Fabrication of Electrospun Juglans regia (Juglone) Loaded Poly(lactic acid) Scaffolds as a Potential Wound Dressing Material
Abstract
:1. Introduction
2. Material and Methods
2.1. Materials
2.2. Preparation of the Electrospinning Solutions
2.3. Fabrication of the Scaffolds with the Electrospinning Method
2.4. Characterization of the Scaffolds
2.5. In Vitro Drug Release Studies
2.6. Assessment of Antimicrobial Activity
2.7. Biocompatibility Tests
3. Results and Discussions
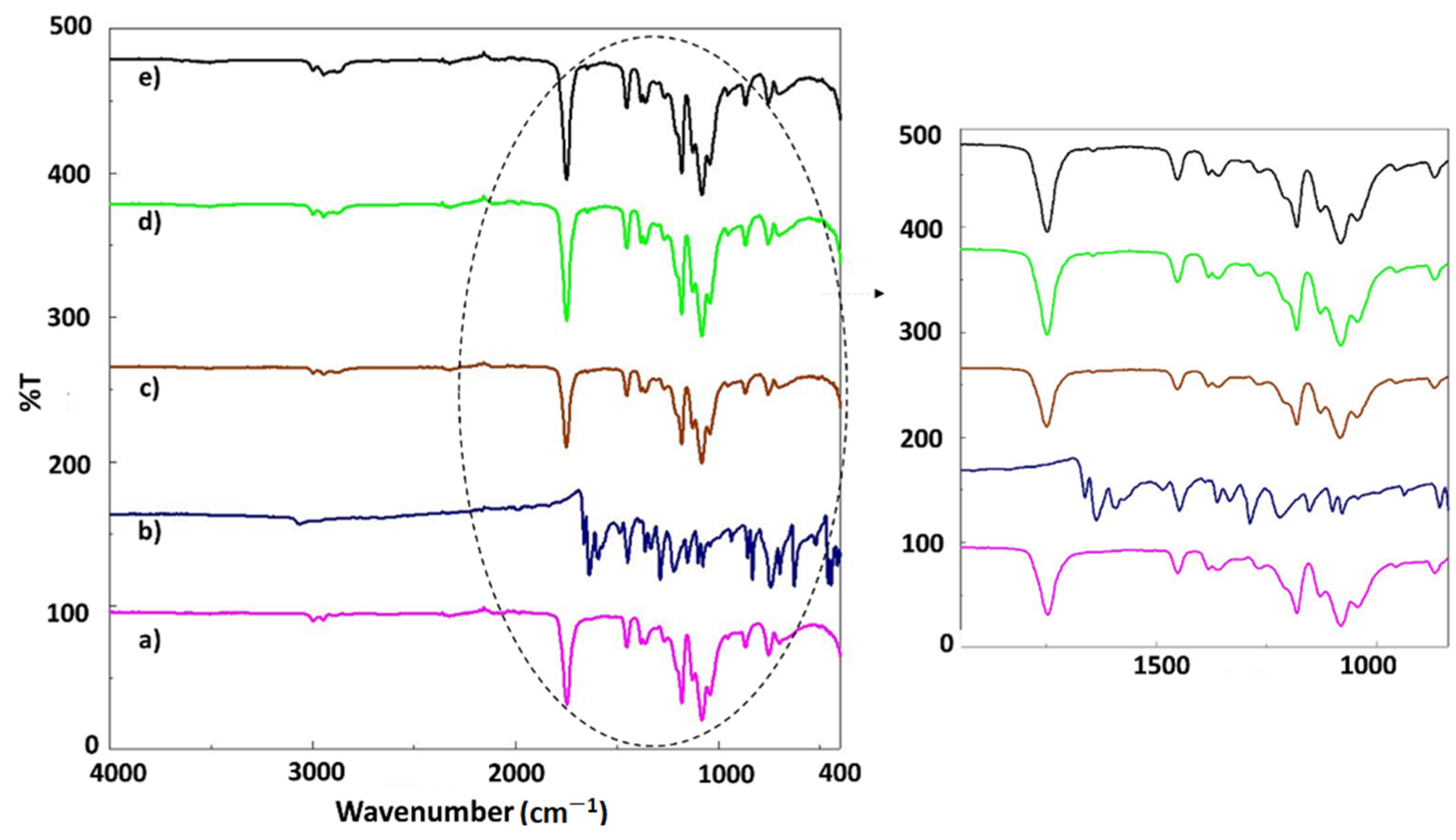
3.1. FT-IR Analysis
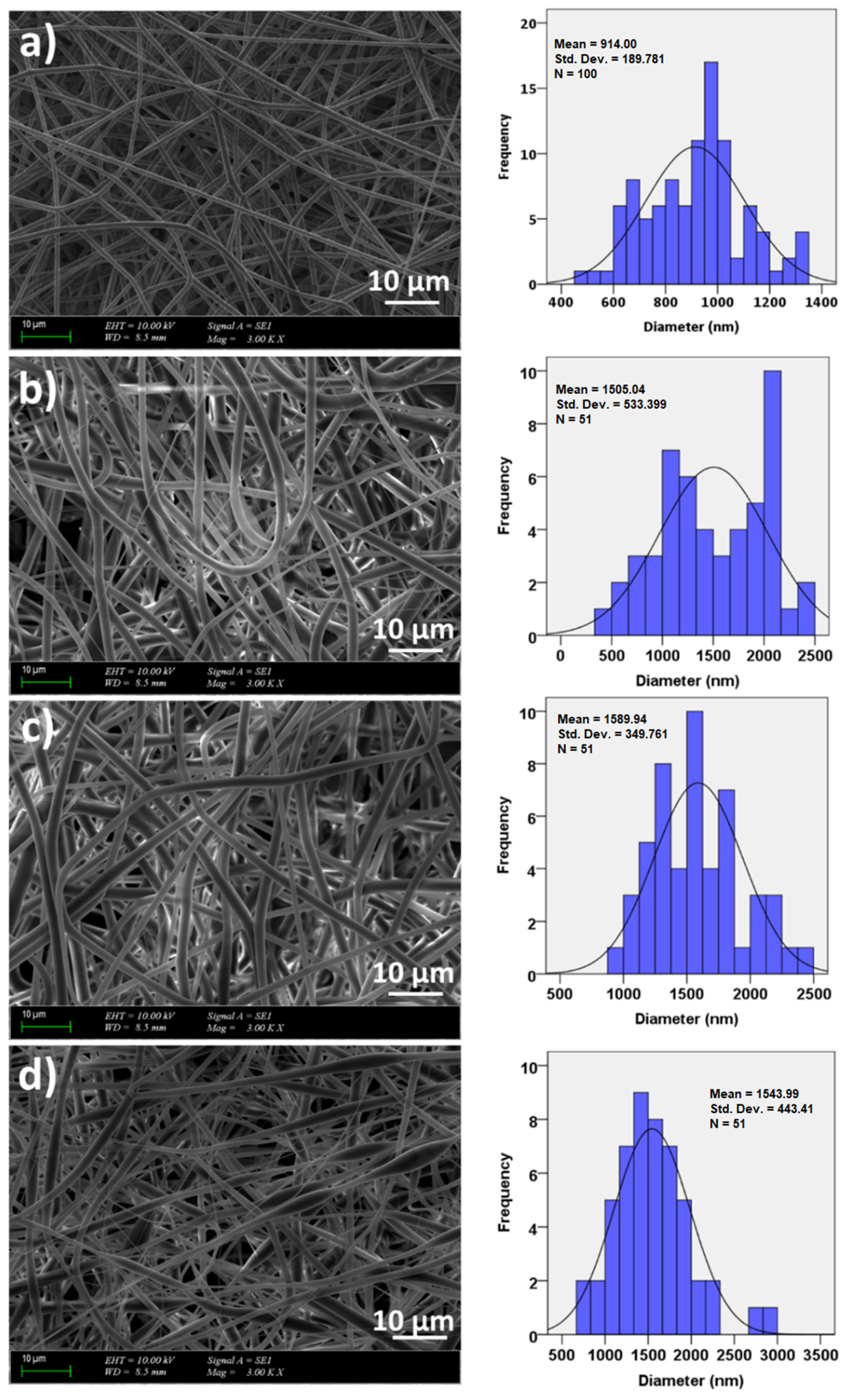
3.2. Morphology and Surface Properties of the Scaffolds
3.3. Mechanical Properties of the Scaffolds
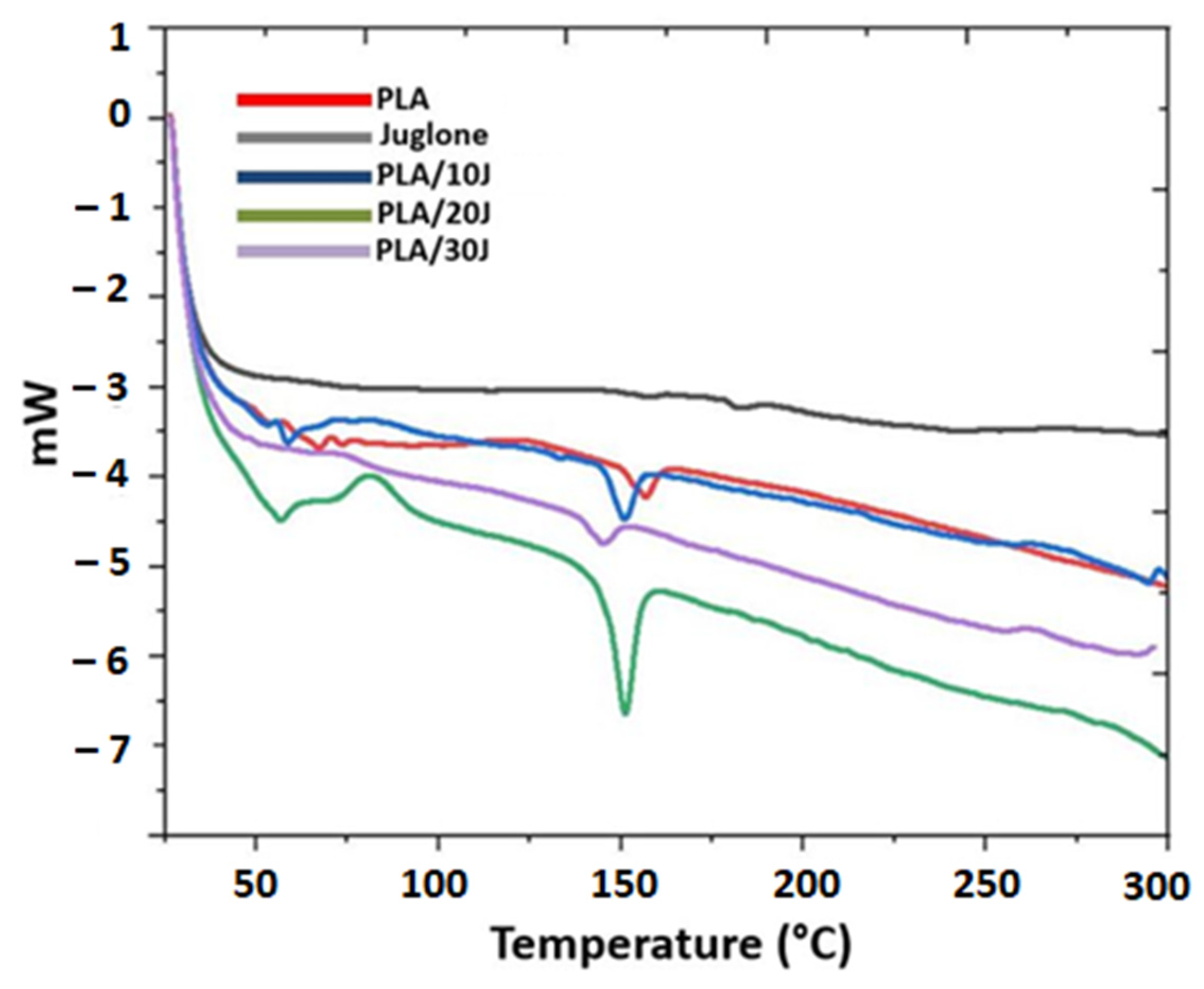
3.4. Thermal Analysis of the Electrospun Scaffolds
3.5. Drug Release Behavior of the Juglone from the Scaffolds
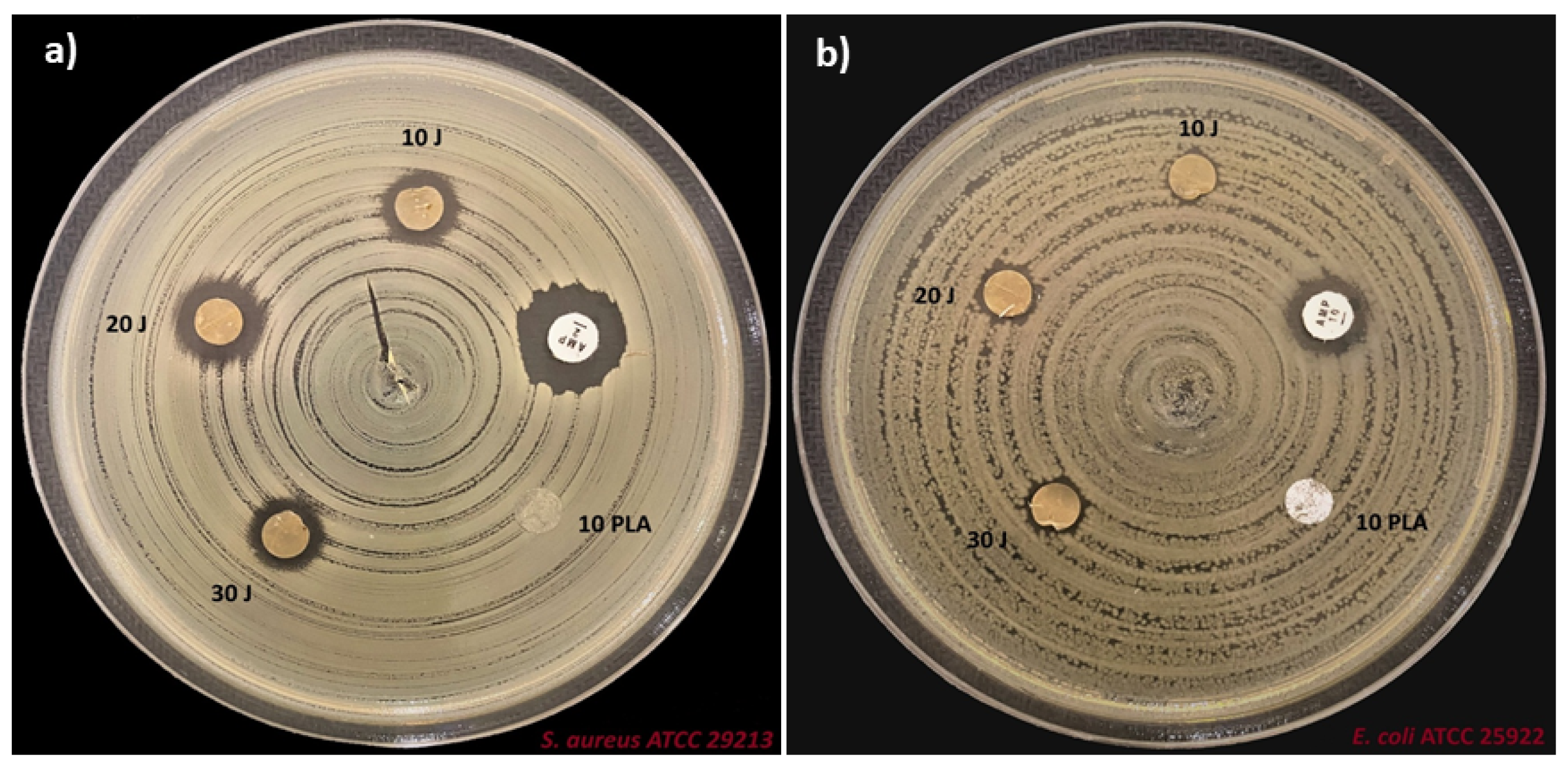
3.6. Antimicrobial Properties of the Scaffolds
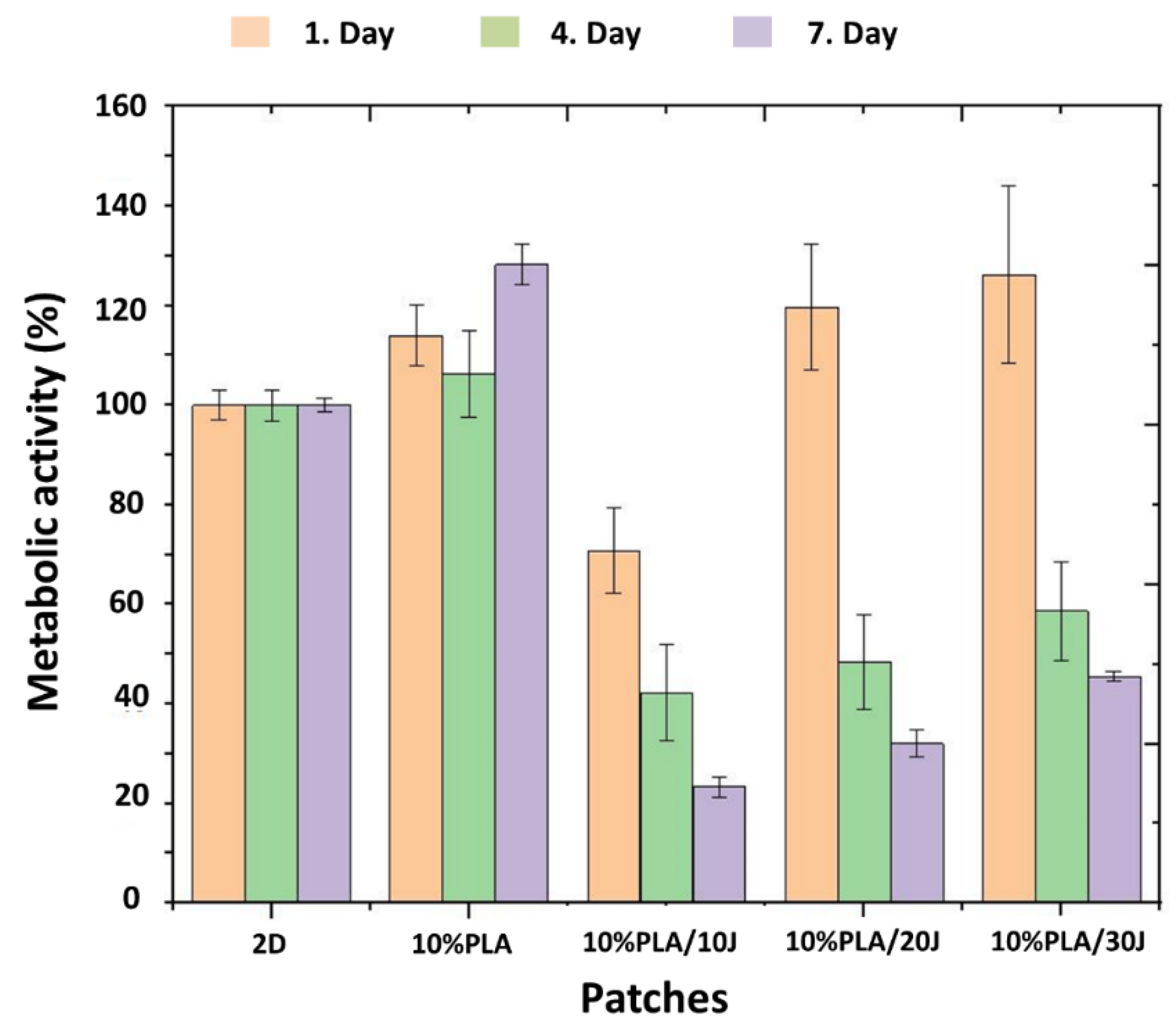
3.7. Cytotoxicity Properties of the Scaffolds
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Do, L.D.; Azizi, N.; Maibach, H. Sensitive Skin Syndrome: An Update. Am. J. Clin. Dermatol. 2020, 21, 401–409. [Google Scholar] [CrossRef] [PubMed]
- Hay, R.J.; Johns, N.E.; Williams, H.C.; Bolliger, I.W.; Dellavalle, R.P.; Margolis, D.J.; Marks, R.; Naldi, L.; Weinstock, M.A.; Wulf, S.K.; et al. The Global Burden of Skin Disease in 2010: An Analysis of the Prevalence and Impact of Skin Conditions. J. Investig. Dermatol. 