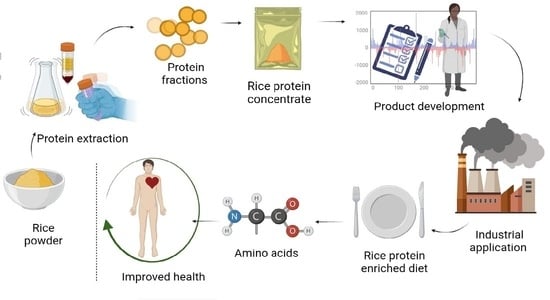
A Narrative Review on Rice Proteins: Current Scenario and Food Industrial Application
Abstract
:1. Introduction
2. Protein Extraction
2.1. Chemical Extraction
2.1.1. Solvent Extraction
2.1.2. Alkali Extraction
2.1.3. Enzymatic Extraction
2.2. Physical Extraction
Ultrasonic-Assisted Alkali Extraction
3. Chemistry of Rice Protein
3.1. Fractions of Rice Protein
3.2. Molecular Weights of Rice Protein Fractions
3.3. Isoelectric Point
3.4. Molecular Structure
3.5. Amino Acid Composition
3.6. Surface Hydrophobicity
4. Characterization of Rice Protein
4.1. Fourier-Transform Infrared Spectroscopy (FTIR)
4.2. Differential Scanning Calorimetry (DSC)
4.3. X-ray Diffraction (XRD)
4.4. Scanning Electron Microscopy (SEM)
5. Techno Functional Properties of Rice Protein
5.1. Solubility
5.2. Buffering Capacity and Viscosity
5.3. Emulsifying Property
5.4. Water and Oil Binding Capacity
5.5. Foaming Capacity
5.6. Polyphenolic Compound’s Binding Properties
6. Application of Rice Seed Protein in Food Industries
6.1. Gluten-Free or Hypoallergic Product
6.2. Protein-Based Meat Products
6.3. Edible Films and Coating
6.4. Baking
6.5. Emulsifier
6.6. Pharmaceutical and Therapeutic Uses
7. Health Benefits
7.1. Anti-Inflammatory and Anti-Cancer Reaction
7.2. Suppression of Hyperglycemia
7.3. Reduction of Cholesterol
7.4. Anti-Oxidative Activity
8. Cytotoxicity of Rice Protein
9. Conclusions and Future Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hossain, M.A.; Hoque, T.S.; Zaid, A.; Wani, S.H.; Mostofa, M.G.; Henry, R. Targeting the Ascorbate-Glutathione Pathway and the Glyoxalase Pathway for Genetic Engineering of Abiotic Stress-Tolerance in Rice. In Molecular Breeding for Rice Abiotic Stress Tolerance and Nutritional Quality, 1st ed.; Hossain, M.A., Hassan, L., Ifterkharuddaula, K.M., Kumar, A., Henry, R., Eds.; Jihn Wiley & Sons: Chichester, UK, 2021; Volume 1, pp. 398–427. [Google Scholar]
- Alyami, J.; Ladd, N.; Pritchard, S.E.; Hoad, C.L.; Sultan, A.A.; Spiller, R.C.; Gowland, P.A.; Macdonald, I.A.; Aithal, G.A.; Marciani, L.; et al. Glycaemic, gastrointestinal and appetite responses to breakfast porridges from ancient cereal grains: A MRI pilot study in healthy humans. Int. Food Res. J. 2019, 118, 49–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Willows, R.D.; Worden, P.; Mirzaei, M. Barley Grain Proteomics. Proteomics. In Proteomics in Food Science, 1st ed.; Colgrave, M.L., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 175–188. [Google Scholar]
- Oghbaei, M.; Prakash, J. Effect of primary processing of cereals and legumes on its nutritional quality: A comprehensive review. Cogent Food Agric. 2016, 2, 1136015. [Google Scholar] [CrossRef] [Green Version]
- Bose, U.; Broadbent, J.A.; Byrne, K.; Hasan, S.; Howitt, C.A.; Colgrave, M.L. Optimization of protein extraction for in-depth profiling of the cereal grain proteome. J. Proteom. 2019, 197, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.K.; Srivastav, P.P. Proximate composition, mineral content and fatty acids analyses of aromatic and non-aromatic Indian rice. Rice Sci. 2017, 24, 21–31. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Composition and protein profile analysis of rice protein ingredients. J. Food Compos. Anal. 2017, 59, 18–26. [Google Scholar] [CrossRef]
