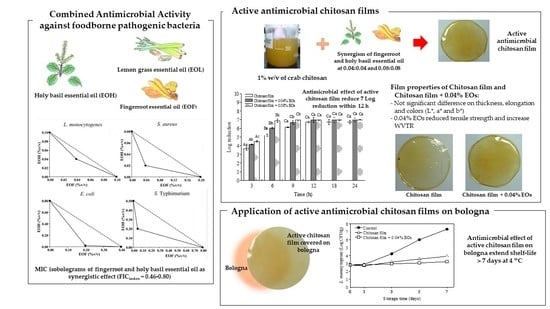
Synergistic Antimicrobial Activities of Thai Household Essential Oils in Chitosan Film
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Materials
2.2. Tested Bacteria
2.3. Agar Dilution Assay
2.4. Antimicrobial Interaction Assessment
2.5. Preparation of Chitosan Film and Chitosan Films Incorporated with Essential Oils of Fingerroot (EOF) and Holy Basil (EOH)
2.6. Evaluation of Viable Cell Count
2.7. Film Applications on Bologna
2.8. Film Thickness
2.9. Tensile Strength (TS) and Percentage Elongation (%E)
2.10. Water Vapor Transmission Rate (WVTR) of Films
2.11. Color Measurement
2.12. Statistical Analysis
3. Results and Discussion
3.1. Minimum Inhibitory Concentration (MIC) of Essential Oils
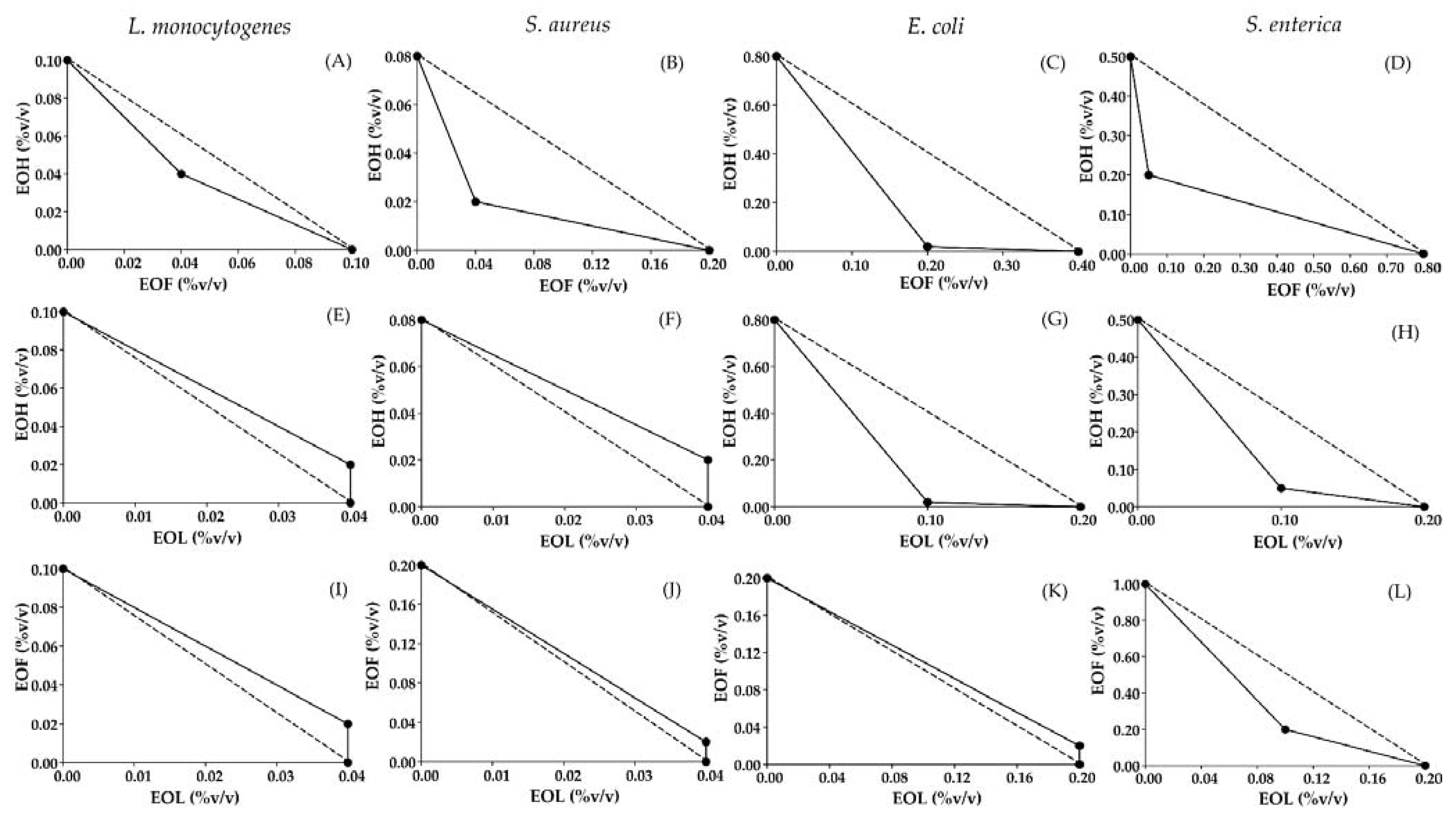
3.2. A Screen of Antimicrobial Combination Effect
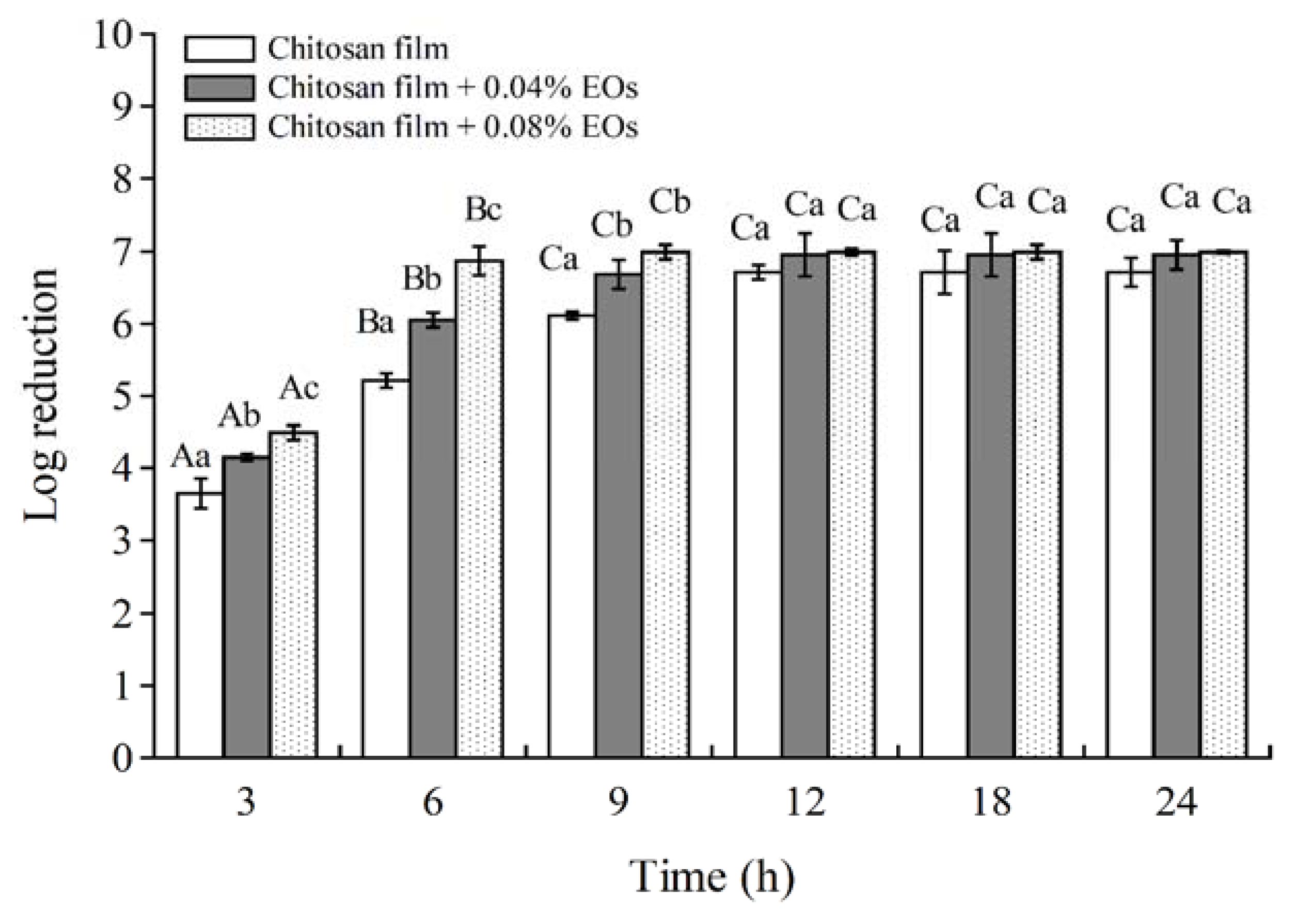
3.3. Antimicrobial Activity of Chitosan Films Combined with EOF and EOH in a Culture Broth Medium
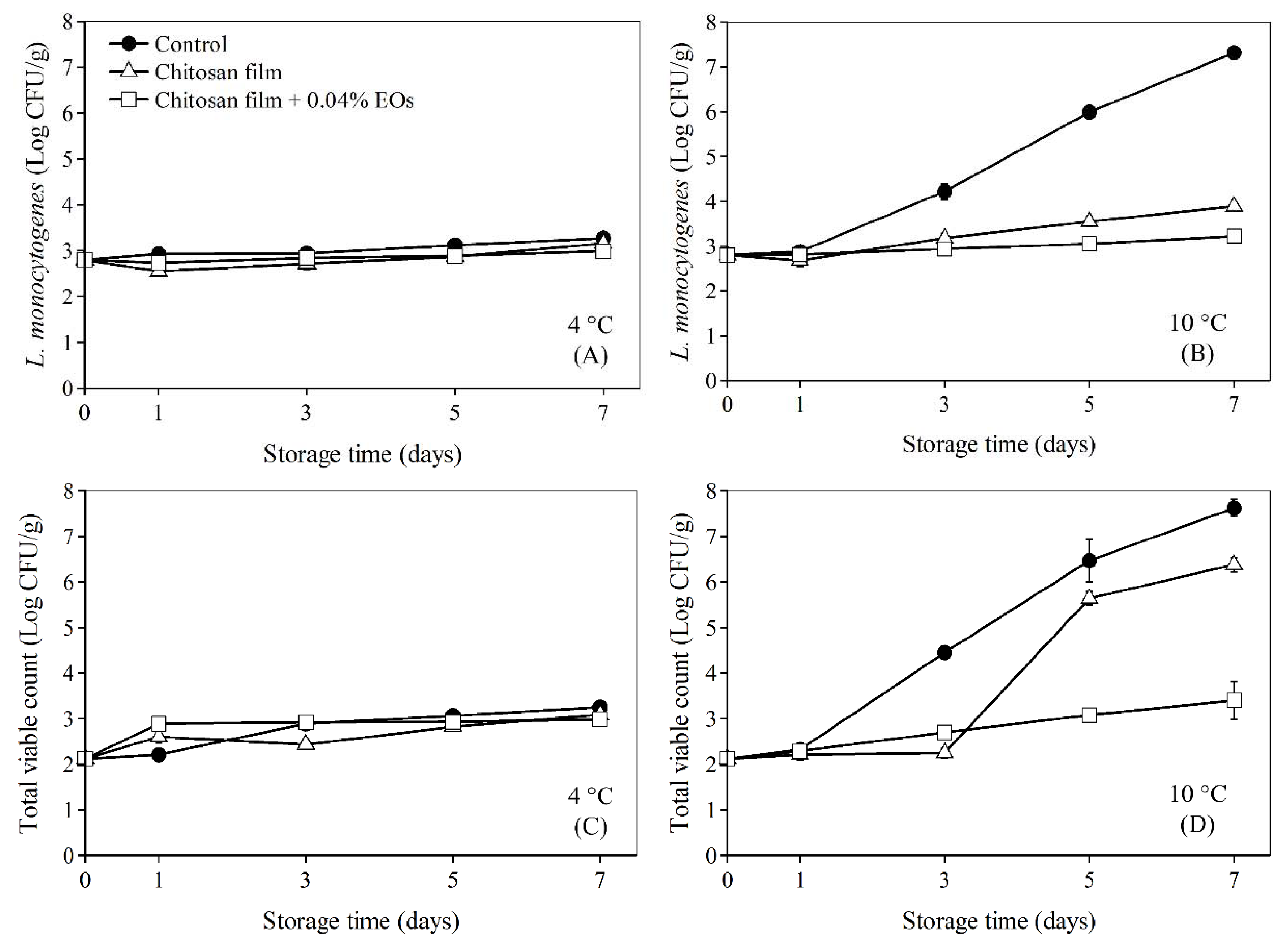
3.4. Application of Chitosan Film Combined with Essential Oils from EOF and EOH on Bologna
3.5. Mechanical Properties
3.6. Water Vapor Transmission Rate (WVTR)
3.7. Color Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EOH | Fingerroot essential oil |
| EOH | Holy basil essential oil |
| EOL | Lemongrass essential oil |
| Chitosan film + 0.04% EOs | Chitosan film incorporated with EOF:EOH at 0.04:0.04%v/v/w |
| Chitosan film + 0.08% EOs | Chitosan film incorporated with EOF:EOH at 0.08:0.08%v/v/w |
