Polyglycerols as Multi-Functional Platforms: Synthesis and Biomedical Applications †
Abstract
:1. Introduction
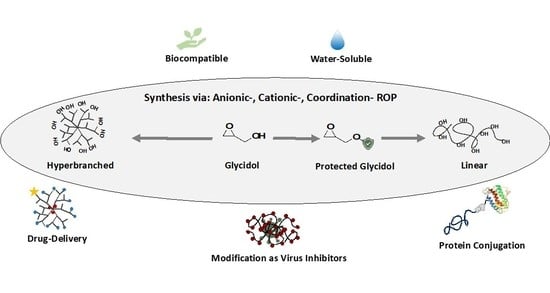
2. Synthetic Strategies for Polyglycidols
2.1. Cationic Ring-Opening Polymerization
2.1.1. Hyperbranched Polyglycidol
2.1.2. Linear Polyglycidol
2.2. Anionic Ring-Opening Polymerization
2.2.1. Dendritic and Hyperbranched Polyglycerol (dPG and HPG)
2.2.2. Linear Polyglycidol
2.3. Coordination Ring-Opening Polymerization
2.3.1. Hyperbranched Polyglycidol
2.3.2. High Molecular Weight Linear Polyglycidol
3. Polyglycidols as a Multiplatform for Biomedical Applications
3.1. Drug Delivery Systems, Protein and Surface Conjugation
3.2. Viral Infection Inhibition
3.3. Antifouling Coatings for Biomedical Application
4. Summary
Author Contributions
Funding
Conflicts of Interest
References
- Mülhaupt, R. Hermann Staudinger and the Origin of Macromolecular Chemistry. Angew. Chem. Int. Ed. 2004, 43, 1054–1063. [Google Scholar] [CrossRef] [PubMed]
- Jiayi, P.; Rostamizadeh, K.; Filipczak, N.; Torchilin, V.P. Polymeric Co-Delivery Systems in Cancer Treatment: An Overview on Component Drugs’ Dosage Ratio Effect. Molecules 2019, 24, 1035. [Google Scholar]
- Bianculli, R.H.; Mase, J.D.; Schulz, M.D. Antiviral Polymers: Past Approaches and Future Possibilities. Macromolecules 2020, 53, 9158–9186. [Google Scholar] [CrossRef]
- Zelikin, A.N.; Stellacci, F. Broad-Spectrum Antiviral Agents Based on Multivalent Inhibitors of Viral Infectivity. Adv. Healthc. Mater. 2021, 10, e2001433. [Google Scholar] [CrossRef]
- Dernedde, J.; Rausch, A.; Weinhart, M.; Enders, S.; Tauber, R.; Licha, K.; Schirner, M.; Zügel, U.; von Bonin, A.; Haag, R. Dendritic polyglycerol sulfates as multivalent inhibitors of inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 19679. [Google Scholar] [CrossRef] [Green Version]
- Bochenek, M.; Oleszko-Torbus, N.; Wałach, W.; Lipowska-Kur, D.; Dworak, A.; Utrata-Wesołek, A. Polyglycidol of Linear or Branched Architecture Immobilized on a Solid Support for Biomedical Applications. Polym. Rev. 2020, 60, 717–767. [Google Scholar] [CrossRef]
- Thomas, A.; Müller, S.S.; Frey, H. Beyond Poly(ethylene glycol): Linear Polyglycerol as a Multifunctional Polyether for Biomedical and Pharmaceutical Applications. Biomacromolecules 2014, 15, 1935–1954. [Google Scholar] [CrossRef]
- Wilms, D.; Stiriba, S.-E.; Frey, H. Hyperbranched Polyglycerols: From the Controlled Synthesis of Biocompatible Polyether Polyols to Multipurpose Applications. Acc. Chem. Res. 2010, 43, 129–141. [Google Scholar] [CrossRef]
- Herzberger, J.; Niederer, K.; Pohlit, H.; Seiwert, J.; Worm, M.; Wurm, F.R.; Frey, H. Polymerization of Ethylene Oxide, Propylene Oxide, and Other Alkylene Oxides: Synthesis, Novel Polymer Architectures, and Bioconjugation. Chem. Rev. 2016, 116, 2170–2243. [Google Scholar] [CrossRef] [Green Version]
- Flory, P.J. Molecular Size Distribution in Three Dimensional Polymers. VI. Branched Polymers Containing A—R—Bf-1 Type Units. J. Am. Chem. Soc. 1952, 74, 2718–2723. [Google Scholar] [CrossRef]
- Dworak, A.; Walach, W.; Trzebicka, B. Cationic polymerization of glycidol. Polymer structure and polymerization mechanism. Macromol. Chem. Phys. 1995, 196, 1963–1970. [Google Scholar] [CrossRef]
- Tokar, R.; Kubisa, P.; Penczek, S.; Dworak, A. Cationic polymerization of glycidol: Coexistence of the activated monomer and active chain end mechanism. Macromolecules 1994, 27, 320–322. [Google Scholar] [CrossRef]
- Mohammadifar, E.; Bodaghi, A.; Dadkhahtehrani, A.; Nemati Kharat, A.; Adeli, M.; Haag, R. Green Synthesis of Hyperbranched Polyglycerol at Room Temperature. ACS Macro Lett. 2017, 6, 35–40. [Google Scholar] [CrossRef]
