Preparation and Release of pH-Sensitive β-Cyclodextrin Derivative Micelles Loaded with Paclitaxel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Equipment
2.2. Synthesis and Characterization of 6-β-CD-N-ODMA
2.2.1. Synthesis of 6-OTs-β-CD
2.2.2. Synthesis of 6-CHO-β-CD
2.2.3. Synthesis of 6-β-CD-N-ODMA
2.3. Preparation of PTX/6-β-CD-N-ODMA Micelles
2.3.1. Single-Factor Study
2.3.2. Orthogonal Test for Optimization of Drug-Loaded Micelles Preparation
2.3.3. Determination of Critical Micellar Concentration (CMC)
2.3.4. Properties and Characterization of PTX/6-β-CD-N-ODMA
2.4. Estimation of Drug Release
3. Results and Discussion
3.1. Synthesis of 6-β-CD-N-ODMA
3.2. Critical Micelle Concentration of 6-β-CD-N-ODMA
3.3. Formulation Optimization of PTX/6-β-CD-N-ODMA
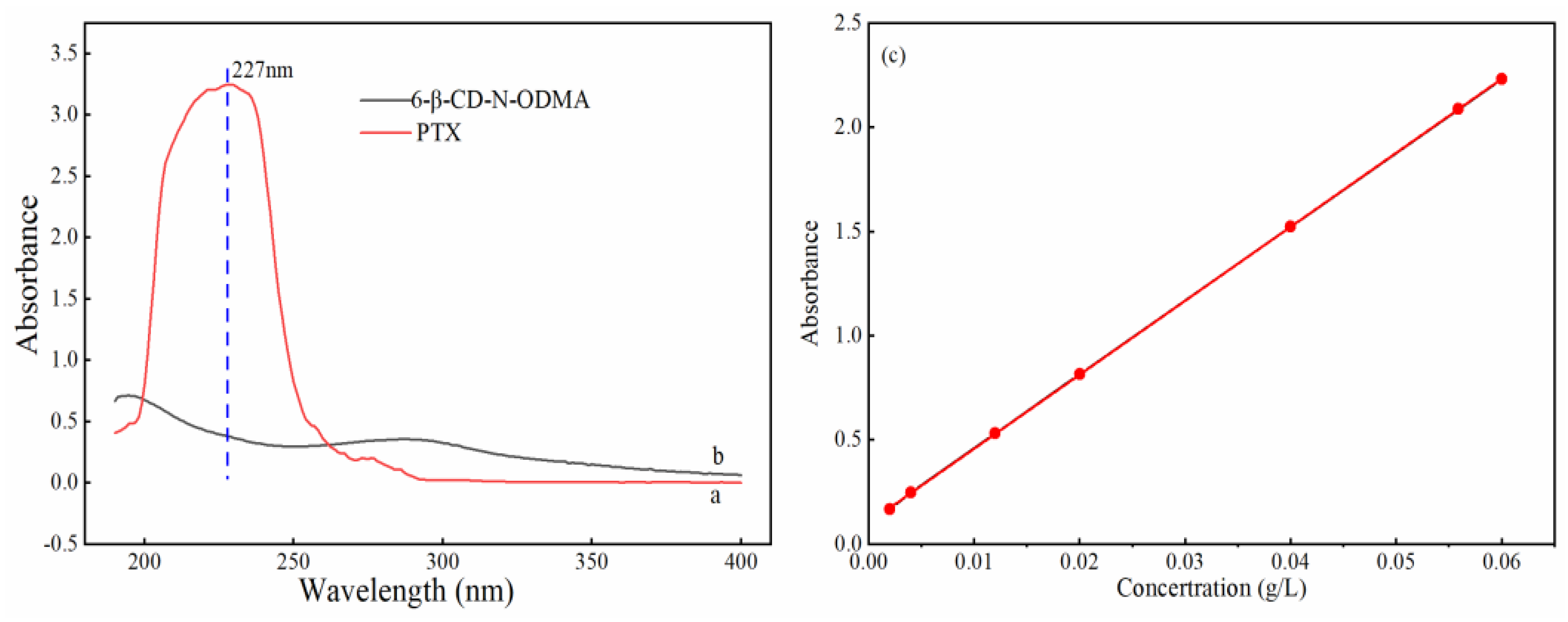
3.3.1. Standard Curve of PTX
3.3.2. Single-Factor Analysis
3.3.3. Orthogonal Result Analysis
3.4. Characterization of PTX/6-β-CD-N-ODMA
3.4.1. FTIR Analysis
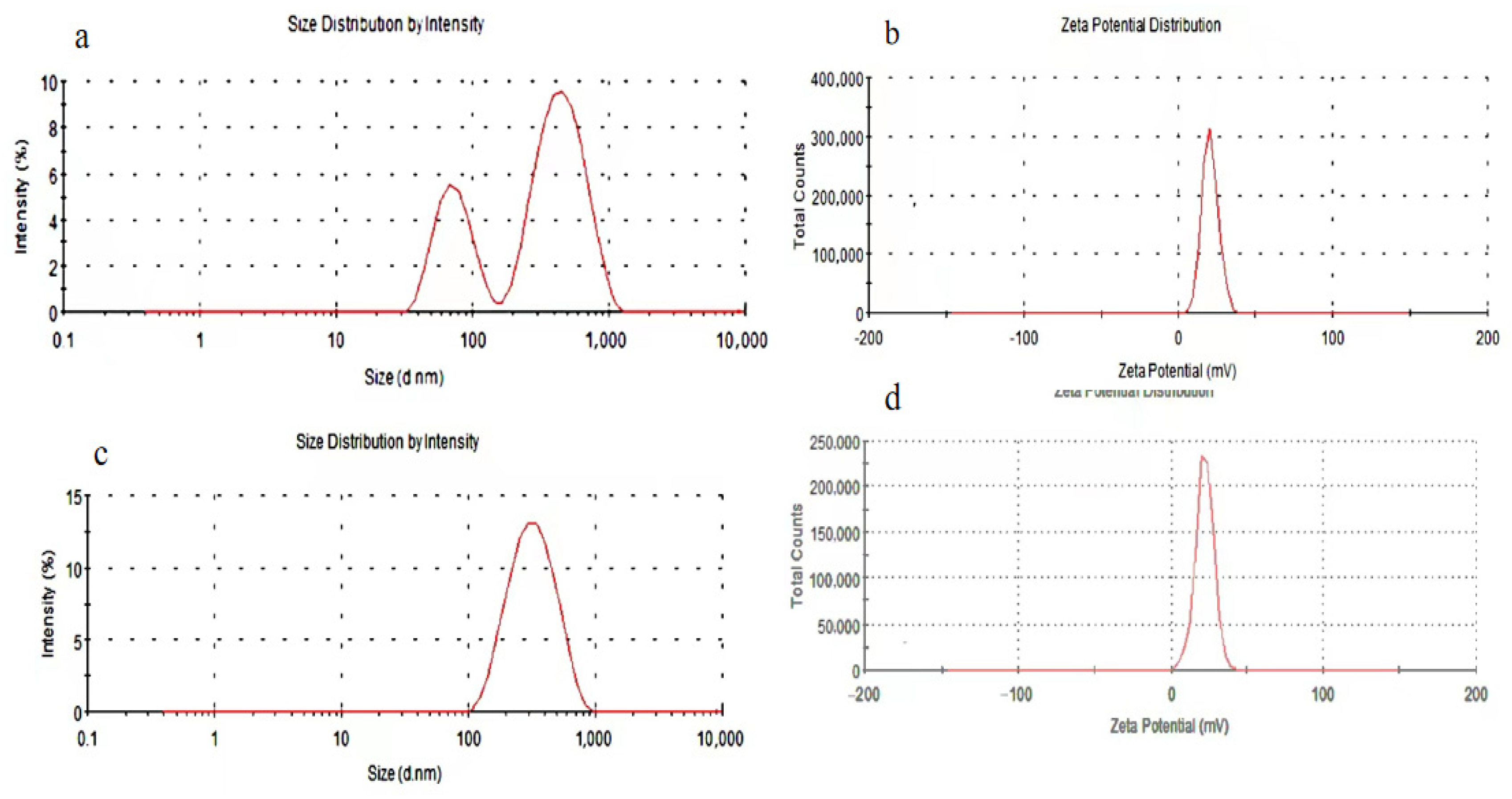
3.4.2. Particle Size Distribution and Zeta Potential Analysis
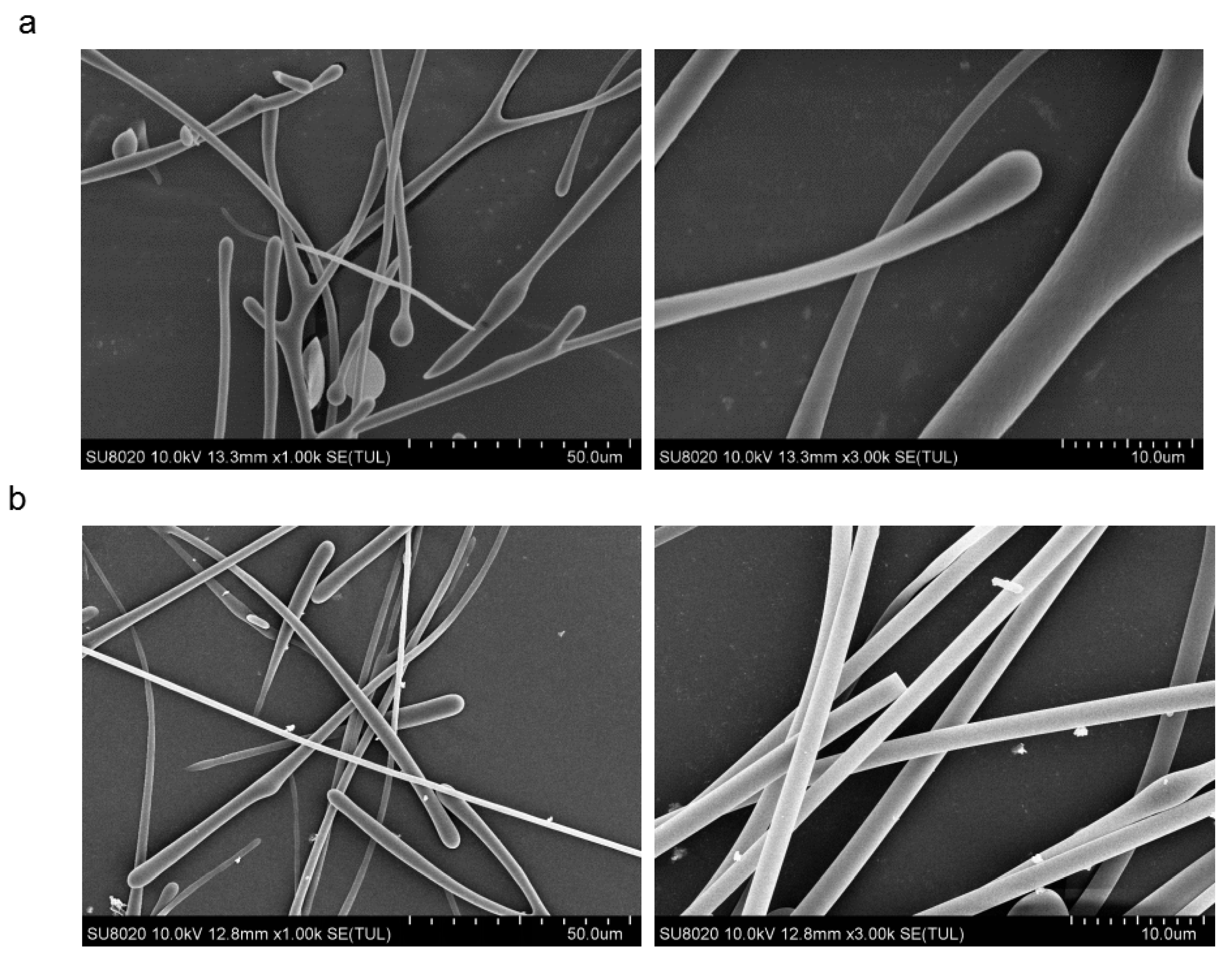
3.4.3. SEM Analysis
3.5. Sustained Release Behavior of PTX
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Croy, S.R.; Kwon, G.S. Polymeric micelles for drug delivery. Curr. Pharm. Des. 2006, 12, 4669–4684. [Google Scholar] [CrossRef] [PubMed]
- Cai, M.T.; Zhu, K.; Qiu, Y.B.; Liu, X.R.; Chen, Y.W.; Luo, X.L. pH and redox-responsive mixed micelles for enhanced intracellular drug release. Colloids Surf. B 2014, 116, 424–431. [Google Scholar] [CrossRef] [PubMed]
- Biswas, S.; Kumari, P.; Lakhani, P.M.; Ghosh, B. Recent advances in polymeric micelles for anti-cancer drug delivery. Eur. J. Pharm. Sci. 2016, 83, 184–202. [Google Scholar] [CrossRef]