- Liu, X.; Shao, W.; Luo, M.; Bian, J.; Yu, D.G. Electrospun blank nanocoating for improved sustained release profiles from medicated gliadin nanofibers. Nanomaterials 2018, 8, 184. [Google Scholar] [CrossRef] [Green Version]
- Balindong, J.L.; Liu, L.; Ward, R.M.; Barkla, B.J.; Waters, D.L. Optimization and standardization of extraction and HPLC analysis of rice grain protein. J. Cereal Sci. 2016, 72, 124–130. [Google Scholar] [CrossRef]
- Labuschagne, M.T. A review of cereal grain proteomics and its potential for sorghum improvement. J. Cereal Sci. 2018, 84, 151–158. [Google Scholar] [CrossRef]
- Ghanghas, N.; Mukilan, M.T.; Sharma, S.; Prabhakar, P.K. Classification, composition, extraction, functional modification and application of rice (Oryza sativa) seed protein: A comprehensive review. Food Rev. Int. 2022, 38, 354–383. [Google Scholar] [CrossRef]
- Sousa, R.; Portmann, R.; Dubois, S.; Recio, I.; Egger, L. Protein digestion of different protein sources using the INFOGEST static digestion model. Int. Food Res. J. 2020, 130, 108996. [Google Scholar] [CrossRef]
- Eakkanaluksamee, K.; Anuntagool, J. Optimization of High-Protein Glutinous Rice Flour Production Using Response Surface Method. Rice Sci. 2020, 27, 75–80. [Google Scholar] [CrossRef]
- Kamjijam, B.; Bednarz, H.; Suwannaporn, P.; Jom, K.N.; Niehaus, K. Localization of amino acids in germinated rice grain: Gamma-aminobutyric acid and essential amino acids production approach. J. Cereal Sci. 2020, 93, 102958. [Google Scholar] [CrossRef]
- Wang, T.; Xu, P.; Chen, Z.; Zhou, X.; Wang, R. Alteration of the structure of rice proteins by their interaction with soy protein isolate to design novel protein composites. Food Funct. 2018, 9, 4282–4291. [Google Scholar] [CrossRef]
- Triratanasirichai, K.; Singh, M.; Anal, A.K. Value-Added By-Products from Rice Processing Industries. In Food Processing By-Products and Their Utilization, 1st ed.; Anal, A.K., Ed.; John Wiley & Sons Ltd.: West Sussex, UK, 2017; pp. 277–293. [Google Scholar]
- Quiñones, R.S.; Macachor, C.P.; Quiñones, H.G. Development of gluten-free composite flour blends. Trop. Technol. J. 2015, 19, 1585. [Google Scholar] [CrossRef]
- Małecki, J.; Muszyński, S.; Sołowiej, B.G. Proteins in food systems—Bionanomaterials, conventional and unconventional sources, functional properties, and development opportunities. Polymers 2021, 13, 2506. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Tomar, M.; Potkule, J.; Verma, R.; Punia, S.; Mahapatra, A.; Dhahuja, A.; Joshi, S.; Berwal, M.K. Advances in the plant protein extraction: Mechanism and recommendations. Food Hydrocoll. 2021, 115, 106595. [Google Scholar] [CrossRef]
- Singh, J.; Karmakar, S.; Banerjee, R. Extraction and nutritional properties of protein derived from rice (Oryza sativa) based distillery byproducts: A potential substrate for food formulation. Biocatal. Agric. Biotechnol. 2019, 22, 101364. [Google Scholar] [CrossRef]
- Likittrakulwong, W.; Poolprasert, P.; Srikaeo, K. Effects of extraction methods on protein properties obtained from paddy rice and germinated paddy rice. PeerJ 2021, 9, e11365. [Google Scholar] [CrossRef]