2014, 134, 1527–1534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nurpeissov, T.; Khan, V. The role of a complex of emollients in the treatment of atopic dermatitis in children. Allergy 2020, 75, 450. [Google Scholar]
- Peyrí, J.; Lleonart, M. Clinical and therapeutic profile and quality of life of patients with seborrheic dermatitis. Actas Dermo-Sifiliogr. 2007, 98, 476–482. [Google Scholar] [CrossRef]
- del Rosso, J.Q. Topical and oral antibiotics for acne vulgaris. Semin. Cutan. Med. Surg. 2016, 35, 57–61. [Google Scholar] [CrossRef]
- Zhong, Y.P.; Yang, B.; Huang, L.N.; Elias, P.M.; Man, M.Q. Lasers for Becker’s nevus. Laser Med. Sci. 2019, 34, 1071–1079. [Google Scholar] [CrossRef]
- Cameron, H.; Yule, S.; Dawe, R.S.; Ibbotson, S.H.; Moseley, H.; Ferguson, J. Review of an established UK home phototherapy service 1998–2011: Improving access to a cost-effective treatment for chronic skin disease. Public Health 2014, 128, 317–324. [Google Scholar] [CrossRef]
- Battle, E.F.; Battle, S. Clinical Evaluation of Safety and Efficacy of Fractional Radiofrequency Facial Treatment of Skin Type VI Patients. J. Drugs Dermatol. 2018, 17, 1169–1172. [Google Scholar]
- Bannasch, H.; Fohn, M.; Unterberg, T.; Bach, A.D.; Weyand, B.; Stark, G.B. Skin tissue engineering. Clin. Plast. Surg. 2003, 30, 573–579. [Google Scholar] [CrossRef]