- Pasut, G.; Veronese, F.M. PEG conjugates in clinical development or use as anticancer agents: An overview. Adv. Drug Del. Rev. 2009, 61, 1177–1188. [Google Scholar] [CrossRef]
- Sun, H.L.; Guo, B.N.; Cheng, R.; Meng, F.H.; Liu, H.Y.; Zhong, Z.Y. Biodegradable micelles with sheddable poly (ethylene glycol) shells for triggered intracellular release of doxorubicin. Biomaterials 2009, 30, 6358–6366. [Google Scholar] [CrossRef] [PubMed]
- Edgar, P.H.; Alberto, F.M. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar]
- Deng, C.; Jiang, Y.J.; Cheng, R.; Meng, F.H.; Zhong, Z.Y. Biodegradable polymeric micelles for targeted and controlled anticancer drug delivery: Promises, progress and prospects. Nano Today 2012, 7, 467–480. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, L.; Li, Y.K.; Wang, C.Q. Reduction and pH dual-responsive nanoparticles based chitooligosaccharide-based graft copolymer for doxorubicin delivery. Colloids Surf. A Physicochem. Eng. Asp. 2016, 497, 8–15. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Guo, X.; Tang, Z.M.; Zhong, Z.D.; Zhou, S.B. Redox-responsive polyanhydride micelles for cancer therapy. Biomaterials 2014, 35, 3080–3090. [Google Scholar] [CrossRef]
- Zhang, J.X.; Ma, P.X. Cyclodextrin-based supramolecular systems for drug delivery: Recent progress and future perspective. Adv. Drug Deliv. Rev. 2013, 65, 1215–1233. [Google Scholar] [CrossRef] [Green Version]
- Mura, P. Analytical techniques for characterization of cyclodextrin complexes in the solid state: A review. J. Pharm. Biomed. Anal. 2014, 101, 238–250. [Google Scholar] [CrossRef]