- Balindong, J.L.; Ward, R.M.; Liu, L.; Rose, T.J.; Pallas, L.A.; Ovenden, B.W.; Snell, P.J.; Waters, D.L. Rice grain protein composition influences instrumental measures of rice cooking and eating quality. J. Cereal Sci. 2018, 79, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Yamuangmorn, S.; Dell, B.; Prom-u-thai, C. Effects of cooking on anthocyanin concentration and bioactive antioxidant capacity in glutinous and non-glutinous purple rice. Rice Sci. 2018, 25, 270–278. [Google Scholar] [CrossRef]
- de Souza, D.; Sbardelotto, A.F.; Ziegler, D.R.; Marczak, L.D.F.; Tessaro, I.C. Characterization of rice starch and protein obtained by a fast-alkaline extraction method. Food Chem. 2016, 191, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Pan, Z.; Shen, K.; Cai, X.; Zheng, B.; Miao, S. Influence of ultrasound-assisted alkali treatment on the structural properties and functionalities of rice protein. J. Cereal Sci. 2018, 79, 204–209. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Zhang, Y.; Ma, H.; Liang, Q.; Qu, W.; He, R.; Zhou, C.; Mahunu, G.K. Effects of ultrasound and ultrasound assisted alkaline pretreatments on the enzymolysis and structural characteristics of rice protein. Ultrason. Sonochem. 2016, 31, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Liu, N.; Lin, P.; Zhang, K.; Yao, X.; Li, D.; Yang, L.; Zhao, M. Combined effects of limited enzymatic hydrolysis and high hydrostatic pressure on the structural and emulsifying properties of rice proteins. Innov. Food Sci. Emerg. Technol. 2022, 77, 102975. [Google Scholar] [CrossRef]
- Hozzein, W.N.; Abuelsoud, W.; Wadaan, M.A.; Shuikan, A.M.; Selim, S.; Al Jaouni, S.; AbdElgawad, H. Exploring the potential of actinomycetes in improving soil fertility and grain quality of economically important cereals. Sci. Total Environ. 2019, 651, 2787–2798. [Google Scholar] [CrossRef]
- Kawakatsu, T.; Takaiwa, F. Rice proteins and essential amino acids. In Rice, 4th ed.; Bao, J., Ed.; AACC International Press: Washington, DC, USA, 2019; pp. 109–130. [Google Scholar]
- Hoogenkamp, H.; Kumagai, H.; Wanasundara, J.P.D. Rice protein and rice protein products. In Sustainable Protein Sources; Nadathur, S.R., Wanasundara, J.P.D., Scanlin, L., Eds.; Academic Press: Cambridge, MA, USA, 2016; pp. 47–65. [Google Scholar]
- Braspaiboon, S.; Osiriphun, S.; Peepathum, P.; Jirarattanarangsri, W. Comparison of the effectiveness of alkaline and enzymatic extraction and the solubility of proteins extracted from carbohydrate-digested rice. Heliyon 2020, 6, e05403. [Google Scholar] [CrossRef]
- Larkins, B.A.; Wu, Y.; Song, R.; Messing, J. 14 Maize Seed Storage Proteins. In Maize Kernel Development; Larkins, B.A., Ed.; CABI International: Wallingford, UK, 2017; p. 175. [Google Scholar]
- Singh, T.P.; Sogi, D.S. Comparative study of structural and functional characterization of bran protein concentrates from superfine, fine and coarse rice cultivars. Int. J. Biol. Macromol. 2018, 111, 281–288. [Google Scholar] [CrossRef]
- Ito, V.C.; Lacerda, L.G. Black rice (Oryza sativa L.): A review of its historical aspects, chemical composition, nutritional and functional properties, and applications and processing technologies. Food Chem. 2019, 301, 125304. [Google Scholar] [CrossRef]
