Photo-Curing Kinetics of 3D-Printing Photo-Inks Based on Urethane-Acrylates
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Instruments
2.3. Methods
3. Results and Discussion
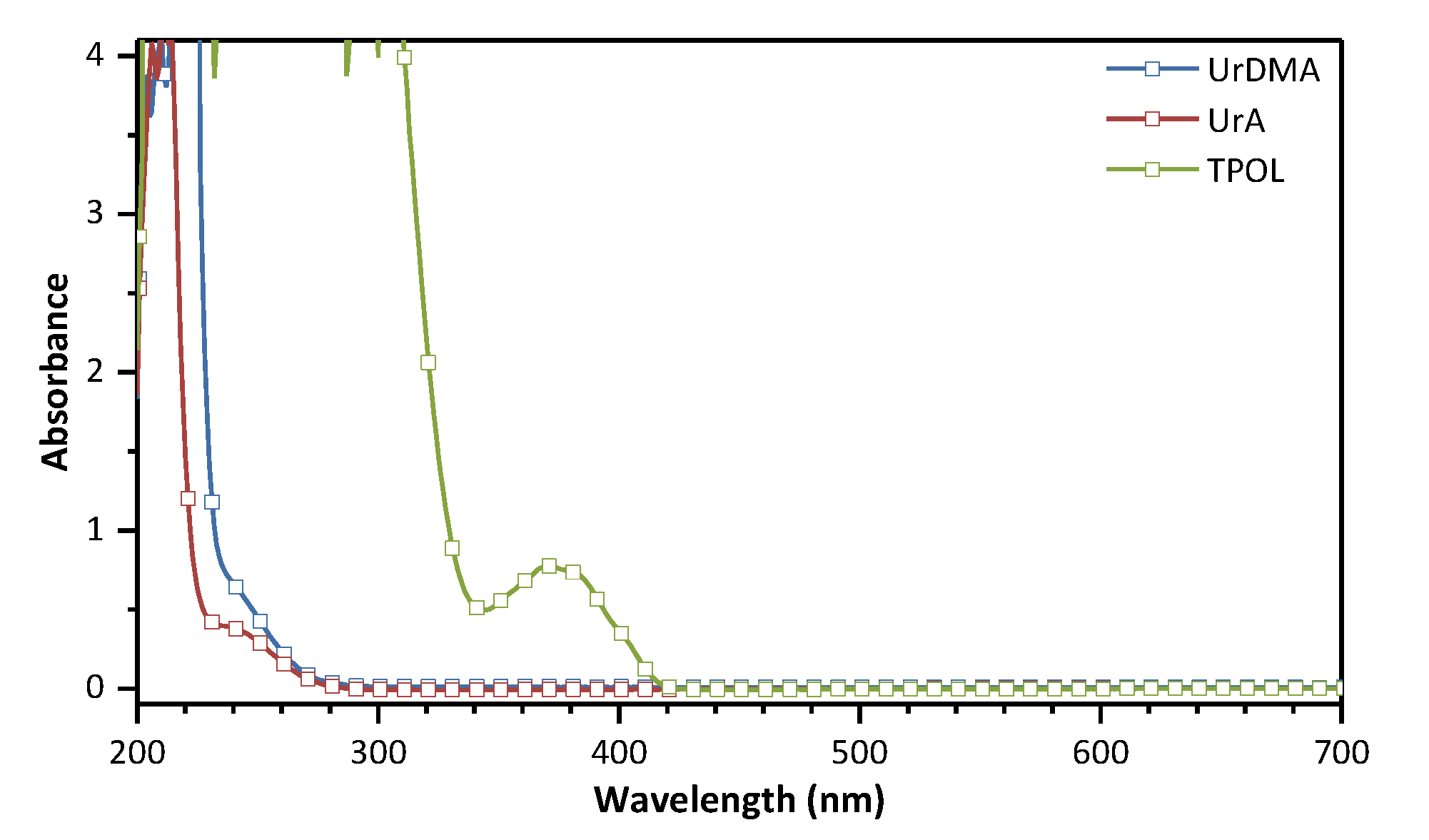
3.1. UV–vis Absorption
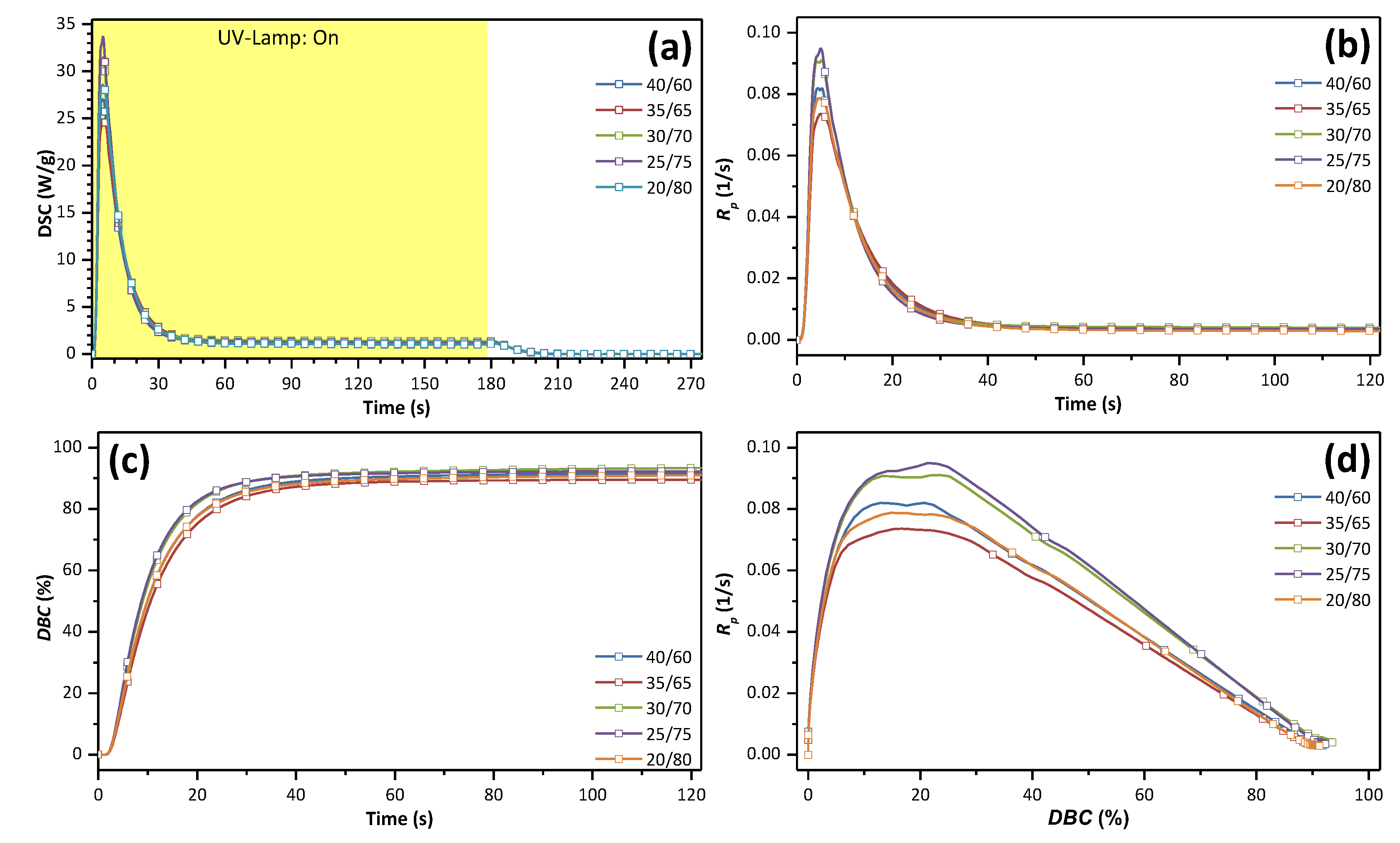
3.2. Photo-Curing
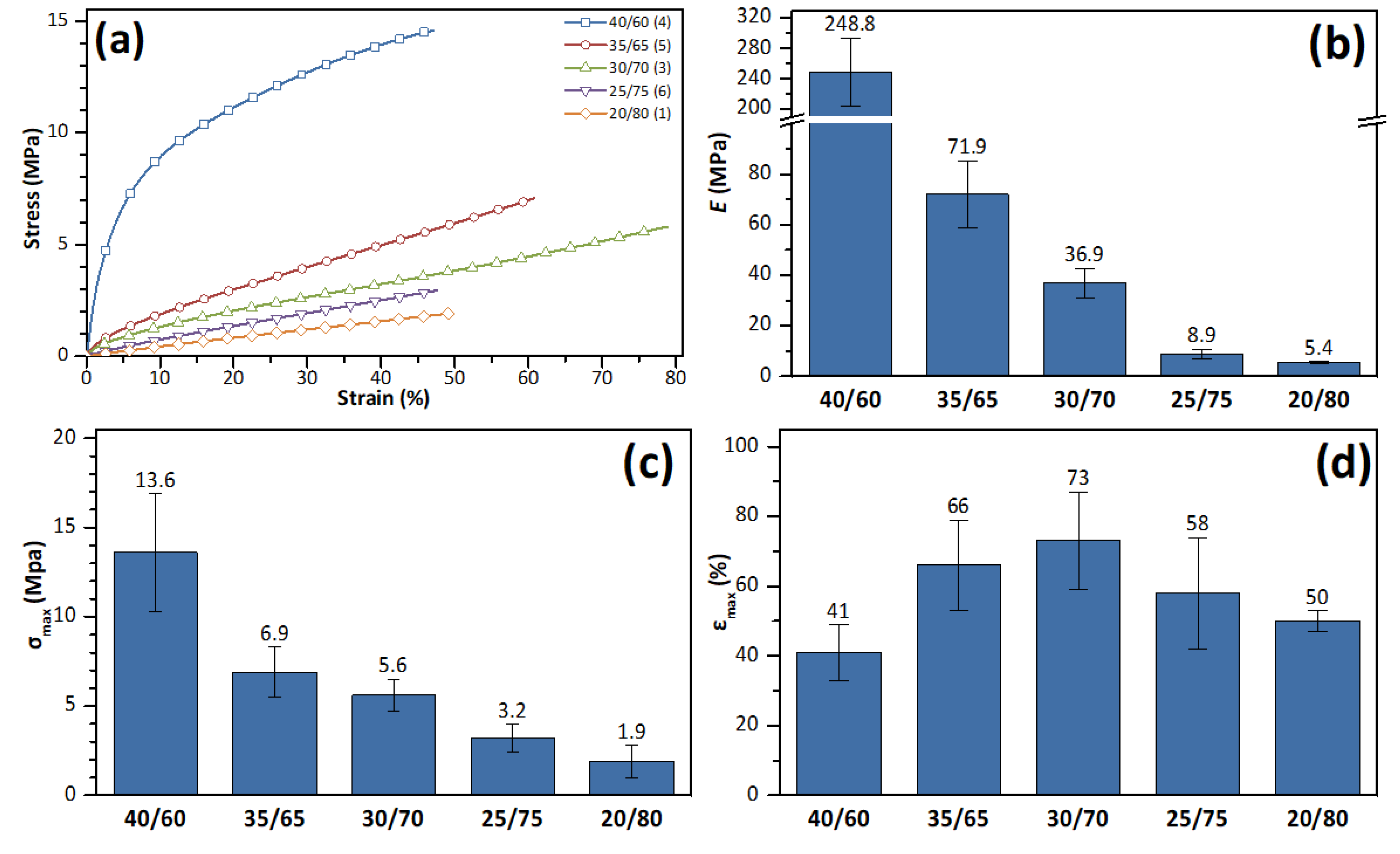
3.3. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Quan, H.; Zhang, T.; Xu, H.; Luo, S.; Nie, J.; Zhu, X. Photo-curing 3D printing technique and its challenges. Bioact. Mater. 2020, 5, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Tzeng, J.-J.; Yang, T.-S.; Lee, W.-F.; Chen, H.; Chang, H.-M. Mechanical Properties and Biocompatibility of Urethane Acrylate-Based 3D-Printed Denture Base Resin. Polymers 2021, 13, 822. [Google Scholar] [CrossRef] [PubMed]
- Buchheit, H.; Bruchmann, B.; Stoll, K.; Mülhaupt, R. Functionalized acrylic polyhydroxy urethanes as molecular tool box for photocurable thermosets and 3D printing. J. Appl. Polym. Sci. 2021, 59, 882–892. [Google Scholar] [CrossRef]
- Peng, S.; Li, Y.; Wu, L.; Zhong, J.; Weng, Z.; Zheng, L.; Yang, Z.; Miao, J.-T. 3D Printing Mechanically Robust and Transparent Polyurethane Elastomers for Stretchable Electronic Sensors. ACS Appl. Mater. Interfaces 2020, 12, 6479–6488. [Google Scholar] [CrossRef]