- Gaucher, G.; Dufresne, M.H.; Sant, V.P.; Kang, N.; Maysinger, D.; Leroux, J.C. Block copolymer micelles: Preparation, characterization and application in drug delivery. J. Control. Release 2005, 109, 169–188. [Google Scholar] [CrossRef] [PubMed]
- Dharmendra, N.; Bhattarai, J.K.; Demchenko, A.V.; Stine, K.J. A pH sensitive thiolated β-cyclodextrin-modified nanoporous gold for controlled release of doxorubicin. J. Drug Deliv. Sci. Technol. 2020, 60, 101985. [Google Scholar]
- Ji, Q.; Qiu, L.Y. Mechanism study of PEGylated polyester and β-cyclodextrin integrated micelles on drug resistance reversal in MRP1-overexpressed HL60/ADR cells. Colloids Surf. B 2016, 144, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Kim, C.; Jeong, D.; Kim, S.; Kim, Y.; Jung, S. Cyclodextrin functionalized agarose gel with low gelling temperature for controlled drug delivery systems. Carbohydr. Polym. 2019, 222, 1–9. [Google Scholar] [CrossRef]
- Zhou, Z.S.; Guo, F.; Wang, N.R.; Meng, M.; Li, G.Y. Dual pH-sensitive Supramolecular micelles from star-shaped PDMAEMA based on β-cyclodextrin for drug release. Int. J. Biol. Macromol. 2018, 116, 911–919. [Google Scholar] [CrossRef]
- Lv, J.Q.; Liang, R.C.; Xia, Z.H.; Li, Y.; Lv, Z.F.; Hou, D.Z.; Yu, L.P.; Chen, G.; Liu, Y.; Yang, F. Synthesis and characterization of Amphiphilic star-shaped copolymers based on β-cyclodextrin for micelles drug delivery. Drug Dev. Ind. Pharm. 2019, 45, 1017–1028. [Google Scholar] [CrossRef]
- Ramesh, K.; Balavigneswaran, C.K.; Siboro, S.A.P.; Muthuvijayan, V.; Lim, K.T. Synthesis of cyclodextrin-derived star poly(N-vinylpyrrolidone)/poly(lactic-co-glycolide) supramolecular micelles via host-guest interaction for delivery of doxorubicin. Polymer 2021, 214, 123243. [Google Scholar] [CrossRef]