- Luo, G.X.; Teh, K.S.; Liu, Y.M.; Zang, X.N.; Wen, Z.Y.; Lin, L.W. Direct-Write, Self-Aligned Electrospinning on Paper for Controllable Fabrication of Three-Dimensional Structures. ACS Appl. Mater. Interfaces 2015, 7, 27765–27770. [Google Scholar] [CrossRef] [Green Version]
- Fatehi, P.; Abbasi, M. Medicinal plants used in wound dressings made of electrospun nanofibers. J. Tissue Eng. Regen. Med. 2020, 14, 1527–1548. [Google Scholar] [CrossRef] [PubMed]
- Suganya, S.; Venugopal, J.; Ramakrishna, S.; Lakshmi, B.S.; Dev, V.R.G. Herbally derived polymeric nanofibrous scaffolds for bone tissue regeneration. J. Appl. Polym. Sci. 2014, 131, 39835. [Google Scholar] [CrossRef]
- Pajoumshariati, S.; Yavari, S.K.; Shokrgozar, M.A. Physical and biological modification of polycaprolactone electrospun nanofiber by panax ginseng extract for bone tissue engineering application. Ann. Biomed. Eng. 2016, 44, 1808–1820. [Google Scholar] [CrossRef] [PubMed]
- Rasal, R.M.; Hirt, D.E. Poly(lactic acid) Toughening with a Better Balance of Properties. Macromol. Mater. Eng. 2010, 295, 204–209. [Google Scholar] [CrossRef]
- XLiu YChen YHZhang JDu YBLv SMMo YCLiu FDing JFWu, J.L.i. Juglone potentiates TRAIL-induced apoptosis in human melanoma cells via activating the ROS-p38-p53 pathway. Mol. Med. Rep. 2017, 16, 9645–9651. [Google Scholar]
- Wahedi, H.M.; Park, Y.U.; Moon, E.Y.; Kim, S.Y. Juglone ameliorates skin wound healing by promoting skin cell migration through Rac1/Cdc42/PAK pathway. Wound Repair Regen. 2016, 24, 786–794. [Google Scholar] [CrossRef]