- Piotrowicz, I.B.B.; Salas-Mellado, M.M. Protein concentrates from defatted rice bran: Preparation and characterization. Food Sci. Technol. 2017, 37, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Wang, L.; Chen, Z.; Zhang, X.; Zhu, Z. Functional properties and structural changes of rice proteins with anthocyanins complexation. Food Chem. 2020, 331, 127336. [Google Scholar] [CrossRef]
- Amagliani, L.; O’Regan, J.; Kelly, A.L.; O’Mahony, J.A. Physical and flow properties of rice protein powders. J. Food Eng. 2016, 190, 1–9. [Google Scholar] [CrossRef]
- Nunes, F.A.; Seferin, M.; Maciel, V.G.; Flôres, S.H.; Ayub, M.A.Z. Life cycle greenhouse gas emissions from rice production systems in Brazil: A comparison between minimal tillage and organic farming. J. Clean. Prod. 2016, 139, 799–809. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Tang, W.J.; Xu, Z.; Wen, L.; Chen, M.L. Structure and functional properties of rice protein–dextran conjugates prepared by the Maillard reaction. Int. J. Food Sci. Technol. 2017, 53, 372–380. [Google Scholar] [CrossRef]
- Hou, F.; Ding, W.; Qu, W.; Oladejo, A.O.; Xiong, F.; Zhang, W.; He, R.; Ma, H. Alkali solution extraction of rice residue protein isolates: Influence of alkali concentration on protein functional, structural properties and lysinoalanine formation. Food Chem. 2017, 218, 207–215. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y.; Zhao, C.; Lin, H.; Wang, Z.; Wu, F. Structural and functional properties of rice bran protein oxidized by peroxyl radicals. Int. J. Food Prop. 2017, 20, 1456–1467. [Google Scholar] [CrossRef] [Green Version]
- Ren, J.; Li, S. Effects of thermal treatment on structure and surface hydrophobicity of sunflower seed protein isolate. China Oils Fats 2016, 41, 24–27. [Google Scholar]
- Qi, B.; Zhao, C.; Jiang, L.; Wang, Z.; Li, Y. Effect of composition and secondary structure of soybean protein isolate on surface hydrophobicity. J. Chin. Inst. Food Sci. Technol. 2018, 18, 288–293. [Google Scholar]
- Wu, X.; Li, F.; Wu, W. Effects of oxidative modification by 13-hydroperoxyoctadecadienoic acid on the structure and functional properties of rice protein. Int. Food Res. J. 2020, 132, 109096. [Google Scholar] [CrossRef]
- Yan, X.; Liang, S.; Peng, T.; Zhang, G.; Zeng, Z.; Yu, P.; Gong, D.; Deng, S. Influence of phenolic compounds on physicochemical and functional properties of protein isolate from Cinnamomum camphora seed kernel. Food Hydrocoll. 2020, 102, 105612. [Google Scholar] [CrossRef]
- Zhao, Q.; Lin, J.; Wang, C.; Yousaf, L.; Xue, Y.; Shen, Q. Protein structural properties and proteomic analysis of rice during storage at different temperatures. Food Chem. 2021, 361, 130028. [Google Scholar] [CrossRef]
- Lu, X.; Chang, R.; Lu, H.; Ma, R.; Qiu, L.; Tian, Y. Effect of amino acids composing rice protein on rice starch digestibility. LWT 2021, 146, 111417. [Google Scholar] [CrossRef]
- Park, J.; Sung, J.M.; Choi, Y.S.; Park, J.D. pH-dependent pasting and texture properties of rice flour subjected to limited protein hydrolysis. Food Hydrocoll. 2021, 117, 106754. [Google Scholar] [CrossRef]