- Gao, Y.R.; Li, G.Y.; Zhou, Z.S.; Gao, L.L.; Tao, Q. Sensitive complex micelles based on host-guest recognition from chitosan-graft-β-cyclodextrin for drug release. Int. J. Biol. Macrom. 2017, 105, 74–80. [Google Scholar] [CrossRef]
- Poudel, A.J.; He, F.; Huang, L.X.; Xiao, L.; Yang, G. Supramolecular hydrogels based on poly (ethylene glycol)-poly (lactic acid) block copolymer micelles and alpha-cyclodextrin for potential injectable drug delivery system. Carbohydr. Polym. 2018, 194, 69–79. [Google Scholar] [CrossRef]
- Huang, X.J.; Xiao, Y.; Lang, M.D. Self-assembly of pH-sensitive mixed micelles based on linear and star copolymers for drug delivery. J. Colloid Interface Sci. 2011, 364, 92–99. [Google Scholar] [CrossRef] [PubMed]
- Jain, V.; Jain, S.; Mahajan, S.C. Nanomedicines based drug delivery systems for anticancer targeting and treatment. Cur. Drug Deliv. 2015, 12, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Ganta, S.; Devalapally, H.; Shahiwala, A.; Amiji, M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J. Control. Release 2008, 126, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Shang, Y.Q.; Guo, L.X.; Wang, Z.G. Tetraphenylsilane Cored Star Shaped Amphiphilic Block Copolymers for pH-Responsive Anticancer Drug Delivery. Macrom. Chem. Phys. 2019, 220, 1900248. [Google Scholar] [CrossRef]
- Mao, J.; Li, Y.; Wu, T.; Yuan, C.H.; Zeng, B.R.; Xu, Y.T.; Dai, L.Z. A simple dual-pH responsive prodrug-based polymeric micelles for drug delivery. ACS Appl. Mater. Interfaces 2016, 8, 17109–171038. [Google Scholar] [CrossRef]
- Dong, P.W.; Wang, X.H.; Gu, Y.C.; Wang, Y.J.; Wang, Y.J.; Gong, C.Y.; Luo, F.; Guo, G.; Zhao, X.; Wei, Y.Q.; et al. Self-assembled biodegradable micelles based on star-shaped PCL-b-PEG copolymers for chemotherapeutic drug delivery. Colloids Surf. A Physicochem. Eng. Asp. 2010, 358, 128–134. [Google Scholar] [CrossRef]