- Kim, S.E.; Yang, H.J.; Choi, S.; Hwang, E.; Kim, M.; Paik, H.-J.; Jeong, J.-E.; Park, Y.I.; Kim, J.C.; Kim, B.-S.; et al. A recyclable metal-free catalytic system for the cationic ring-opening polymerization of glycidol under ambient conditions. Green Chem. 2022, 24, 251–258. [Google Scholar] [CrossRef]
- Abbina, S.; Vappala, S.; Kumar, P.; Siren, E.M.J.; La, C.C.; Abbasi, U.; Brooks, D.E.; Kizhakkedathu, J.N. Hyperbranched polyglycerols: Recent advances in synthesis, biocompatibility and biomedical applications. J. Mater. Chem. B 2017, 5, 9249–9277. [Google Scholar] [CrossRef] [PubMed]
- Haag, R.; Sunder, A.; Stumbé, J.-F. An Approach to Glycerol Dendrimers and Pseudo-Dendritic Polyglycerols. J. Am. Chem. Soc. 2000, 122, 2954–2955. [Google Scholar] [CrossRef]
- Sunder, A.; Hanselmann, R.; Frey, H.; Mülhaupt, R. Controlled Synthesis of Hyperbranched Polyglycerols by Ring-Opening Multibranching Polymerization. Macromolecules 1999, 32, 4240–4246. [Google Scholar] [CrossRef]
- Schmitt, V.; Rodríguez-Rodríguez, C.; Hamilton, J.L.; Shenoi, R.A.; Schaffer, P.; Sossi, V.; Kizhakkedathu, J.N.; Saatchi, K.; Häfeli, U.O. Quantitative SPECT imaging and biodistribution point to molecular weight independent tumor uptake for some long-circulating polymer nanocarriers. RSC Adv. 2018, 8, 5586–5595. [Google Scholar] [CrossRef] [Green Version]
- Kautz, H.; Sunder, A.; Frey, H. Control of the molecular weight of hyperbranched polyglycerols. Macromol. Symp. 2001, 163, 67–74. [Google Scholar] [CrossRef]
- Wallert, M.; Plaschke, J.; Dimde, M.; Ahmadi, V.; Block, S.; Haag, R. Automated Solvent-Free Polymerization of Hyperbranched Polyglycerol with Tailored Molecular Weight by Online Torque Detection. Macromol. Mater. Eng. 2021, 306, 2000688. [Google Scholar] [CrossRef]
- Wilms, D.; Wurm, F.; Nieberle, J.; Böhm, P.; Kemmer-Jonas, U.; Frey, H. Hyperbranched Polyglycerols with Elevated Molecular Weights: A Facile Two-Step Synthesis Protocol Based on Polyglycerol Macroinitiators. Macromolecules 2009, 42, 3230–3236. [Google Scholar] [CrossRef]
- Moore, E.; Zill, A.T.; Anderson, C.A.; Jochem, A.R.; Zimmerman, S.C.; Bonder, C.S.; Kraus, T.; Thissen, H.; Voelcker, N.H. Synthesis and Conjugation of Alkyne-Functional Hyperbranched Polyglycerols. Macromol. Chem. Phys. 2016, 217, 2252–2261. [Google Scholar] [CrossRef]
- Kainthan, R.K.; Muliawan, E.B.; Hatzikiriakos, S.G.; Brooks, D.E. Synthesis, Characterization, and Viscoelastic Properties of High Molecular Weight Hyperbranched Polyglycerols. Macromolecules 2006, 39, 7708–7717. [Google Scholar] [CrossRef]
- Ul-haq, M.I.; Shenoi, R.A.; Brooks, D.E.; Kizhakkedathu, J.N. Solvent-assisted anionic ring opening polymerization of glycidol: Toward medium and high molecular weight hyperbranched polyglycerols. J. Polym. Sci. Part A Polym. Chem. 2013, 51, 2614–2621. [Google Scholar] [CrossRef]
- Anilkumar, P.; Lawson, T.B.; Abbina, S.; Mäkelä, J.T.A.; Sabatelle, R.C.; Takeuchi, L.E.; Snyder, B.D.; Grinstaff, M.W.; Kizhakkedathu, J.N. Mega macromolecules as single molecule lubricants for hard and soft surfaces. Nat. Commun. 2020, 11, 2139. [Google Scholar] [CrossRef]
- Kainthan, R.K.; Brooks, D.E. In Vivo Biological Evaluation of High Molecular Weight Hyperbranched Polyglycerols. no. 0142-9612 (Print). Biomaterials 2007, 28, 4779–4787. [Google Scholar] [CrossRef]
- Shenoi, R.A.; Narayanannair, J.K.; Hamilton, J.L.; Lai, B.F.L.; Horte, S.; Kainthan, R.K.; Varghese, J.P.; Rajeev, K.G.; Manoharan, M.; Kizhakkedathu, J.N. Branched Multifunctional Polyether Polyketals: Variation of Ketal Group Structure Enables Unprecedented Control over Polymer Degradation in Solution and within Cells. J. Am. Chem. Soc. 2012, 134, 14945–14957. [Google Scholar] [CrossRef]