- Liu, K.; Zheng, J.; Chen, F. Heat-induced changes in the physicochemical properties and in vitro digestibility of rice protein fractions. J. Food Sci. Technol. 2021, 58, 1368–1377. [Google Scholar] [CrossRef] [PubMed]
- Amagliani, L.; O’Regan, J.; Schmitt, C.; Kelly, A.L.; O’Mahony, J.A. Characterization of the physicochemical properties of intact and hydrolyzed rice protein ingredients. J. Cereal Sci. 2019, 88, 16–23. [Google Scholar] [CrossRef]
- Wang, R.; Li, L.; Feng, W.; Wang, T. Fabrication of hydrophilic composites by bridging the secondary structures between rice proteins and pea proteins toward enhanced nutritional properties. Food Funct. 2020, 11, 7446–7455. [Google Scholar] [CrossRef] [PubMed]
- Azizi, R.; Capuano, E.; Nasirpour, A.; Pellegrini, N.; Golmakani, M.T.; Hosseini, S.M.H.; Farahnaky, A. Varietal differences in the effect of rice ageing on starch digestion. Food Hydrocoll. 2019, 95, 358–366. [Google Scholar] [CrossRef]
- Ling, B.; Ouyang, S.; Wang, S. Effect of radio frequency treatment on functional, structural and thermal behaviors of protein isolates in rice bran. Food Chem. 2019, 289, 537–544. [Google Scholar] [CrossRef]
- Jia, X.; Zhao, M.; Xia, N.; Teng, J.; Jia, C.; Wei, B.; Chen, D. Interaction between plant phenolics and rice protein improved oxidative stabilities of emulsion. J. Cereal Sci. 2019, 89, 102818. [Google Scholar] [CrossRef]
- Jiménez-Munoz, L.M.; Tavares, G.M.; Corredig, M. Design future foods using plant protein blends for best nutritional and technological functionality. Trends Food Sci. Technol. 2021, 113, 139–150. [Google Scholar] [CrossRef]
- Moirangthem, K.; Jenkins, D.; Ramakrishna, P.; Rajkumari, R.; Cook, D. Indian black rice: A brewing raw material with novel functionality. J. Inst. Brew. 2020, 126, 35–45. [Google Scholar] [CrossRef]
- Schefer, S.; Oest, M.; Rohn, S. Interactions between Phenolic Acids, Proteins, and Carbohydrates—Influence on Dough and Bread Properties. Foods 2021, 10, 2798. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.H.; Benjakul, S.; Sae-leaw, T.; Balange, A.K.; Maqsood, S. Protein–polyphenol conjugates: Antioxidant property, functionalities and their applications. Trends Food Sci. Technol. 2019, 91, 507–517. [Google Scholar] [CrossRef]
- Kelemen, V.; Pichler, A.; Ivić, I.; Buljeta, I.; Šimunović, J.; Kopjar, M. Brown rice proteins as delivery system of phenolic and volatile compounds of raspberry juice. Int. J. Food Sci. 2022, 57, 1866–1874. [Google Scholar] [CrossRef]
- Pham, L.B.; Wang, B.; Zisu, B.; Adhikari, B. Covalent modification of flaxseed protein isolate by phenolic compounds and the structure and functional properties of the adducts. Food Chem. 2019, 293, 463–471. [Google Scholar] [CrossRef]
- Gao, X.; Xu, Z.; Liu, G.; Wu, J. Polyphenols as a versatile component in tissue engineering. Acta Biomater. 2021, 119, 57–74. [Google Scholar] [CrossRef]
- De Blas, C.; Mateos, G.G. 12 Feed Formulation. In Nutrition of the Rabbit, 3rd ed.; De Blas, C., Wisewan, J., Eds.; CAB International: Pondicherry, India, 2020; p. 243. [Google Scholar]
- Munir, S.; Javed, M.; Hu, Y.; Liu, Y.; Xiong, S. The Effect of Acidic and Alkaline pH on the Physico-Mechanical Properties of Surimi-Based Edible Films Incorporated with Green Tea Extract. Polymers 2020, 12, 2281. [Google Scholar] [CrossRef]