- Chen, H.; Lee, S.-Y.; Lin, Y.-M. Synthesis and Formulation of PCL-Based Urethane Acrylates for DLP 3D Printers. Polymers 2020, 12, 1500. [Google Scholar] [CrossRef]
- Kim, S.; Lee, J.; Han, H. Synthesis of UV Curable, Highly Stretchable, Transparent Poly(urethane-acrylate) Elastomer and Applications Toward Next Generation Technology. Macromol. Res. 2020, 28, 896–902. [Google Scholar] [CrossRef]
- Singh, N.; Bakhshi, H.; Meyer, W. Developing non-isocyanate urethane-methacrylate photo-monomers for 3D printing application. RSC Adv. 2020, 10, 44103–44110. [Google Scholar] [CrossRef]
- Baker, M.; Wang, R.; Damanik, F.; Kuhnt, T.; Ippel, H.; Dijkstra, P.; ten Cate, T.; Dias, A.; Moroni, L. Biodegradable Poly(Ester) Urethane Acrylate Resins for Digital Light Processing: From Polymer Synthesis to 3D Printed Tissue Engineering Constructs. ChemRxiv 2020. [Google Scholar]
- Soreni-Harari, M.; Pierre, R.S.; McCue, C.; Moreno, K.; Bergbreiter, S. Multimaterial 3D Printing for Microrobotic Mechanisms. Soft Robot. 2020, 7, 59–67. [Google Scholar] [CrossRef]
- Deng, Y.; Li, J.; He, Z.; Hong, J.; Bao, J. Urethane acrylate-based photosensitive resin for three-dimensional printing of stereolithographic elastomer. J. Appl. Polym. Sci. 2020, 137, 49294. [Google Scholar] [CrossRef]