- Hubbell, J.A.; Thomas, S.N.; Swartz, M.A. Materials engineering for immunomodulation. Nature 2009, 462, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Son, S.; Shin, E.; Kim, B.-S. Redox-Degradable Biocompatible Hyperbranched Polyglycerols: Synthesis, Copolymerization Kinetics, Degradation, and Biocompatibility. Macromolecules 2015, 48, 600–609. [Google Scholar] [CrossRef]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-responsive nanocarriers for drug delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Sun, H.; Meng, F.; Cheng, R.; Deng, C.; Zhong, Z. Reduction-sensitive degradable micellar nanoparticles as smart and intuitive delivery systems for cancer chemotherapy. Expert Opin. Drug Deliv. 2013, 10, 1109–1122. [Google Scholar] [CrossRef] [PubMed]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in Drug Delivery: Pros and Cons as Well as Potential Alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Wurm, F.; Dingels, C.; Frey, H.; Klok, H.-A. Squaric Acid Mediated Synthesis and Biological Activity of a Library of Linear and Hyperbranched Poly(Glycerol)–Protein Conjugates. Biomacromolecules 2012, 13, 1161–1171. [Google Scholar] [CrossRef] [PubMed]
- Fitton, A.O.; Hill, J.; Jane, D.E.; Millar, R. Synthesis of Simple Oxetanes Carrying Reactive 2-Substituents. Synthesis 1987, 1987, 1140–1142. [Google Scholar] [CrossRef]
- Taton, D.; Le Borgne, A.; Sepulchre, M.; Spassky, N. Synthesis of chiral and racemic functional polymers from glycidol and thioglycidol. Macromol. Chem. Phys. 1994, 195, 139–148. [Google Scholar] [CrossRef]
- Gervais, M.; Brocas, A.-L.; Cendejas, G.; Deffieux, A.; Carlotti, S. Synthesis of Linear High Molar Mass Glycidol-Based Polymers by Monomer-Activated Anionic Polymerization. Macromolecules 2010, 43, 1778–1784. [Google Scholar] [CrossRef]
- Baek, J.; Kim, M.; Park, Y.; Kim, B.-S. Acetal-Based Functional Epoxide Monomers: Polymerizations and Applications. Macromol. Biosci. 2021, 21, 2100251. [Google Scholar] [CrossRef]
- Walach, W.; Kowalczuk, A.; Trzebicka, B.; Dworak, A. Synthesis of High-Molar Mass Arborescent-Branched Polyglycidol via Sequential Grafting. Macromol. Rapid Commun. 2001, 22, 1272–1277. [Google Scholar] [CrossRef]
- Dworak, A.; Panchev, I.; Trzebicka, B.; Walach, W. Hydrophilic and amphiphilic copolymers of 2,3-epoxypropanol-1. Macromol. Symp. 2000, 153, 233–242. [Google Scholar] [CrossRef]
- Hans, M.; Keul, H.; Moeller, M. Chain transfer reactions limit the molecular weight of polyglycidol prepared via alkali metal based initiating systems. Polymer 2009, 50, 1103–1108. [Google Scholar] [CrossRef]
- Hans, M.; Gasteier, P.; Keul, H.; Moeller, M. Ring-Opening Polymerization of ε-Caprolactone by Means of Mono- and Multifunctional Initiators: Comparison of Chemical and Enzymatic Catalysis. Macromolecules 2006, 39, 3184–3193. [Google Scholar] [CrossRef]
- Erberich, M.; Keul, H.; Möller, M. Polyglycidols with Two Orthogonal Protective Groups: Preparation, Selective Deprotection, and Functionalization. Macromolecules 2007, 40, 3070–3079. [Google Scholar] [CrossRef]
- Anja, T.; Niederer, K.; Wurm, F.; Frey, H. Combining Oxyanionic Polymerization and Click-Chemistry: A General Strategy for the Synthesis of Polyether Polyol Macromonomers. Polym. Chem. 2014, 5, 899–909. [Google Scholar]
- Gesine, G.; Weinhart, M.; Becherer, T.; Haag, R.; Huck, W.T.S. Effect of Polymer Brush Architecture on Antibiofouling Properties. Biomacromolecules 2011, 12, 4169–4172. [Google Scholar]
- Toy, A.A.; Reinicke, S.; Müller, A.H.E.; Schmalz, H. One-Pot Synthesis of Polyglycidol-Containing Block Copolymers with Alkyllithium Initiators Using the Phosphazene Base t-BuP4. Macromolecules 2007, 40, 5241–5244. [Google Scholar] [CrossRef]
- Dimitrov, I.; Tsvetanov, C.B. 4.21—High-Molecular-Weight Poly(ethylene oxide). In Polymer Science: A Comprehensive Reference; Matyjaszewski, K., Möller, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2012; pp. 551–569. [Google Scholar]