References
- Appendini, P.; Hotchkiss, J.H. Review of antimicrobial food packaging. Innov. Food Sci. Emerg. Technol. 2002, 3, 113–126. [Google Scholar] [CrossRef]
- Tantala, J.; Rachtanapun, C.; Rachtanapun, P. Effect of molecular sizes, sources of chitosan and plasticizer types on properties of carboxymethyl chitosan films. Adv. Mat. Res. 2012, 506, 611–614. [Google Scholar] [CrossRef]
- Park, S.Y.; Marsh, K.S.; Rhim, J.W. Characteristics of different molecular weight chitosan films affected by the type of organic solvents. J. Food Sci. 2002, 67, 194–197. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A. Chemistry and bioactivity of essential oils. In Lipids and Essential Oils as Antimicrobial Agents; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2011; pp. 203–238. [Google Scholar]
- Burt, S. Essential oils: Their antibacterial properties and potential applications in foods—A review. Int. J. Food Microbiol. 2004, 94, 223–253. [Google Scholar] [CrossRef] [PubMed]
- Nazzaro, F.; Fratianni, F.; De Martino, L.; Coppola, R.; De Feo, V. Effect of essential oils on pathogenic bacteria. Pharmaceuticals 2013, 6, 1451–1474. [Google Scholar] [CrossRef] [PubMed]
- Thongson, C. Antimicrobial Activity of Thai Spices against Foodborne Pathogens. Ph.D. Dissertation, Kasetsart University, Bangkok, Thailand, 2005. [Google Scholar]
- Lv, F.; Liang, H.; Yuan, Q.; Li, C. In vitro antimicrobial effects and mechanism of action of selected plant essential oil combinations against four food-related microorganisms. Food Res. Int. 2011, 44, 3057–3064. [Google Scholar] [CrossRef]
