karimzadeh, I.; Aghazadeh, M.; Doroudi, T.; Ganjali, M.R.; Kolivand, P.H. Electrochemical preparation and characterization of chitosan-coated superparamagnetic iron oxide (Fe3O4) nanoparticles. Mater. Res. Innov. 2018, 22, 352–360. [Google Scholar] [CrossRef]
- Unsoy, G.; Yalcin, S.; Khodadust, R.; Gunduz, G.; Gunduz, U. Synthesis optimization and characterization of chitosancoated iron oxide nanoparticles produced for biomedical applications. J. Nanoparticle Res. 2012, 14, 964. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef]
- Gao, L.; Zhuang, J.; Nie, L.; Zhang, J.; Zhang, Y.; Gu, N.; Wang, T.; Feng, J.; Yang, D.; Perrett, S.; et al. Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nat. Nanotechnol. 2007, 2, 577–583. [Google Scholar] [CrossRef]
- Canals, M.; Gonzalez-Olmos, R.; Costas, M.; Company, A. Robust iron coordination complexes with N-based neutral ligands as efficient fenton-like catalysts at neutral pH. Environ. Sci. Technol. 2013, 47, 9918–9927. [Google Scholar] [CrossRef]
- Mumtaz, S.; Wang, L.S.; Abdullah, M.; Hussain, S.Z.; Iqbal, Z.; Rotello, V.M.; Hussain, I. Facile method to synthesize dopamine-capped mixed ferrite nanoparticles and their peroxidase-like activity. J. Phys. D Appl. Phys. 2017, 50, 11LT02. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From New Concepts, Mechanisms, and Standards to Applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef]
- Fang, H.; Huang, J.; Ding, L.; Li, M.; Chen, Z. Preparation of magnetic chitosan nanoparticles and immobilization of laccase. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2009, 24, 42–47. [Google Scholar] [CrossRef]
- Shleev, S.; Reimann, C.T.; Serezhenkov, V.; Burbaev, D.; Yaropolov, A.I.; Gorton, L.; Ruzgas, T. Autoreduction and aggregation of fungal laccase in solution phase: Possible correlation with a resting form of laccase. Biochimie 2006, 88, 1275–1285. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wang, Q.; Jiang, Y.; Gao, J. Biomimetic synthesis of magnetic composite particles for laccase immobilization. Ind. Eng. Chem. Res. 2012, 51, 10140–10146. [Google Scholar] [CrossRef]
- Shi, L.; Ma, F.; Han, Y.; Zhang, X.; Yu, H. Removal of sulfonamide antibiotics by oriented immobilized laccase on Fe3O4 nanoparticles with natural mediators. J. Hazard. Mater. 2014, 279, 203–211. [Google Scholar] [CrossRef]
- Secundo, F. Conformational changes of enzymes upon immobilisation. Chem. Soc. Rev. 2013, 42, 6250–6261. [Google Scholar] [CrossRef] [PubMed]
- Vera, M.; Rivas, B.L. Immobilization of Trametes versicolor laccase on different PGMA-based polymeric microspheres using response surface methodology: Optimization of conditions. J. Appl. Polym. Sci. 2017, 134, 45249. [Google Scholar] [CrossRef]
- Yinghui, D.; Qiuling, W.; Shiyu, F. Laccase stabilization by covalent binding immobilization on activated polyvinyl alcohol carrier. Lett. Appl. Microbiol. 2002, 35, 451–456. [Google Scholar] [CrossRef]
- Kalkan, N.A.; Aksoy, S.; Aksoy, E.A.; Hasirci, N. Preparation of chitosan--coated magnetite nanoparticles and application for immobilization of laccase. J. Appl. Polym. Sci. 2012, 123, 707–716. [Google Scholar] [CrossRef]
- Zhang, D.; Deng, M.; Cao, H.; Zhang, S.; Zhao, H. Laccase immobilized on magnetic nanoparticles by dopamine polymerization for 4-chlorophenol removal. Green Energy Environ. 2017, 2, 393–400. [Google Scholar] [CrossRef]
- Majeau, J.A.; Brar, S.K.; Tyagi, R.D. Laccases for removal of recalcitrant and emerging pollutants. Bioresour. Technol. 2010, 101, 2331–2350. [Google Scholar] [CrossRef]







| Sample | C (%) | H (%) | N (%) | Polymer Amount (%) * |
|---|---|---|---|---|
| CS | 45 | 7 | 8 | - |
| MNPs-CS | 0.5 | 0.4 | 0.2 | 1 |
| MNPs-CS in situ | 1.8 | 1.0 | 0.9 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Apriceno, A.; Silvestro, I.; Girelli, A.; Francolini, I.; Pietrelli, L.; Piozzi, A. Preparation and Characterization of Chitosan-Coated Manganese-Ferrite Nanoparticles Conjugated with Laccase for Environmental Bioremediation. Polymers 2021, 13, 1453. https://doi.org/10.3390/polym13091453
Apriceno A, Silvestro I, Girelli A, Francolini I, Pietrelli L, Piozzi A. Preparation and Characterization of Chitosan-Coated Manganese-Ferrite Nanoparticles Conjugated with Laccase for Environmental Bioremediation. Polymers. 2021; 13(9):1453. https://doi.org/10.3390/polym13091453
Chicago/Turabian StyleApriceno, Azzurra, Ilaria Silvestro, Annamaria Girelli, Iolanda Francolini, Loris Pietrelli, and Antonella Piozzi. 2021. "Preparation and Characterization of Chitosan-Coated Manganese-Ferrite Nanoparticles Conjugated with Laccase for Environmental Bioremediation" Polymers 13, no. 9: 1453. https://doi.org/10.3390/polym13091453