- Arbuck, S.G.; Christian, M.C.; Fisherman, J.S.; Cazenave, L.A.; Sarosy, G.; Suffness, M.; Adams, J.; Canetta, R.; Cole, K.E.; Friedman, M.A. Clinical development of Taxol. J. Natl. Cancer Inst. Monogr. 1993, 15, 11–24. [Google Scholar]
- Wang, L.; Li, H.; Ren, Y.; Zou, S.; Fang, W.; Jiang, X.; Jia, L.; Li, L.; Liu, X.; Yuan, X.; et al. Targeting HDAC with a novel inhibitor effectively reverses paclitaxel resistance in non-small cell lung cancer via multiple mechanisms. Cell Death Dis. 2016, 7, e2063. [Google Scholar] [CrossRef]
- Zhong, N.; Byun, H.S.; Bittrnan, R. An Improved Synthesis of 6-O-Monotosyi-6-deoxy-β-cyclodextrin. Tetrahedron Lett. 1998, 39, 2919–2920. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Hong, S.Y.; Martin, K.A.; Czarnik, A.W. A General Method for the Synthesis of Cyclodextrinyl Aldehydes and Carboxylic Acids. J. Org. Chem. 1995, 60, 2792–2795. [Google Scholar] [CrossRef]
- Malenkovskaya, M.A.; Shipilov, D.A.; Vasyanina, L.K.; Grachev, M.K. Synthesis of 6-Mono-aldehyde of β-cyclodextrin and imino derivatives on its basis. Rus. J. Gen. Chem. 2016, 86, 2725–2727. [Google Scholar] [CrossRef]
- Xu, M.Y.; Wu, S.Z.; Zeng, F.; Yu, C.M. Cyclodextrin supramolecular complex as a water-soluble ratiometric sensor for ferric ion sensing. Langmuir 2010, 26, 4529–4534. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Li, Y.; Kim, M.S.; Kang, S.W.; Jeong, J.H.; Lee, D.S. Synthesis and evaluation of biotin-conjugated pH-responsive polymeric micelles as drug carriers. Int. J. Pharm. 2012, 427, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.B.; Jin, Y.; Qi, R.; Peng, S.J.; Fan, B.Z. Oxidation responsive monocleavable amphiphilic di-block polymer micelles labeled with a single diselenide. Polym. Chem. 2013, 4, 4017–4023. [Google Scholar] [CrossRef]