- Chouhan, S.; Sharma, K.; Guleria, S. Antimicrobial activity of some essential oils—Present status and future perspectives. Medicines 2017, 4, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Man, A.; Santacroce, L.; Iacob, R.; Mare, A.; Man, L. Antimicrobial activity of six essential oils against a group of human pathogens: A comparative study. Pathogens 2019, 8, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shojaee-Aliabadi, S.; Hosseini, S.M.; Mirmoghtadaie, L. Antimicrobial activity of essential oil. In Essential Oils in Food Processing; Hashemi, S.M.B., Khaneghah, A.M., Sant’Ana, A.d.S., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2017; pp. 191–229. [Google Scholar]
- Murbach Teles Andrade, B.F.; Nunes Barbosa, L.; da Silva Probst, I.; Fernandes Júnior, A. Antimicrobial activity of essential oils. J. Essent. Oil Res. 2014, 26, 34–40. [Google Scholar] [CrossRef]
- Ma, Q.; Davidson, P.M.; Zhong, Q. Antimicrobial properties of lauric arginate alone or in combination with essential oils in tryptic soy broth and 2% reduced fat milk. Int. J. Food Microbiol. 2013, 166, 77–84. [Google Scholar] [CrossRef]
- Ma, Q.; Davidson, P.M.; Critzer, F.; Zhong, Q. Antimicrobial activities of lauric arginate and cinnamon oil combination against foodborne pathogens: Improvement by ethylenediaminetetraacetate and possible mechanisms. LWT 2016, 72, 9–18. [Google Scholar] [CrossRef] [Green Version]