- Cesur, S.; Ulag, S.; Ozak, L.; Gumussoy, A.; Arslan, S.; Yilmaz, B.K.; Ekren, N.; Agirbasli, M.; Kalaskar, D.M.; Gunduz, O. Production and characterization of elastomeric cardiac tissue-like patches for Myocardial Tissue Engineering. Polym. Test. 2020, 90, 106613. [Google Scholar] [CrossRef]
- Mamipour, Z.; Moghadaszadeh, M.; Habibi, B.; Mahkam, M. Preparation and effect of juglone on the antibacterial activity of the polycaprolactone nanofibers. In Proceedings of the 24th Iranian Seminar of Organic, Chemistry, Tabriz, Iran, 24–26 August 2016. [Google Scholar]
- Wang, N.; Liu, Z.; Yang, J.; Song, Y. Investigation of antibacterial activity of one-dimensional electrospun Walnut green husk extract-PVP nanofibers. Iran. Polym. J. 2022, 31, 779–785. [Google Scholar] [CrossRef]
- Zaheer, Z. Eco-friendly walnut shell powder based facile fabrication of biogenic Ag-nanodisks, and their interaction with bovine serum albumin. J. Photochem. Photobiol. B 2019, 193, 8–17. [Google Scholar] [CrossRef]
- Guo, L.L.; Wang, A.F.; Hu, P.F.; Tian, A.H.; Hao, R.; Yu, D.D.; Yang, J.; Chen, D.Z.; Wang, H. Renewable juglone nanowires with size-dependent charge storage properties. RSC Adv. 2018, 8, 2077–2081. [Google Scholar] [CrossRef] [Green Version]
- Gumus, B.; Acar, T.; Atabey, T.; Derman, S.; Sahin, F.; Arasoglu, T. The battle against biofilm infections: Juglone loaded nanoparticles as an anticandidal agent. J. Biotechnol. 2020, 316, 17–26. [Google Scholar] [CrossRef] [PubMed]