- Kaewprachu, P.; Jaisan, C.; Klunklin, W.; Phongthai, S.; Rawdkuen, S.; Tongdeesoontorn, W. Mechanical and Physicochemical Properties of Composite Biopolymer Films Based on Carboxymethyl Cellulose from Young Palmyra Palm Fruit Husk and Rice Flour. Polymers 2022, 14, 1872. [Google Scholar] [CrossRef]
- Dhond, U.V.; Devangare, A.A.; Chappalwar, A.M. Effect of addition of different levels of soya flour and rice flour as extenders on quality of quail meatballs and economics. Meat Sci. 2017, 12, 11–16. [Google Scholar]
- Al-Doury, M.K.; Hettiarachchy, N.S.; Horax, R. Rice-Endosperm and Rice-Bran Proteins: A Review. J. Am. Oil Chem. Soc. 2018, 95, 943–956. [Google Scholar] [CrossRef]
- Marconi, O.; Sileoni, V.; Ceccaroni, D.; Perretti, G. The use of rice in brewing. In Advances in International Rice Research; Li, J.Q., Ed.; InTech: Janeza, Croatia, 2017; pp. 49–66. [Google Scholar]
- Shoaib, A.; Sahar, A.; Sameen, A.; Saleem, A.; Tahir, A.T. Use of pea and rice protein isolates as source of meat extenders in the development of chicken nuggets. J. Food Process. Preserv. 2018, 42, e13763. [Google Scholar] [CrossRef]
- Wang, L.; Ding, J.; Fang, Y.; Pan, X.; Fan, F.; Li, P.; Hu, Q. Effect of ultrasonic power on properties of edible composite films based on rice protein hydrolysates and chitosan. Ultrason. Sonochem. 2020, 65, 105049. [Google Scholar] [CrossRef] [PubMed]
- Silva-Rodrigues, H.C.; Silveira, M.P.; Helm, C.V.; de Matos Jorge, L.M.; Jorge, R.M. Gluten free edible film based on rice flour reinforced by guabiroba (Campomanesia xanthocarpa) pulp. J. Appl. Polym. Sci. 2020, 137, 49254. [Google Scholar] [CrossRef]
- Xie, H.; Ouyang, K.; Zhang, L.; Hu, J.; Huang, S.; Sun, W.; Xiong, H.; Zhao, Q. Chitosan/rice hydrolysate/curcumin composite film. Effect of chitosan molecular weight. Int. J. Biol. Macromol. 2022, 210, 53–62. [Google Scholar] [CrossRef] [PubMed]
- de la Horra, A.E.; Steffolani, E.M.; Barrera, G.N.; Ribotta, P.D.; León, A.E. The role of cyclodextrinase and glucose oxidase in obtaining gluten-free laminated baked products. Eur. Food Res. Technol. 2018, 244, 1341–1351. [Google Scholar] [CrossRef]
- Yano, H.; Fu, W. Effective Use of Plant Proteins for the Development of “New” Foods. Foods 2022, 11, 1185. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Fang, Y.; Wang, L.; Xie, M.; Hu, B.; Zhu, Y.; Zhao, E.; Pei, F.; Shen, F.; Li, P.; et al. Effect of enzyme types on the stability of oil-in-water emulsions formed with rice protein hydrolysates. J. Sci. Food Agric. 2019, 99, 6731–6740. [Google Scholar] [CrossRef]
- Qamar, S.; Manrique, Y.J.; Parekh, H.; Falconer, J.R. Nuts, cereals, seeds and legumes proteins derived emulsifiers as a source of plant protein beverages: A review. Crit. Rev. Food Sci. Nutr. 2020, 60, 2742–2762. [Google Scholar] [CrossRef]
- Sen, S.; Chakraborty, R.; Kalita, P. Rice-not just a staple food: A comprehensive review on its phytochemicals and therapeutic potential. Trends Food Sci. Technol. 2020, 97, 265–285. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, J.; Wang, J.; Sun, B. The anti-cancer activity and potential clinical application of rice bran extracts and fermentation products. RSC Adv. 2019, 9, 18060–18069. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.; Cui, X.; Duan, Y.; Yang, S.; Wang, P.; Saleh, A.S.; Xiao, Z. Potential health benefits and food applications of rice bran protein: Research advances and challenges. Food Rev. Int. 2021, 1–24. [Google Scholar] [CrossRef]