- Bassole, I.H.; Lamien-Meda, A.; Bayala, B.; Tirogo, S.; Franz, C.; Novak, J.; Nebie, R.C.; Dicko, M.H. Composition and antimicrobial activities of Lippia multiflora Moldenke, Mentha x piperita L. and Ocimum basilicum L. essential oils and their major monoterpene alcohols alone and in combination. Molecules 2010, 15, 7825–7839. [Google Scholar] [CrossRef] [PubMed]
- Pei, R.S.; Zhou, F.; Ji, B.P.; Xu, J. Evaluation of combined antibacterial effects of eugenol, cinnamaldehyde, thymol, and carvacrol against E. coli with an improved method. J. Food Sci. 2009, 74, M379–M383. [Google Scholar] [CrossRef] [PubMed]
- Thongson, C.; Davidson, P.M.; Mahakarnchanakul, W.; Vibulsresth, P. Antimicrobial effect of Thai spices against Listeria monocytogenes and Salmonella Typhimurium DT104. J. Food Prot. 2005, 68, 2054–2058. [Google Scholar] [CrossRef] [PubMed]
- Jarriyawattanachaikul, W.; Chaveerach, P.; Chokesajjawatee, N. Antimicrobial activity of Thai-herbal plants against food-borne pathogens E. coli, S. aureus and C. jejuni. Agric. Agric. Sci. Procedia 2016, 11, 20–24. [Google Scholar] [CrossRef]
- Akbar, A.; Anal, A.K. Isolation of Salmonella from ready-to-eat poultry meat and evaluation of its survival at low temperature, microwaving and simulated gastric fluids. J. Food Sci. Technol. 2015, 52, 3051–3057. [Google Scholar] [CrossRef] [Green Version]
- Huang, L.; Hwang, C.A. In-package pasteurization of ready-to-eat meat and poultry products. In Advances in Meat, Poultry and Seafood Packaging; Kerry, J.P., Ed.; Woodhead Publishing: Cambridge, UK, 2012; pp. 437–450. [Google Scholar]