- Ulag, S.; Sahin, A.; Guncu, M.M.; Aksu, B.; Ekren, N.; Sengor, M.; Kalaskar, D.M.; Gunduz, O. A novel approach to treat the Thiel-Behnke corneal dystrophy using 3D printed honeycomb-shaped polymethylmethacrylate (PMMA)/Vancomycin (VAN) scaffolds. Bioprinting 2021, 24, e00173. [Google Scholar] [CrossRef]
- Liu, C.; Wong, H.M.; Yeung, K.W.K.; Tjong, S.C. Novel Electrospun Polylactic Acid Nanocomposite Fiber Mats with Hybrid Graphene Oxide and Nanohydroxyapatite Reinforcements Having Enhanced Biocompatibility. Polymers 2016, 8, 287. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arbeiter, D.; Reske, T.; Teske, M.; Bajer, D.; Senz, V.; Schmitz, K.P.; Grabow, N.; Oschatz, S. Influence of Drug Incorporation on the Physico-Chemical Properties of Poly(l-Lactide) Implant Coating Matrices-A Systematic Study. Polymers 2021, 13, 292. [Google Scholar] [CrossRef] [PubMed]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- Ilhan, E.; Ulag, S.; Sahin, A.; Yilmaz, B.K.; Ekren, N.; Kilic, O.; Sengor, M.; Kalaskar, D.M.; Oktar, F.N.; Gunduz, O. Fabrication of tissue-engineered tympanic membrane patches using 3D-Printing technology. J. Mech. Behav. Biomed. Mater. 2021, 114, 104219. [Google Scholar] [CrossRef]
- Iannace, S.; Sorrentino, L.; di Maio, E. 6—Biodegradable biomedical foam scaffolds. In Biomedical Foams for Tissue Engineering Applications; Netti, P.A., Ed.; Woodhead Publishing: Sawston, UK, 2014; pp. 163–187. [Google Scholar]