- Li, H.; Wang, Z.; Liang, M.; Cai, L.; Yang, L. Methionine augments antioxidant activity of rice protein during gastrointestinal digestion. Int. J. Mol. Sci. 2019, 20, 868. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hossny, E.; Ebisawa, M.; El-Gamal, Y.; Arasi, S.; Dahdah, L.; El-Owaidy, R.; Galvan, C.A.; Lee, B.W.; Levin, M.; Martinez, S.; et al. Challenges of managing food allergy in the developing world. World Allergy Organ. J. 2019, 12, 100089. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Q.; Woo, M.W.; Hu, J.; Xiong, H.; Zhao, Q. The role of heating time on the characteristics, functional properties and antioxidant activity of enzyme-hydrolyzed rice proteins-glucose Maillard reaction products. Food Biosci. 2021, 43, 101225. [Google Scholar] [CrossRef]
- Ekezie, F.G.C.; Cheng, J.H.; Sun, D.W. Effects of nonthermal food processing technologies on food allergens: A review of recent research advances. Trends Food Sci. Technol. 2018, 74, 12–25. [Google Scholar] [CrossRef]
- Birla, D.S.; Malik, K.; Sainger, M.; Chaudhary, D.; Jaiwal, R.; Jaiwal, P.K. Progress and challenges in improving the nutritional quality of rice (Oryza sativa L.). Crit. Rev. Food Sci. Nutr. 2017, 57, 2455–2481. [Google Scholar] [CrossRef]
- Brestenský, M.; Nitrayová, S.; Patráš, P.; Nitray, J. Dietary requirements for proteins and amino acids in human nutrition. Curr. Nutr. Food Sci. 2019, 15, 638–645. [Google Scholar] [CrossRef]




| Amino Acids | Rice Protein (g/100 g) | Reference | |
|---|---|---|---|
| Essential amino acid | threonine | 2.09–5.06 | |
| Valine | 3.78–6.80 | ||
| Isoleucine | 2.69–5.18 | ||
| Leucine | 5.30–9.51 | ||
| Lysine | 2.2–6.24 | ||
| Histidine | 1.19–3.49 | [7,15,18,22,24,31] | |
| Methionine | 0.65–3.49 | ||
| Cystine | 0.13–3.42 | ||
| Tyrosine | 1.33–6.0 | ||
| Phenylalanine | 3.5–6.30 | ||
| Methionine + cystine | 2.35–3.88 | ||
| Phenylalanine + tyrosine | 6.80–10.33 | ||
| Non-essential amino acid | Aspartic acid | 8.10–10.98 | |
| Serine | 2.96–5.64 | ||
| Glutamic acid | 13.36–22.42 | ||
| Glycine | 4.21–5.98 | [33,34,35,36] | |
| Alanine | 3.69–6.20 | ||
| Arginine | 5.30–9.84 | ||
| proline | 2.70–14.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jayaprakash, G.; Bains, A.; Chawla, P.; Fogarasi, M.; Fogarasi, S. A Narrative Review on Rice Proteins: Current Scenario and Food Industrial Application. Polymers 2022, 14, 3003. https://doi.org/10.3390/polym14153003
Jayaprakash G, Bains A, Chawla P, Fogarasi M, Fogarasi S. A Narrative Review on Rice Proteins: Current Scenario and Food Industrial Application. Polymers. 2022; 14(15):3003. https://doi.org/10.3390/polym14153003
Chicago/Turabian StyleJayaprakash, Gopika, Aarti Bains, Prince Chawla, Melinda Fogarasi, and Szabolcs Fogarasi. 2022. "A Narrative Review on Rice Proteins: Current Scenario and Food Industrial Application" Polymers 14, no. 15: 3003. https://doi.org/10.3390/polym14153003