- Lee, T.Y.; Roper, T.M.; Jönsson, E.S.; Guymon, C.A.; Hoyle, C.E. Influence of Hydrogen Bonding on Photopolymerization Rate of Hydroxyalkyl Acrylates. Macromolecules 2004, 37, 3659–3665. [Google Scholar] [CrossRef]
- Jansen, J.F.G.A.; Dias, A.A.; Dorschu, M.; Coussens, B. Fast Monomers: Factors Affecting the Inherent Reactivity of Acrylate Monomers in Photoinitiated Acrylate Polymerization. Macromolecules 2003, 36, 3861–3873. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I.M. Structure–property relationships in dimethacrylate networks based on Bis-GMA, UDMA and TEGDMA. Dent. Mater. 2009, 25, 1082–1089. [Google Scholar] [CrossRef]
- Lemon, M.T.; Jones, M.S.; Stansbury, J.W. Hydrogen bonding interactions in methacrylate monomers and polymers. J. Biomed. Mater. Res. Part A 2007, 83, 734–746. [Google Scholar] [CrossRef]
- Bagheri, A.; Jin, J. Photopolymerization in 3D Printing. ACS Appl. Polym. Mater. 2019, 1, 593–611. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, J.; Schmölzer, S.; Ruderer, M.A.; Fruhmann, G.; Hinrichsen, O. Photo-differential scanning calorimetry parameter study of photopolymers used in digital light synthesis. SPE Polym. 2021, 3, 41–53. [Google Scholar] [CrossRef]