- Arasoglu, T.; Derman, S.; Mansuroglu, B.; Yelkenci, G.; Kocyigit, B.; Gumus, B.; Acar, T.; Kocacaliskan, I. Synthesis, characterization and antibacterial activity of juglone encapsulated PLGA nanoparticles. J. Appl. Microbiol. 2017, 123, 1407–1419. [Google Scholar] [CrossRef]
- Radusin, T.; Torres-Giner, S.; Stupar, A.; Ristic, I.; Miletic, A.; Novakovic, A.; Lagaron, J.M. Preparation, characterization and antimicrobial properties of electrospun polylactide films containing Allium ursinum L. extract. Food Packag. Shelf Life 2019, 21, 100357. [Google Scholar] [CrossRef]
- Abrigo, M.; Kingshott, P.; McArthur, S.L. Electrospun Polystyrene Fiber Diameter Influencing Bacterial Attachment, Proliferation, and Growth. ACS Appl. Mater. Interfaces 2015, 7, 7644–7652. [Google Scholar] [CrossRef]
- Moriarty, T.F.; Zaat, S.A.; Busscher, H.J. Biomaterials Associated Infection: Immunological Aspects and Antimicrobial Strategies; Springer: New York, NY, USA, 2013. [Google Scholar]
- Delaviz, Y.; Santerre, J.P.; Cvitkovitch, D.G. Infection resistant biomaterials. In Biomaterials and Medical Device—Associated Infections; Barnes, L., Cooper, I.R., Eds.; Woodhead Publishing: Oxford, UK, 2015; pp. 223–254. [Google Scholar]