- Mor-Mur, M.; Yuste, J. Emerging bacterial pathogens in meat and poultry: An overview. Food Bioprocess Technol. 2009, 3, 24. [Google Scholar] [CrossRef]
- Syne, S.M.; Ramsubhag, A.; Adesiyun, A.A. Microbiological hazard analysis of ready-to-eat meats processed at a food plant in Trinidad, West Indies. Infect. Ecol. Epidemiol. 2013, 3. [Google Scholar] [CrossRef]
- Adzitey, F.; Ekli, R.; Abu, A. Prevalence and antibiotic susceptibility of Staphylococcus aureus isolated from raw and grilled beef in Nyankpala community in the Northern Region of Ghana. Cogent Food Agric. 2019, 5, 1671115. [Google Scholar] [CrossRef]
- Adzitey, F.; Ekli, R.; Aduah, M. Incidence and antibiotic susceptibility of Staphylococcus aureus isolated from ready-to-eat meats in the environs of Bolgatanga Municipality of Ghana. Cogent Environ. Sci. 2020, 6, 1791463. [Google Scholar] [CrossRef]
- CDC. Listeria (Listeriosis). Available online: https://www.cdc.gov/listeria/technical.html (accessed on 22 November 2020).
- Larsen, M.H.; Dalmasso, M.; Ingmer, H.; Langsrud, S.; Malakauskas, M.; Mader, A.; Møretrø, T.; Smole Možina, S.; Rychli, K.; Wagner, M.; et al. Persistence of foodborne pathogens and their control in primary and secondary food production chains. Food Control 2014, 44, 92–109. [Google Scholar] [CrossRef] [Green Version]
- CDC. Outbreak of Listeria Infections Linked to Pork Products. Available online: https://www.cdc.gov/listeria/outbreaks/porkproducts-11-18/index.html (accessed on 22 January 2021).