- Lin, W.J.; Yang, C.F.; Xue, Z.L.; Huang, Y.H.; Luo, H.S.; Zu, X.H.; Zhang, L.J.; Yi, G.B. Controlled construction of gold nanoparticles in situ from β-cyclodextrin based unimolecular micelles for in vitro computed tomography imaging. J. Colloid Interface Sci. 2018, 528, 135–144. [Google Scholar] [CrossRef]
- Mastromatteo, M.; Conte, A.; Del Nobile, M.A. Advances in controlled release devices for food packaging applications. Trends Food Sci. Tech. 2010, 21, 591–598. [Google Scholar] [CrossRef]




| Level | PTX/mg A | 6-β-CD-N-ODMA/mg B | DMF/mL C | Water/mL D |
|---|---|---|---|---|
| 1 | 1 | 30 | 3 | 1 |
| 2 | 2 | 40 | 5 | 2 |
| 3 | 3 | 50 | 7 | 3 |
| 4 | 4 | 60 | 10 | 4 |
| No. | A | B | C | D | Blank | Drug Loading (%) |
|---|---|---|---|---|---|---|
| 1 | 1 | 1 | 1 | 1 | 1 | 0.51 |
| 2 | 1 | 2 | 2 | 2 | 2 | 1.08 |
| 3 | 1 | 3 | 3 | 3 | 3 | 0.99 |
| 4 | 1 | 4 | 4 | 4 | 4 | 0.66 |
| 5 | 2 | 1 | 2 | 3 | 4 | 1.27 |
| 6 | 2 | 2 | 1 | 4 | 3 | 1.14 |
| 7 | 2 | 3 | 4 | 1 | 2 | 1.03 |
| 8 | 2 | 4 | 3 | 2 | 1 | 0.724 |
| 9 | 3 | 1 | 3 | 4 | 2 | 0.86 |
| 10 | 3 | 2 | 4 | 3 | 1 | 0.62 |
| 11 | 3 | 3 | 1 | 2 | 4 | 1.96 |
| 12 | 3 | 4 | 2 | 1 | 3 | 1.45 |
| 13 | 4 | 1 | 4 | 2 | 3 | 0.91 |
| 14 | 4 | 2 | 3 | 1 | 4 | 1.12 |
| 15 | 4 | 3 | 2 | 4 | 1 | 1.24 |
| 16 | 4 | 4 | 1 | 3 | 2 | 1.06 |
| K1 | 0.810 | 0.888 | 1.167 | 1.028 | 0.774 | |
| K2 | 1.041 | 0.990 | 1.260 | 1.169 | 1.008 | |
| K3 | 1.222 | 1.305 | 0.923 | 0.985 | 1.123 | |
| K4 | 1.083 | 0.973 | 0.805 | 0.975 | 1.252 | |
| R | 0.412 | 0.417 | 0.455 | 0.194 | 0.478 |
| Factors | Sum of Squares of Deviations | Degrees of Freedom | Ratio of F | The Critical Value of F |