- Jiang, F.; Drummer, D. Curing Kinetic Analysis of Acrylate Photopolymer for Additive Manufacturing by Photo-DSC. Polymers 2020, 12, 1080. [Google Scholar] [CrossRef]
- Kim, Y.C.; Hong, S.; Sun, H.; Kim, M.G.; Choi, K.; Cho, J.; Choi, H.R.; Koo, J.C.; Moon, H.; Byun, D.; et al. UV-curing kinetics and performance development of in situ curable 3D printing materials. Eur. Polym. J. 2017, 93, 140–147. [Google Scholar] [CrossRef]
- Assumption, H.J.; Mathias, L.J. Photopolymerization of urethane dimethacrylates synthesized via a non-isocyanate route. Polymer 2003, 44, 5131–5136. [Google Scholar] [CrossRef]
- Anseth, K.S.; Wang, C.M.; Bowman, C.N. Kinetic evidence of reaction diffusion during the polymerization of multi(meth)acrylate monomers. Macromolecules 1994, 27, 650–655. [Google Scholar] [CrossRef]
- Harikrishna, R.; Ponrathnam, S.; Rajan, C.R.; Tambe, S.S. Photopolymerization of bis-aromatic and alicyclic based solid urethane acrylate macromonomer in the presence of large excess of reactive diluent. J. Therm. Anal. 2012, 112, 805–813. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, H.; Xie, D. Preparation of new low viscosity urethane dimethacrylates for dental composites. J. Biomater. Sci. Polym. Ed. 2017, 29, 1011–1025. [Google Scholar] [CrossRef]