- Brocas, A.-L.; Mantzaridis, C.; Tunc, D.; Carlotti, S. Polyether synthesis: From activated or metal-free anionic ring-opening polymerization of epoxides to functionalization. Prog. Polym. Sci. 2013, 38, 845–873. [Google Scholar] [CrossRef]
- Spears, B.R.; Waksal, J.; McQuade, C.; Lanier, L.; Harth, E. Controlled branching of polyglycidol and formation of protein–glycidol bioconjugates via a graft-from approach with “PEG-like” arms. Chem. Commun. 2013, 49, 2394–2396. [Google Scholar] [CrossRef]
- Nijenhuis, A.J.; Grijpma, D.W.; Pennings, A.J. Lewis acid catalyzed polymerization of L-lactide. Kinetics and mechanism of the bulk polymerization. Macromolecules 1992, 25, 6419–6424. [Google Scholar] [CrossRef]
- Cherri, M.; Ferraro, M.; Mohammadifar, E.; Quaas, E.; Achazi, K.; Ludwig, K.; Grötzinger, C.; Schirner, M.; Haag, R. Biodegradable Dendritic Polyglycerol Sulfate for the Delivery and Tumor Accumulation of Cytostatic Anticancer Drugs. ACS Biomater. Sci. Eng. 2021, 7, 2569–2579. [Google Scholar] [CrossRef]
- Zabihi, F.; Graff, P.; Schumacher, F.; Kleuser, B.; Hedtrich, S.; Haag, R. Synthesis of poly(lactide-co-glycerol) as a biodegradable and biocompatible polymer with high loading capacity for dermal drug delivery. Nanoscale 2018, 10, 16848–16856. [Google Scholar] [CrossRef]
- Reisbeck, F.; Ozimkovski, A.; Cherri, M.; Dimde, M.; Quaas, E.; Mohammadifar, E.; Achazi, K.; Haag, R. Gram Scale Synthesis of Dual-Responsive Dendritic Polyglycerol Sulfate as Drug Delivery System. Polymers 2021, 13, 982. [Google Scholar] [CrossRef] [PubMed]
- Jamróz-Piegza, M.; Utrata-Wesołek, A.; Trzebicka, B.; Dworak, A. Hydrophobic modification of high molar mass polyglycidol to thermosensitive polymers. Eur. Polym. J. 2006, 42, 2497–2506. [Google Scholar] [CrossRef]
- Haouet, A.; Sepulchre, M.; Spassky, N. Preparation et proprietes des poly(R)-glycidols. Eur. Polym. J. 1983, 19, 1089–1098. [Google Scholar] [CrossRef]
- Utrata-Wesołek, A.; Żymełka-Miara, I.; Kowalczuk, A.; Trzebicka, B.; Dworak, A. Photocrosslinking of Polyglycidol and Its Derivative: Route to Thermoresponsive Hydrogels. Photochem. Photobiol. 2018, 94, 52–60. [Google Scholar] [CrossRef]
- Labbé, A.; Carlotti, S.; Billouard, C.; Desbois, P.; Deffieux, A. Controlled High-Speed Anionic Polymerization of Propylene Oxide Initiated by Onium Salts in the Presence of Triisobutylaluminum. Macromolecules 2007, 40, 7842–7847. [Google Scholar] [CrossRef]
- Kowalczuk, A.; Trzcinska, R.; Trzebicka, B.; Müller, A.H.E.; Dworak, A.; Tsvetanov, C.B. Loading of polymer nanocarriers: Factors, mechanisms and applications. Prog. Polym. Sci. 2014, 39, 43–86. [Google Scholar] [CrossRef]
- Mohammadifar, E.; Nemati Kharat, A.; Adeli, M. Polyamidoamine and polyglycerol; their linear, dendritic and linear–dendritic architectures as anticancer drug delivery systems. J. Mater. Chem. B 2015, 3, 3896–3921. [Google Scholar] [CrossRef] [PubMed]
- Koehler, J.; Marquardt, F.; Keul, H.; Moeller, M. Phosphonoethylated Polyglycidols: A Platform for Tunable Enzymatic Grafting Density. Macromolecules 2013, 46, 3708–3718. [Google Scholar] [CrossRef]
- Zhong, Y.; Dimde, M.; Stöbener, D.; Meng, F.; Deng, C.; Zhong, Z.; Haag, R. Micelles with Sheddable Dendritic Polyglycerol Sulfate Shells Show Extraordinary Tumor Targetability and Chemotherapy in Vivo. ACS Appl. Mater. Interfaces 2016, 8, 27530–27538. [Google Scholar] [CrossRef]
- Braatz, D.; Dimde, M.; Ma, G.; Zhong, Y.; Tully, M.; Grötzinger, C.; Zhang, Y.; Mavroskoufis, A.; Schirner, M.; Zhong, Z.; et al. Toolbox of Biodegradable Dendritic (Poly glycerol sulfate)–SS-poly(ester) Micelles for Cancer Treatment: Stability, Drug Release, and Tumor Targeting. Biomacromolecules 2021, 22, 2625–2640. [Google Scholar] [CrossRef]