- Zadeh, K.M.; Luyt, A.S.; Zarif, L.; Augustine, R.; Hasan, A.; Messori, M.; Hassan, M.K.; Yalcin, H.C. Electrospun polylactic acid/date palm polyphenol extract nanofibres for tissue engineering applications. Emergent Mater. 2019, 2, 141–151. [Google Scholar] [CrossRef] [Green Version]
- Bernard, M.; Jubeli, E.; Pungente, M.D.; Yagoubi, N. Biocompatibility of polymer-based biomaterials and medical devices—regulations, in vitro screening and risk-management. Biomater. Sci. 2018, 6, 2025–2053. [Google Scholar] [CrossRef] [PubMed]
- Yue, W.H.; Qin, L.Q.; Cai, J.; Mei, R.; Qian, H.Q.; Zou, Z.Y. Jug-PLGA-NPs, a New Form of Juglone with Enhanced Efficiency and Reduced Toxicity on Melanoma. Chin. J. Integr. Med. 2021. [Google Scholar] [CrossRef] [PubMed]
- Gokturk, F.; Erkoc-Kaya, D.; Arikoglu, H. Juglone can inhibit angiogenesis and metastasis in pancreatic cancer cells by targeting Wnt/beta-catenin signaling. Bratisl. Med. J. 2021, 122, 132–137. [Google Scholar] [CrossRef] [PubMed]
- Pavan, V.; Ribaudo, G.; Zorzan, M.; Redaelli, M.; Pezzani, R.; Mucignat-Caretta, C.; Zagotto, G. Antiproliferative activity of Juglone derivatives on rat glioma. Nat. Prod. Res. 2017, 31, 632–638. [Google Scholar] [CrossRef]
- Ahmad, T.; Khan, T.; Alamgeer; Shah, A.J. Juglone as antihypertensive agent acts through multiple vascular mechanisms. Clin. Exp. Hypertens. 2020, 42, 335–344. [Google Scholar] [CrossRef]



| Patch | Tensile Strength (MPa) | Strain at Break (%) |
|---|---|---|
| PLA (w/v) | 3.06 ± 0.32 | 4.90 ± 0.63 |
| PLA (w/v)/10 J | 1.084 ± 0.21 | 40.72 ± 11.17 |