- Barszczewska-Rybarek, I. The role of molecular structure on impact resistance and bending strength of photocured urethane-dimethacrylate polymer networks. Polym. Bull. 2017, 74, 4023–4040. [Google Scholar] [CrossRef]
- Weigand, J.J.; Miller, C.I.; Janisse, A.P.; McNair, O.D.; Kim, K.; Wiggins, J.S. 3D printing of dual-cure benzoxazine networks. Polymer 2020, 189, 122193. [Google Scholar] [CrossRef]
- Kim, G.-T.; Go, H.-B.; Yu, J.-H.; Yang, S.-Y.; Kim, K.-M.; Choi, S.-H.; Kwon, J.-S. Cytotoxicity, Colour Stability and Dimensional Accuracy of 3D Printing Resin with Three Different Photoinitiators. Polymers 2022, 14, 979. [Google Scholar] [CrossRef]
- Zeng, B.; Cai, Z.; Lalevée, J.; Yang, Q.; Lai, H.; Xiao, P.; Liu, J.; Xing, F. Cytotoxic and cytocompatible comparison among seven photoinitiators-triggered polymers in different tissue cells. Toxicol. Vitr. 2021, 72, 105103. [Google Scholar] [CrossRef]
- Steyrer, B.; Neubauer, P.; Liska, R.; Stampfl, J. Visible Light Photoinitiator for 3D-Printing of Tough Methacrylate Resins. Materials 2017, 10, 1445. [Google Scholar] [CrossRef] [Green Version]