- Baabur-Cohen, H.; Vossen, L.I.; Krüger, H.R.; Eldar-boock, A.; Yeini, E.; Landa-Rouben, N.; Tiram, G.; Wedepohl, S.; Markovsky, E.; Leor, J.; et al. In vivo comparative study of distinct polymeric architectures bearing a combination of paclitaxel and doxorubicin at a synergistic ratio. J. Control. Release 2017, 257, 118–131. [Google Scholar] [CrossRef] [PubMed]
- Rancan, F.; Asadian-Birjand, M.; Dogan, S.; Graf, C.; Cuellar, L.; Lommatzsch, S.; Blume-Peytavi, U.; Calderón, M.; Vogt, A. Effects of thermoresponsivity and softness on skin penetration and cellular uptake of polyglycerol-based nanogels. J. Control. Release 2016, 228, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Gerecke, C.; Edlich, A.; Giulbudagian, M.; Schumacher, F.; Zhang, N.; Said, A.; Yealland, G.; Lohan, S.B.; Neumann, F.; Meinke, M.C.; et al. Biocompatibility and characterization of polyglycerol-based thermoresponsive nanogels designed as novel drug-delivery systems and their intracellular localization in keratinocytes. Nanotoxicology 2017, 11, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Edlich, A.; Gerecke, C.; Giulbudagian, M.; Neumann, F.; Hedtrich, S.; Schäfer-Korting, M.; Ma, N.; Calderon, M.; Kleuser, B. Specific uptake mechanisms of well-tolerated thermoresponsive polyglycerol-based nanogels in antigen-presenting cells of the skin. Eur. J. Pharm. Biopharm. 2017, 116, 155–163. [Google Scholar] [CrossRef]
- Sahle, F.F.; Giulbudagian, M.; Bergueiro, J.; Lademann, J.; Calderón, M. Dendritic polyglycerol and N-isopropylacrylamide based thermoresponsive nanogels as smart carriers for controlled delivery of drugs through the hair follicle. Nanoscale 2017, 9, 172–182. [Google Scholar] [CrossRef]
- Molina, M.; Wedepohl, S.; Miceli, E.; Calderón, M. Overcoming drug resistance with on-demand charged thermoresponsive dendritic nanogels. Nanomedicine 2017, 12, 117–129. [Google Scholar] [CrossRef]
- Miceli, E.; Wedepohl, S.; Osorio Blanco, E.R.; Rimondino, G.N.; Martinelli, M.; Strumia, M.; Molina, M.; Kar, M.; Calderón, M. Semi-interpenetrated, dendritic, dual-responsive nanogels with cytochrome c corona induce controlled apoptosis in HeLa cells. Eur. J. Pharm. Biopharm. 2018, 130, 115–122. [Google Scholar] [CrossRef]
- Hofmann, A.M.; Wurm, F.; Frey, H. Rapid Access to Polyfunctional Lipids with Complex Architecture via Oxyanionic Ring-Opening Polymerization. Macromolecules 2011, 44, 4648–4657. [Google Scholar] [CrossRef]
- Jamróz-Piegza, M.; Wałach, W.; Dworak, A.; Trzebicka, B. Polyether nanoparticles from covalently crosslinked copolymer micelles. J. Colloid Interface Sci. 2008, 325, 141–148. [Google Scholar] [CrossRef]
- Haji Abdolvahab, M.; Venselaar, H.; Fazeli, A.; Arab, S.S.; Behmanesh, M. Point Mutation Approach to Reduce Antigenicity of Interferon Beta. Int. J. Pept. Res. Ther. 2020, 26, 1353–1361. [Google Scholar] [CrossRef]
- Bailon, P.; Palleroni, A.; Schaffer, C.A.; Spence, C.L.; Fung, W.J.; Porter, J.E.; Ehrlich, G.K.; Pan, W.; Xu, Z.X.; Modi, M.W.; et al. Rational design of a potent, long-lasting form of interferon: A 40 kDa branched polyethylene glycol-conjugated interferon alpha-2a for the treatment of hepatitis C. no. 1043-1802 (Print). Bioconjugate Chem. 2001, 12, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.R.; Modi, M.W.; Pedder, S. Use of peginterferon alfa-2a (40 KD) (Pegasys) for the treatment of hepatitis C. no. 0169-409X (Print). Adv. Drug Deliv. Rev. 2002, 54, 571–586. [Google Scholar]
- Kozma, G.T.; Shimizu, T.; Ishida, T.; Szebeni, J. Anti-PEG antibodies: Properties, formation, testing and role in adverse immune reactions to PEGylated nano-biopharmaceuticals. Adv. Drug Deliv. Rev. 2020, 154–155, 163–175. [Google Scholar] [CrossRef]