- Kurpas, M.; Wieczorek, K.; Osek, J. Ready-to-eat meat products as a source of Listeria monocytogenes. J. Vet. Res. 2018, 62, 49–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricci, A.; Allende, A.; Bolton, D.; Chemaly, M.; Davies, R.; Fernández Escámez, P.S.; Girones, R.; Herman, L.; Koutsoumanis, K.; Nørrung, B.; et al. Listeria monocytogenes contamination of ready-to-eat foods and the risk for human health in the EU. EFSA 2018, 16, e05134. [Google Scholar] [CrossRef]
- Lin, C.M.; Takeuchi, K.; Zhang, L.; Dohm, C.B.; Meyer, J.D.; Hall, P.A.; Doyle, M.P. Cross-contamination between processing equipment and deli meats by Listeria monocytogenes. J. Food Prot. 2006, 69, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Begin, A.; Van Calsteren, M.R. Antimicrobial films produced from chitosan. Int. J. Biol. Macromol. 1999, 26, 63–67. [Google Scholar] [CrossRef]
- Brul, S.; Coote, P. Preservative agents in foods. Mode of action and microbial resistance mechanisms. Int. J. Food Microbiol. 1999, 50, 1–17. [Google Scholar] [CrossRef]
- Sánchez-González, L.; Cháfer, M.; Hernández, M.; Chiralt, A.; González-Martínez, C. Antimicrobial activity of polysaccharide films containing essential oils. Food Control 2011, 22, 1302–1310. [Google Scholar] [CrossRef]
- FDA. E. coli and Foodborne Illness. Available online: https://www.fda.gov/news-events/public-health-focus/e-coli-and-foodborne-illness#:~:text=Escherichia%20coli%20(E.,mild%20to%20severe%20gastrointestinal%20illness (accessed on 26 April 2020).
- Mamber, S.W.; Mohr, T.; Leathers, C.; Mbandi, E.; Bronstein, P.; Barlow, K. Occurrence of Salmonella in ready-to-eat meat and poultry product samples from U.S. Department of Agriculture-Regulated Producing Establishments. I. Results from the ALLRTE and RTE001 random and risk-based sampling projects, from 2005 to 2012. J. Food Prot. 2018, 81, 1729–1736. [Google Scholar] [CrossRef] [PubMed]
- American Meat Science Association. Salmonella Fact Sheet. Available online: https://meatscience.org/docs/default-source/publications-resources/fact-sheets/salmonella-fact-sheet-2015.pdf?sfvrsn=0 (accessed on 1 May 2021).
- Abass, A.; Adzitey, F.; Huda, N. Escherichia coli of ready-to-eat (RTE) meats origin showed resistance to antibiotics used by farmers. Antibiotics 2020, 9, 869. [Google Scholar] [CrossRef] [PubMed]
- Henriques, A.R.; Fraqueza, M.J. Listeria monocytogenes and ready-to-eat meat-based food products: Incidence and control. In Listeria monocytogenes: Incidence, Growth Behavior and Control; Vicario, T., Ed.; Nova Science Publishers, Incorporated: New York, USA, USA, 2015; pp. 71–103. [Google Scholar]
- Pranoto, Y.; Rakshit, S.K.; Salokhe, V.M. Enhancing antimicrobial activity of chitosan films by incorporating garlic oil, potassium sorbate and nisin. LWT 2005, 38, 859–865. [Google Scholar] [CrossRef]
- NCCLS. Performance standards for antimicrobial susceptibility testing. In Twelfth Informational Supplement. NCCLS Document M100-S12; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 2002. [Google Scholar]
- Davidson, P.M.; Branen, A.L. Antimicrobials in Foods, 2nd ed.; Marcel Dekker, Inc.: New York, NY, USA, 1993. [Google Scholar]