- Kardar, P.; Ebrahimi, M.; Bastani, S. Influence of temperature and light intensity on the photocuring process and kinetics parameters of a pigmented UV curable system. J. Therm. Anal. 2014, 118, 541–549. [Google Scholar] [CrossRef]
- Sideridou, I.; Tserki, V.; Papanastasiou, G. Effect of chemical structure on degree of conversion in light-cured dimethacrylate-based dental resins. Biomaterials 2002, 23, 1819–1829. [Google Scholar] [CrossRef]
- Kousaalya, A.B.; Ayalew, B.; Pilla, S. Photopolymerization of Acrylated Epoxidized Soybean Oil: A Photocalorimetry-Based Kinetic Study. ACS Omega 2019, 4, 21799–21808. [Google Scholar] [CrossRef] [Green Version]
- Rusu, M.C.; Block, C.; Van Assche, G.; Van Mele, B. Influence of temperature and UV intensity on photo-polymerization reaction studied by photo-DSC. J. Therm. Anal. 2012, 110, 287–294. [Google Scholar] [CrossRef]
- Macarie, L.; Ilia, G. The influence of temperature and photoinitiator concentration on photoinitiated polymerization of diacrylate monomer. Open Chem. 2005, 3, 721–730. [Google Scholar] [CrossRef]
- Zhou, H.; Li, Q.; Lee, T.Y.; Guymon, C.A.; Jönsson, E.S.; Hoyle, C.E. Photopolymerization of Acid Containing Monomers: Real-Time Monitoring of Polymerization Rates. Macromolecules 2006, 39, 8269–8273. [Google Scholar] [CrossRef]