- Aldosari, B.N.; Alfagih, I.M.; Almurshedi, A.S. Lipid Nanoparticles as Delivery Systems for RNA-Based Vaccines. Pharmaceutics 2021, 13, 206. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Gao, G.F. mRNA vaccines: A matter of delivery. EClinicalMedicine 2021, 32, 100746. [Google Scholar] [CrossRef] [PubMed]
- Lubich, C.; Allacher, P.; de la Rosa, M.; Bauer, A.; Prenninger, T.; Horling, F.M.; Siekmann, J.; Oldenburg, J.; Scheiflinger, F.; Reipert, B.M. The Mystery of Antibodies Against Polyethylene Glycol (PEG)—What do we Know? Pharm. Res. 2016, 33, 2239–2249. [Google Scholar] [CrossRef]
- Licha, K.; Welker, P.; Weinhart, M.; Wegner, N.; Kern, S.; Reichert, S.; Gemeinhardt, I.; Weissbach, C.; Ebert, B.; Haag, R.; et al. Fluorescence Imaging with Multifunctional Polyglycerol Sulfates: Novel Polymeric near-IR Probes Targeting Inflammation. Bioconjugate Chem. 2011, 22, 2453–2460. [Google Scholar] [CrossRef]
- Krüger, H.R.; Schütz, I.; Justies, A.; Licha, K.; Welker, P.; Haucke, V.; Calderón, M. Imaging of doxorubicin release from theranostic macromolecular prodrugs via fluorescence resonance energy transfer. J. Control. Release 2014, 194, 189–196. [Google Scholar] [CrossRef]
- Imran ul-haq, M.; Lai, B.F.L.; Chapanian, R.; Kizhakkedathu, J.N. Influence of architecture of high molecular weight linear and branched polyglycerols on their biocompatibility and biodistribution. Biomaterials 2012, 33, 9135–9147. [Google Scholar] [CrossRef]
- Kainthan, R.K.; Janzen, J.; Levin, E.; Devine, D.V.; Brooks, D.E. Biocompatibility Testing of Branched and Linear Polyglycidol. Biomacromolecules 2006, 7, 703–709. [Google Scholar] [CrossRef]
- Abu Lila, A.S.; Nawata, K.; Shimizu, T.; Ishida, T.; Kiwada, H. Use of polyglycerol (PG), instead of polyethylene glycol (PEG), prevents induction of the accelerated blood clearance phenomenon against long-circulating liposomes upon repeated administration. Int. J. Pharm. 2013, 456, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Gervais, M.; Labbé, A.; Carlotti, S.; Deffieux, A. Direct Synthesis of α-Azido,ω-hydroxypolyethers by Monomer-Activated Anionic Polymerization. Macromolecules 2009, 42, 2395–2400. [Google Scholar] [CrossRef]
- Bej, R.; Achazi, K.; Haag, R.; Ghosh, S. Polymersome Formation by Amphiphilic Polyglycerol-b-polydisulfide-b-polyglycerol and Glutathione-Triggered Intracellular Drug Delivery. Biomacromolecules 2020, 21, 3353–3363. [Google Scholar] [CrossRef] [PubMed]
- Mendrek, S.; Mendrek, A.; Adler, H.-J.; Walach, W.; Dworak, A.; Kuckling, D. Synthesis of poly(glycidol)-block-poly(N-isopropylacrylamide) copolymers using new hydrophilic poly(glycidol) macroinitiator. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 2488–2499. [Google Scholar] [CrossRef]
- Tully, M.; Hauptstein, N.; Licha, K.; Meinel, L.; Lühmann, T.; Haag, R. Linear Polyglycerol for N-terminal-selective Modification of Interleukin-4. J. Pharm. Sci. 2021, 111, 1642–1651. [Google Scholar] [CrossRef]
- Tully, M.; Dimde, M.; Weise, C.; Pouyan, P.; Licha, K.; Schirner, M.; Haag, R. Polyglycerol for Half-Life Extension of Proteins—Alternative to PEGylation? Biomacromolecules 2021, 22, 1406–1416. [Google Scholar] [CrossRef]
- Hauptstein, N.; Pouyan, P.; Kehrein, J.; Dirauf, M.; Driessen, M.D.; Raschig, M.; Licha, K.; Gottschaldt, M.; Schubert, U.S.; Haag, R.; et al. Molecular Insights into Site-Specific Interferon-α2a Bioconjugates Originated from PEG, LPG, and PEtOx. Biomacromolecules 2021, 22, 4521–4534. [Google Scholar] [CrossRef]
- Fonkwo, P.N. Pricing infectious disease. EMBO Rep. 2008, 9, S13–S17. [Google Scholar] [CrossRef] [Green Version]
- Soria-Martinez, L.; Bauer, S.; Giesler, M.; Schelhaas, S.; Materlik, J.; Janus, K.; Pierzyna, P.; Becker, M.; Snyder, N.L.; Hartmann, L.; et al. Prophylactic Antiviral Activity of Sulfated Glycomimetic Oligomers and Polymers. J. Am. Chem. Soc. 2020, 142, 5252–5265. [Google Scholar] [CrossRef]