| PLA (w/v)/20 J | 1.045 ± 0.24 | 28.16 ± 7.24 |
| PLA (w/v)/30 J | 0.49 ± 0.052 | 20.67 ± 4.90 |
| Scaffolds | E. coli ATCC 25922 Zone Diameter (mm) | S. aureus ATCC 29213 Zone Diameter (mm) |
|---|---|---|
| PLA (w/v) | 0 | 0 |
| Ampiciline (AMP) | 12 | 14 |
| PLA (w/v)/10 J | 6 | 10 |
| PLA (w/v)/20 J | 7 | 11 |
| PLA (w/v)/30 J | 7 | 9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Altan, E.; Karacelebi, Y.; Saatcioglu, E.; Ulag, S.; Sahin, A.; Aksu, B.; Croitoru, A.-M.; Codrea, C.I.; Ficai, D.; Gunduz, O.; et al. Fabrication of Electrospun Juglans regia (Juglone) Loaded Poly(lactic acid) Scaffolds as a Potential Wound Dressing Material. Polymers 2022, 14, 1971. https://doi.org/10.3390/polym14101971
Altan E, Karacelebi Y, Saatcioglu E, Ulag S, Sahin A, Aksu B, Croitoru A-M, Codrea CI, Ficai D, Gunduz O, et al. Fabrication of Electrospun Juglans regia (Juglone) Loaded Poly(lactic acid) Scaffolds as a Potential Wound Dressing Material. Polymers. 2022; 14(10):1971. https://doi.org/10.3390/polym14101971
Chicago/Turabian StyleAltan, Eray, Yasin Karacelebi, Elif Saatcioglu, Songul Ulag, Ali Sahin, Burak Aksu, Alexa-Maria Croitoru, Cosmin Iulian Codrea, Denisa Ficai, Oguzhan Gunduz, and et al. 2022. "Fabrication of Electrospun Juglans regia (Juglone) Loaded Poly(lactic acid) Scaffolds as a Potential Wound Dressing Material" Polymers 14, no. 10: 1971. https://doi.org/10.3390/polym14101971