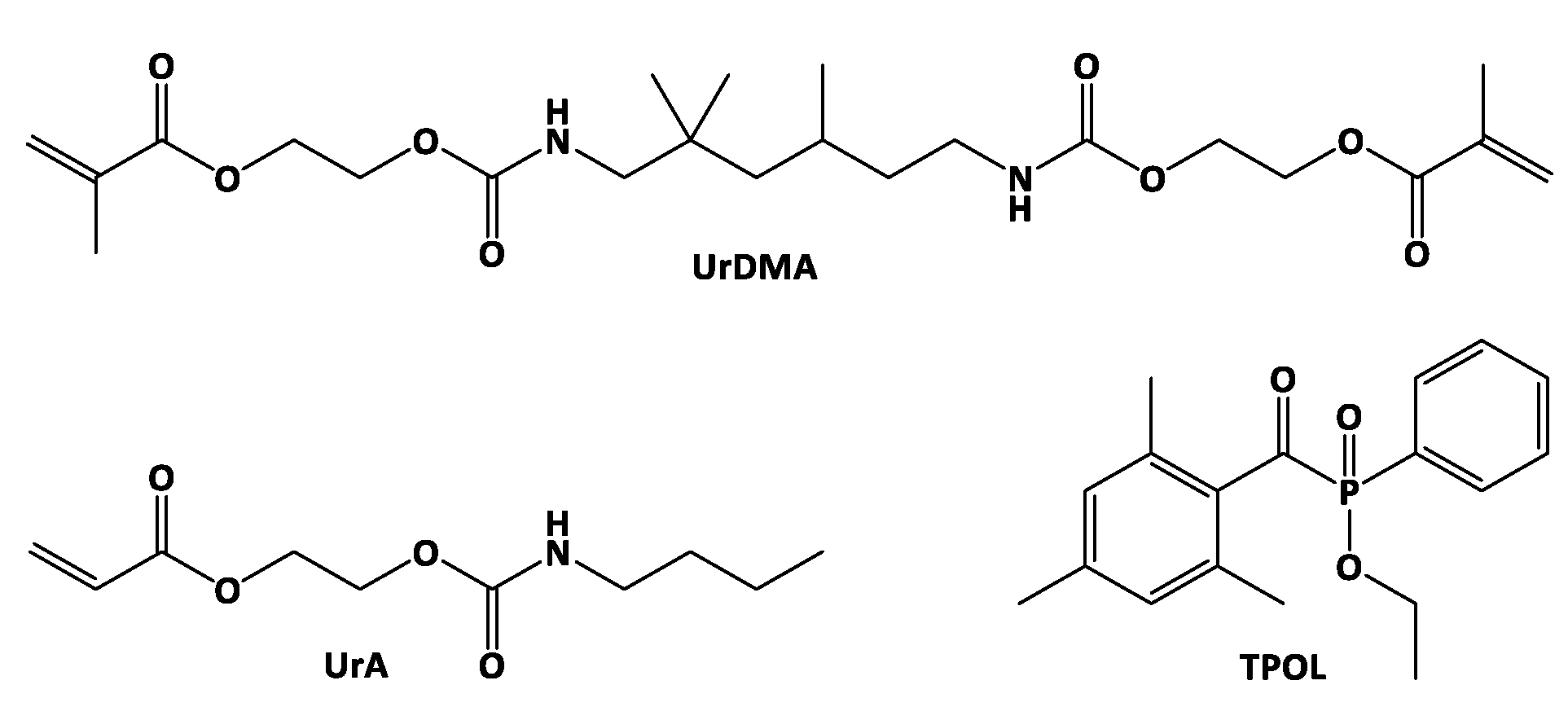
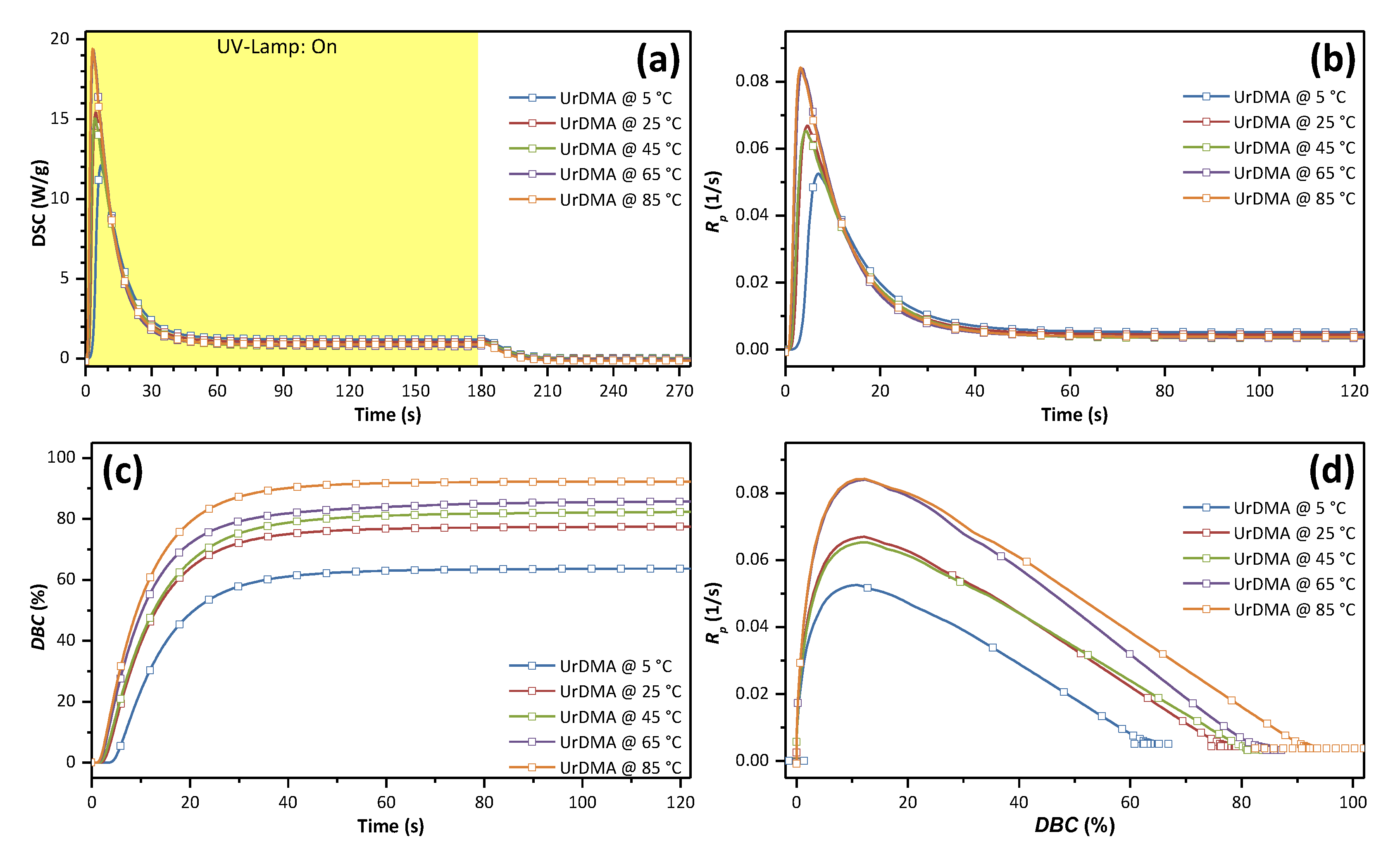
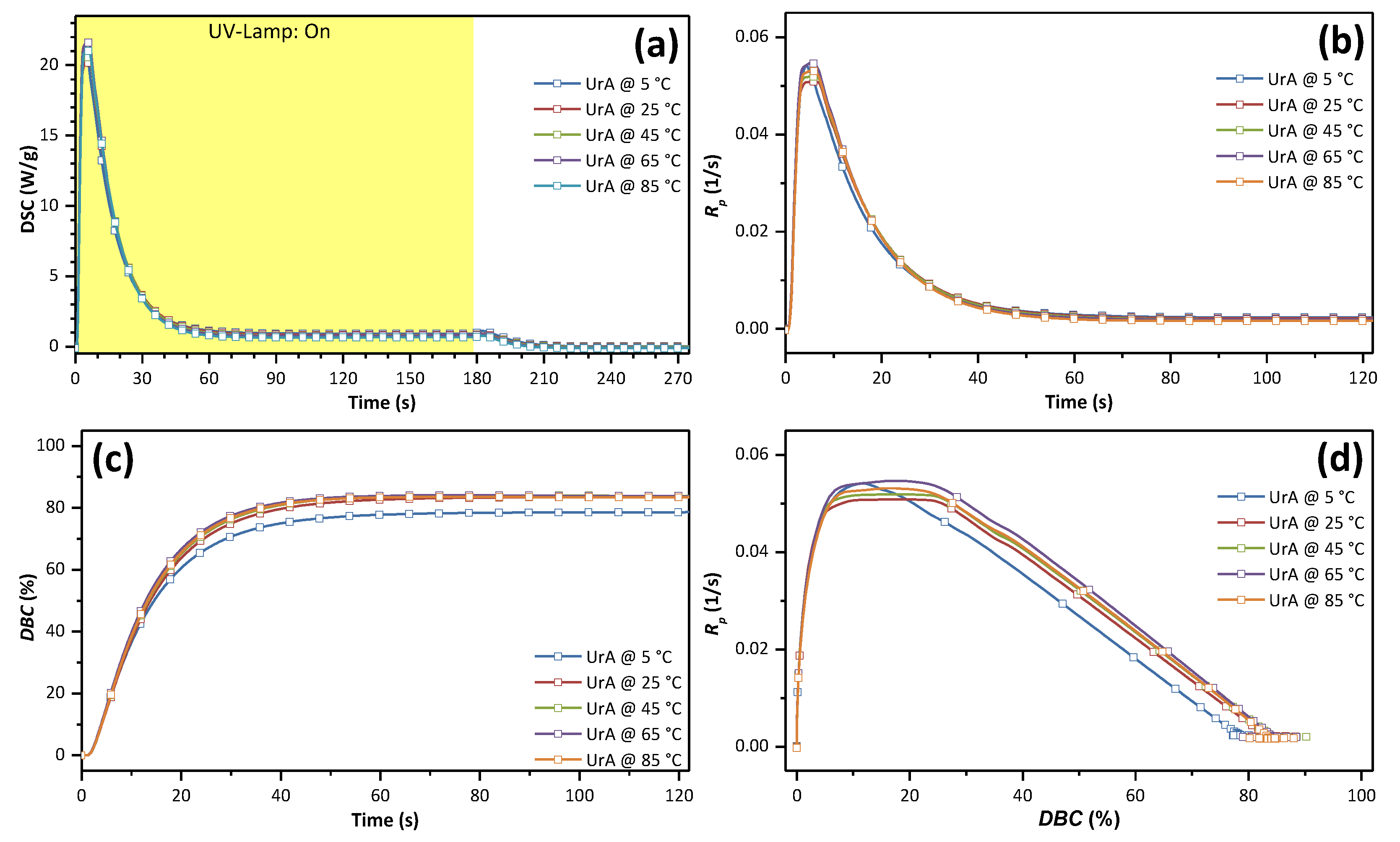
| Tp (°C) | Rp,max (1/s) | tGP (s) | DBCGP (%) | ΔHp,total (J/g) | DBCtotal (%) | t50% (s) | t90% (s) | t95% (s) | Tg (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 5 | 5.25 × 10−2 | 7.0 | 10.9 | 148.6 | 63.8 | 12.4 | 29.2 | 37.8 | 63 |
| 25 | 6.70 × 10−2 | 4.7 | 12.1 | 180.8 | 77.6 | 9.9 | 26.3 | 34.5 | 77 |
| 45 | 6.52 × 10−2 | 4.5 | 12.7 | 192.0 | 82.4 | 10.2 | 28.5 | 38.2 | 101 |
| 65 | 8.40 × 10−2 | 3.6 | 12.3 | 199.9 | 85.8 | 8.8 | 26.2 | 38.4 | 106 |
| 85 | 8.42 × 10−2 | 3.3 | 12.3 | 214.8 | 92.2 | 8.5 | 23.7 | 30.9 | 122 |
| Tp (°C) | Rp,max (1/s) | tGP (s) | DBCGP (%) | ΔHp,total (J/g) | DBCtotal (%) | t50% (s) | t90% (s) | t95% (s) | Tg (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 5 | 5.42 × 10−2 | 4.4 | 11.9 | 315.2 | 78.7 | 10.0 | 30.4 | 39.2 | −11 |
| 25 | 5.08 × 10−2 | 5.7 | 17.8 | 334.8 | 83.6 | 11.2 | 30.4 | 39.3 | −11 |
| 45 | 5.19 × 10−2 | 5.7 | 18.5 | 335.7 | 83.8 | 11.1 | 28.8 | 36.3 | −11 |
| 65 | 5.47 × 10−2 | 5.2 | 17.1 | 335.2 | 83.7 | 10.6 | 27.3 | 33.9 | −11 |
| 85 | 5.31 × 10−2 | 5.3 | 16.5 | 335.5 | 83.3 | 10.9 | 27.7 | 34.3 | −8 |