- Strasfeld, L.; Chou, S. Antiviral drug resistance: Mechanisms and clinical implications. Infect. Dis. Clin. N. Am. 2010, 24, 413–437. [Google Scholar] [CrossRef] [Green Version]
- Achazi, K.; Haag, R.; Ballauff, M.; Dernedde, J.; Kizhakkedathu, J.N.; Maysinger, D.; Multhaup, G. Understanding the Interaction of Polyelectrolyte Architectures with Proteins and Biosystems. Angew. Chem. Int. Ed. 2021, 60, 3882–3904. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Perrett, H.R.; Painter, C.D.; Narayanan, A.; Majowicz, S.A.; Kwong, E.M.; McVicar, R.N.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. Cell 2020, 183, 1043–1057.e1015. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Chen, C.Z.; Swaroop, M.; Xu, M.; Wang, L.; Lee, J.; Wang, A.Q.; Pradhan, M.; Hagen, N.; Chen, L.; et al. Heparan sulfate assists SARS-CoV-2 in cell entry and can be targeted by approved drugs in vitro. Cell Discov. 2020, 6, 80. [Google Scholar] [CrossRef] [PubMed]
- Cagno, V.; Tseligka, E.D.; Jones, S.T.; Tapparel, C. Heparan Sulfate Proteoglycans and Viral Attachment: True Receptors or Adaptation Bias? Viruses 2019, 11, 596. [Google Scholar] [CrossRef] [Green Version]
- Conzelmann, C.; Müller, J.A.; Perkhofer, L.; Sparrer, K.M.J.; Zelikin, A.N.; Münch, J.; Kleger, A. Inhaled and systemic heparin as a repurposed direct antiviral drug for prevention and treatment of COVID-19. Clin. Med. 2020, 20, e218. [Google Scholar] [CrossRef]
- Nahmias, A.J.; Kibrick, S. Inhibitory effect of heparin on herpes simplex virus. J. Bacteriol. 1964, 87, 1060–1066. [Google Scholar] [CrossRef] [Green Version]
- Folkman, J.; Langer, R.; Linhardt, R.J.; Haudenschild, C.; Taylor, S. Angiogenesis Inhibition and Tumor Regression Caused by Heparin or a Heparin Fragment in the Presence of Cortisone. no. 0036-8075 (Print). Science 1983, 221, 719–725. [Google Scholar] [CrossRef]
- Lubor, B.; Wong, R.; Feramisco, J.; Nadeau, D.R.; Varki, N.M.; Varki, A. Heparin and Cancer Revisited: Mechanistic Connections Involving Platelets, P-Selectin, Carcinoma Mucins, and Tumor Metastasis. Proc. Natl. Acad. Sci. USA 2001, 98, 3352. [Google Scholar]
- Paluck Samantha, J.; Nguyen, T.H.; Maynard, H.D. Heparin-Mimicking Polymers: Synthesis and Biological Applications. Biomacromolecules 2016, 17, 3417–3440. [Google Scholar] [CrossRef]
- Kim, S.; Niesler, N.; Rades, N.; Haag, R.; Dernedde, J. Sulfated Dendritic Polyglycerol Is a Potent Complement Inhibitor. Biomacromolecules 2019, 20, 3809–3818. [Google Scholar]
- Pujol, C.A.; Ray, S.; Ray, B.; Damonte, E.B. Antiviral Activity against Dengue Virus of Diverse Classes of Algal Sulfated Polysaccharides. Int. J. Biol. Macromol. 2012, 51, 412–416. [Google Scholar] [CrossRef] [PubMed]
- Edward, Y. The Anti-Inflammatory Effects of Heparin and Related Compounds. Thromb. Res. 2008, 122, 743–752. [Google Scholar]
- Francisco, P.; Arqué, G. Influence of Thromboembolic Events in the Prognosis of Covid-19 Hospitalized Patients. Results from a Cross Sectional Study. PLoS ONE 2021, 16, e0252351. [Google Scholar]
- Miriam, M.; Martin, J.C. Pathological Inflammation in Patients with Covid-19: A Key Role for Monocytes and Macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar]
- Sumati, B.; Hilsch, M.; Cuellar-Camacho, J.L.; Ludwig, K.; Nie, C.; Parshad, B.; Wallert, M.; Block, S.; Lauster, D.; Böttcher, C.; et al. Adaptive Flexible Sialylated Nanogels as Highly Potent Influenza a Virus Inhibitors. Angew. Chem. Int. Ed. 2020, 59, 12417–12422. [Google Scholar]
- Pradip, D.; Bergmann, T.; Cuellar-Camacho, J.L.; Ehrmann, S.; Chowdhury, M.S.; Zhang, M.; Dahmani, I.; Haag, R.; Azab, W. Multivalent Flexible Nanogels Exhibit Broad-Spectrum Antiviral Activity by Blocking Virus Entry. ACS Nano 2018, 12, 6429–6442. [Google Scholar]