- Davidson, P.M.; Sofos, J.N.; Branen, A.L. Antimicrobials in Food; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Branen, J.K.; Davidson, P.M. Enhancement of nisin, lysozyme, and monolaurin antimicrobial activities by ethylenediaminetetraacetic acid and lactoferrin. Int. J. Food Microbiol. 2004, 90, 63–74. [Google Scholar] [CrossRef]
- Vigil, A.L.-M.; Palou, E.; Parish, M.E.; Davidson, P.M. Methods for activity assay and evaluation of results. In Antimicrobials in Food, 3rd ed.; Davidson, P.M., Sofos, J.N., Branen, A.L., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 659–680. [Google Scholar]
- Tantala, J.; Thongngam, M.; Rachtanapun, P.; Rachtanapun, C. Antimicrobial activity of chitosan and carboxymethyl chitosan from differences types and sources of chitosan. Ital. J. Food Sci. 2012, XXIV, 97–101. [Google Scholar]
- NCCLS. Methods for Determining Bactericidal Activity of Antimicrobial Agents: Approved Guideline; National Committee for Clinical Laboratory Standards: Wayne, PA, USA, 1999; Volume 19. [Google Scholar]
- ASTM. Standard test methods for tensile properties of thin plastic sheeting (D882-95). In Annual Book of ASTM Standard; American Society for Testing and Materials: Philadelphia, PA, USA, 2000. [Google Scholar]
- Rachtanapun, P.; Tongdeesoontorn, W. Effect of antioxidants on properties of rice flour/cassava starch film blends plasticized with sorbitol. Kasetsart J. 2009, 43, 252–258. [Google Scholar]
- ASTM. Standard test method for water vapor transmission of material (ASTM E96-80). In Annual Book of ASTM Standard; American Society for Testing and Materials: Philadelphia, PA, USA, 1980. [Google Scholar]
- Rachtanapun, P. Shelf life study of salted crackers in pouch by using computer simulation models. Chiang Mai J. Sci. 2007, 34, 1–10. [Google Scholar]
- Gao, Y.; van Belkum, M.J.; Stiles, M.E. The outer membrane of gram-negative bacteria inhibits antibacterial activity of brochocin-C. Appl. Environ. Microbiol. 1999, 65, 4329–4333. [Google Scholar] [CrossRef] [Green Version]
- Lis-Balchin, M.; Deans, S.G. Bioactivity of selected plant essential oils against Listeria monocytogenes. J. Appl. Microbiol. 1997, 82, 759–762. [Google Scholar] [CrossRef]
- Viyoch, J.; Pisutthanan, N.; Faikreua, A.; Nupangta, K.; Wangtorpol, K.; Ngokkuen, J. Evaluation of in vitro antimicrobial activity of Thai basil oils and their micro-emulsion formulas against Propionibacterium acnes. Int. J. Cosmet. Sci. 2006, 28, 125–133. [Google Scholar] [CrossRef]
- Somolinos, M.; Garcia, D.; Condon, S.; Mackey, B.; Pagan, R. Inactivation of Escherichia coli by citral. J. Appl. Microbiol. 2010, 108, 1928–1939. [Google Scholar] [CrossRef]
- Walsh, S.E.; Maillard, J.Y.; Russell, A.D.; Catrenich, C.E.; Charbonneau, D.L.; Bartolo, R.G. Activity and mechanisms of action of selected biocidal agents on Gram-positive and -negative bacteria. J. Appl. Microbiol. 2003, 94, 240–247. [Google Scholar] [CrossRef]
- Hemaiswarya, S.; Doble, M. Synergistic interaction of eugenol with antibiotics against Gram negative bacteria. Phytomedicine 2009, 16, 997–1005. [Google Scholar] [CrossRef]
- Şimşek, M.; Duman, R. Investigation of effect of 1,8-cineole on antimicrobial activity of chlorhexidine gluconate. Pharmacognosy Res. 2017, 9, 234–237. [Google Scholar] [CrossRef] [Green Version]
- Kanatt, S.R.; Chander, R.; Sharma, A. Chitosan and mint mixture: A new preservative for meat and meat products. Food Chem. 2008, 107, 845–852. [Google Scholar] [CrossRef]