| UrDMA/UrA | Rp,max (1/s) | tGP (s) | DBCGP (%) | ΔHp,total (J/g) | DBCtotal (%) | t50% (s) | t90% (s) | t95% (s) | Tg (°C) |
|---|---|---|---|---|---|---|---|---|---|
| 40/60 | 8.20 × 10−2 | 4.2 | 12.9 | 303.1 | 91.8 | 9.3 | 24.6 | 32.8 | 23 |
| 35/65 | 7.35 × 10−2 | 5.0 | 16.7 | 302.9 | 89.5 | 9.6 | 24.8 | 32.1 | 17 |
| 30/70 | 9.10 × 10−2 | 5.2 | 23.5 | 324.3 | 93.5 | 8.4 | 22.2 | 30.4 | 9 |
| 25/75 | 9.50 × 10−2 | 4.9 | 21.3 | 327.5 | 92.3 | 8.1 | 20.5 | 27.1 | 9 |
| 20/80 | 7.87 × 10−2 | 4.6 | 15.6 | 331.4 | 91.2 | 9.0 | 24.4 | 33.3 | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakhshi, H.; Kuang, G.; Wieland, F.; Meyer, W. Photo-Curing Kinetics of 3D-Printing Photo-Inks Based on Urethane-Acrylates. Polymers 2022, 14, 2974. https://doi.org/10.3390/polym14152974
Bakhshi H, Kuang G, Wieland F, Meyer W. Photo-Curing Kinetics of 3D-Printing Photo-Inks Based on Urethane-Acrylates. Polymers. 2022; 14(15):2974. https://doi.org/10.3390/polym14152974
Chicago/Turabian StyleBakhshi, Hadi, Guanxing Kuang, Franziska Wieland, and Wolfdietrich Meyer. 2022. "Photo-Curing Kinetics of 3D-Printing Photo-Inks Based on Urethane-Acrylates" Polymers 14, no. 15: 2974. https://doi.org/10.3390/polym14152974