|---|---|---|---|---|
| A | 0.352 | 3 | 0.710 | 9.280 |
| B | 0.402 | 3 | 0.810 | 9.280 |
| C | 0.534 | 3 | 1.077 | 9.280 |
| D | 0.096 | 3 | 0.194 | 9.280 |
| Error | 0.500 | 3 |
| pH | Model | Equation | r2 a |
|---|---|---|---|
| 7.4 | Zero-order kinetics | Q1 = 6.131t1 + 2.917 | 0.881 |
| First-order kinetics | ln(100 − Q1) = −0.079t1 + 4.585 | 0.895 | |
| Higuhi equation | Q1 = 17.358t11/2 − 4.869 | 0.892 | |
| Weibull equation | lnln[1/(1 − Q1/100)] = 1.134lnt1 − 2.664 | 0.929 | |
| Ritger–Peppas equation | lnQ1 = 1.015ln t1 + 1.923 | 0.922 | |
| 5.0 | Zero-order kinetics | Q2 = 15.821t2 − 2.5 | 0.860 |
| First-order kinetics | ln(100 − Q2) = −0.444t2 + 4.899 | 0.850 | |
| Higuhi equation | Q2 = 42.887t21/2 − 19.376 | 0.784 | |
| Weibull equation | lnln[1/(1 − Q2/100)] = 2.001lnt2 − 2.658 | 0.914 | |
| Ritger–Peppas equation | lnQ2 = 1.403lnt2 + 2.082 | 0.925 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, M.; Jiang, W.; Xie, X.; Jaiswal, Y.; Williams, L.; Wei, M.; Mo, Y.; Guan, Y.; Yang, H. Preparation and Release of pH-Sensitive β-Cyclodextrin Derivative Micelles Loaded with Paclitaxel. Polymers 2022, 14, 2482. https://doi.org/10.3390/polym14122482
Zhao M, Jiang W, Xie X, Jaiswal Y, Williams L, Wei M, Mo Y, Guan Y, Yang H. Preparation and Release of pH-Sensitive β-Cyclodextrin Derivative Micelles Loaded with Paclitaxel. Polymers. 2022; 14(12):2482. https://doi.org/10.3390/polym14122482
Chicago/Turabian StyleZhao, Meirong, Weiwei Jiang, Xinrong Xie, Yogini Jaiswal, Leonard Williams, Mei Wei, Ying Mo, Yifu Guan, and Hua Yang. 2022. "Preparation and Release of pH-Sensitive β-Cyclodextrin Derivative Micelles Loaded with Paclitaxel" Polymers 14, no. 12: 2482. https://doi.org/10.3390/polym14122482