- Thai Industrial Standard Institute. Standard of Ham. In TIST848-1989; Thai Industrial Standard Institute: Bangkok, Thailand, 1989. [Google Scholar]
- Sofos, J. Safety of food and beverages: Meat and meat products. Encycl. Food Saf. 2014, 3, 268–279. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef]
- Zivanovic, S.; Chi, S.; Draughon, A.F. Antimicrobial activity of chitosan films enriched with essential oils. J. Food Sci. 2005, 70, M45–M51. [Google Scholar] [CrossRef]
- Liu, W.; Xie, J.; Li, L.; Xue, B.; Li, X.; Gan, J.; Shao, Z.; Sun, T. Properties of phenolic acid-chitosan composite films and preservative effect on Penaeus vannamei. J. Mol. Struct. 2021, 130531. [Google Scholar] [CrossRef]
- Rachtanapun, P.; Klunklin, W.; Jantrawut, P.; Jantanasakulwong, K.; Phimolsiripol, Y.; Seesuriyachan, P.; Leksawasdi, N.; Chaiyaso, T.; Ruksiriwanich, W.; Phongthai, S.; et al. Characterization of chitosan film incorporated with curcumin extract. Polymers 2021, 13, 963. [Google Scholar] [CrossRef] [PubMed]

| Bacterium | MIC (%) of Essential Oils | ||
|---|---|---|---|
| Fingerroot | Holy Basil | Lemongrass | |
| L. monocytogenes | 0.30 | 0.10 | 0.10 |
| S. aureus | 0.10 | 0.10 | 0.10 |
| E. coli | 0.40 | 0.50 | 0.20 |
| S. enterica | 0.40 | 0.50 | 0.50 |
| Microorganism | Ratio of Essential Oil Combinations | FIC | FICindex | |||
|---|---|---|---|---|---|---|
| EOF | EOH | EOL | ||||
| L. monocytogenes | EOF:EOH | 0.50:0.50 | 0.04 | 0.04 | - | 0.80(S) b |
| EOH:EOL | 0.33:0.67 | - | 0.20 | 0.10 | 1.20 (AN) | |
| EOF:EOL | 0.33:0.67 | 0.20 | - | 0.10 | 1.20 (AN) | |
| S. aureus | EOF:EOH | 0.33:0.67 | 0.20 | 0.10 | - | 0.45(S) |
| EOH:EOL | 0.33:0.67 | - | 0.25 | 0.10 | 1.25 (AN) | |
| EOF:EOL | 0.33:0.67 | 0.10 | - | 0.10 | 1.10 (AN) | |
| E. coli | EOF:EOH | 0.09:0.91 | 0.50 | 0.30 | - | 0.53 (S) |
| EOH:EOL | 0.17:0.83 | - | 0.03 | 0.50 | 0.53 (S) | |
| EOF:EOL | 0.09:0.91 | 0.10 | - | 1.10 | 1.10 (AN) | |
| S. enterica | EOF:EOH | 0.80:0.20 | 0.06 | 0.40 | - | 0.46 (S) |
| EOH:EOL | 0.33:0.67 | - | 0.10 | 0.50 | 0.60 (S) | |
| EOF:EOL | 0.67:0.33 | 0.20 | - | 0.50 | 0.70 (S) | |
| TYPE OF FILM | Mechanical Properties | Color | |||||
|---|---|---|---|---|---|---|---|
| Thickness (mm) | Tensile Strength (MPa) | Elongation at break (%) | WVTR (g·m−2·d−1) | L* | a* | b* | |
| Chitosan | 0.10 ± 0.01 a | 14.78 ± 2.71 b | 13.49 ± 6.59 a | 16.22 ± 0.62 a | 25.24 ± 3.52 a | −1.81 ± 0.21 a | 2.91 ± 0.75 a |
| Chitosan film + 0.04% EOs | 0.11 ± 0.01 a | 7.88 ± 1.54 a | 5.61 ± 0.55 a | 25.88 ± 5.19 b | 26.51 ± 6.38 a | −1.90 ± 0.56 a | 4.24 ± 2.01 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tantala, J.; Rachtanapun, P.; Rachtanapun, C. Synergistic Antimicrobial Activities of Thai Household Essential Oils in Chitosan Film. Polymers 2021, 13, 1519. https://doi.org/10.3390/polym13091519
Tantala J, Rachtanapun P, Rachtanapun C. Synergistic Antimicrobial Activities of Thai Household Essential Oils in Chitosan Film. Polymers. 2021; 13(9):1519. https://doi.org/10.3390/polym13091519
Chicago/Turabian StyleTantala, Juthamas, Pornchai Rachtanapun, and Chitsiri Rachtanapun. 2021. "Synergistic Antimicrobial Activities of Thai Household Essential Oils in Chitosan Film" Polymers 13, no. 9: 1519. https://doi.org/10.3390/polym13091519