- Blossom, D.B.; Kallen, A.J.; Patel, P.R.; Elward, A.; Robinson, L.; Gao, G.; Langer, R.; Perkins, K.M.; Jaeger, J.L.; Kurkjian, K.M.; et al. Outbreak of Adverse Reactions Associated with Contaminated Heparin. N. Engl. J. Med. 2008, 359, 2674–2684. [Google Scholar] [CrossRef] [Green Version]
- Oduah, E.I.; Linhardt, R.J.; Sharfstein, S.T. Heparin: Past, Present, and Future. Pharmaceuticals 2016, 9, 38. [Google Scholar] [CrossRef] [Green Version]
- Chuanxiong, N.; Pouyan, P.; Lauster, D.; Trimpert, J.; Kerkhoff, Y.; Szekeres, G.P.; Wallert, M.; Block, S.; Sahoo, A.K.; Dernedde, J.; et al. Polysulfates Block Sars-Cov-2 Uptake through Electrostatic Interactions. Angew. Chem. Int. Ed. 2021, 60, 15870–15878. [Google Scholar]
- Sumati, B.; Lauster, D.; Bardua, M.; Ludwig, K.; Angioletti-Uberti, S.; Popp, N.; Hoffmann, U.; Paulus, F.; Budt, M.; Stadtmüller, M.; et al. Linear Polysialoside Outperforms Dendritic Analogs for Inhibition of Influenza Virus Infection In vitro and In vivo. Biomaterials 2017, 138, 22–34. [Google Scholar]
- Pouyan, P.; Nie, C.; Bhatia, S.; Wedepohl, S.; Achazi, K.; Osterrieder, N.; Haag, R. Inhibition of Herpes Simplex Virus Type 1 Attachment and Infection by Sulfated Polyglycerols with Different Architectures. Biomacromolecules 2021, 22, 1545–1554. [Google Scholar] [CrossRef]
- Mohammadifar, E.; Ahmadi, V.; Gholami, M.F.; Oehrl, A.; Kolyvushko, O.; Nie, C.; Donskyi, I.S.; Herziger, S.; Radnik, J.; Ludwig, K.; et al. Graphene-Assisted Synthesis of 2d Polyglycerols as Innovative Platforms for Multivalent Virus Interactions. Adv. Funct. Mater. 2021, 31, 2009003. [Google Scholar] [CrossRef] [PubMed]
- Pavithra, D.; Doble, M. Biofilm Formation, Bacterial Adhesion and Host Response on Polymeric Implants—Issues and Prevention. Biomed. Mater. 2008, 3, 034003. [Google Scholar] [CrossRef] [PubMed]
- Prime, K.L.; Whitesides, G.M. Self-Assembled Organic Monolayers: Model Systems for Studying Adsorption of Proteins at Surfaces. Science 1991, 252, 1164–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gombotz, W.R.; Guanghui, W.; Horbett, T.A.; Hoffman, A.S. Protein adsorption to poly(ethylene oxide) surfaces. J. Biomed. Mater. Res. 1991, 25, 1547–1562. [Google Scholar] [CrossRef]
- Han, S.; Kim, C.; Kwon, D. Thermal/oxidative degradation and stabilization of polyethylene glycol. Polym. Int. J. Sci. Technol. Polym. 1997, 38, 317–323. [Google Scholar] [CrossRef]
- Kulka, M.W.; Smatty, S.; Hehnen, F.; Bierewirtz, T.; Silberreis, K.; Nie, C.; Kerkhoff, Y.; Grötzinger, C.; Friedrich, S.; Dahms, L.I.; et al. The Application of Dual-Layer, Mussel-Inspired, Antifouling Polyglycerol-Based Coatings in Ventricular Assist Devices. Adv. Mater. Interfaces 2020, 7, 2000272. [Google Scholar] [CrossRef]
- Kulka, M.W.; Nie, C.; Nickl, P.; Kerkhoff, Y.; Garg, A.; Salz, D.; Radnik, J.; Grunwald, I.; Haag, R. Surface-Initiated Grafting of Dendritic Polyglycerol from Mussel-Inspired Adhesion-Layers for the Creation of Cell-Repelling Coatings. Adv. Mater. Interfaces 2020, 7, 2000931. [Google Scholar] [CrossRef]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pouyan, P.; Cherri, M.; Haag, R. Polyglycerols as Multi-Functional Platforms: Synthesis and Biomedical Applications. Polymers 2022, 14, 2684. https://doi.org/10.3390/polym14132684
Pouyan P, Cherri M, Haag R. Polyglycerols as Multi-Functional Platforms: Synthesis and Biomedical Applications. Polymers. 2022; 14(13):2684. https://doi.org/10.3390/polym14132684
Chicago/Turabian StylePouyan, Paria, Mariam Cherri, and Rainer Haag. 2022. "Polyglycerols as Multi-Functional Platforms: Synthesis and Biomedical Applications" Polymers 14, no. 13: 2684. https://doi.org/10.3390/polym14132684