Advanced Collagen-Based Composites as Fertilizers Obtained by Recycling Lime Pelts Waste Resulted during Leather Manufacture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physical–Chemical Analyses of Soils, Leachate, and Harvested Crops
2.3. Biochemical Analysis of Soils
2.4. Evaluation of Exchangeable NPK Nutrients of Agro-Hydrogels in Soils
2.5. Determination of Leached Ammonium and Phosphate Ions
3. Results
3.1. Characterization of Compounded Hydrogels with Nutrients Encapsulated
3.2. Biochemical Characterization of Soils
3.3. Agrochemical Tests of New Fertilizers on Soils
4. Discussion
4.1. Physical–Chemical Interactions Inside Polymeric Composites
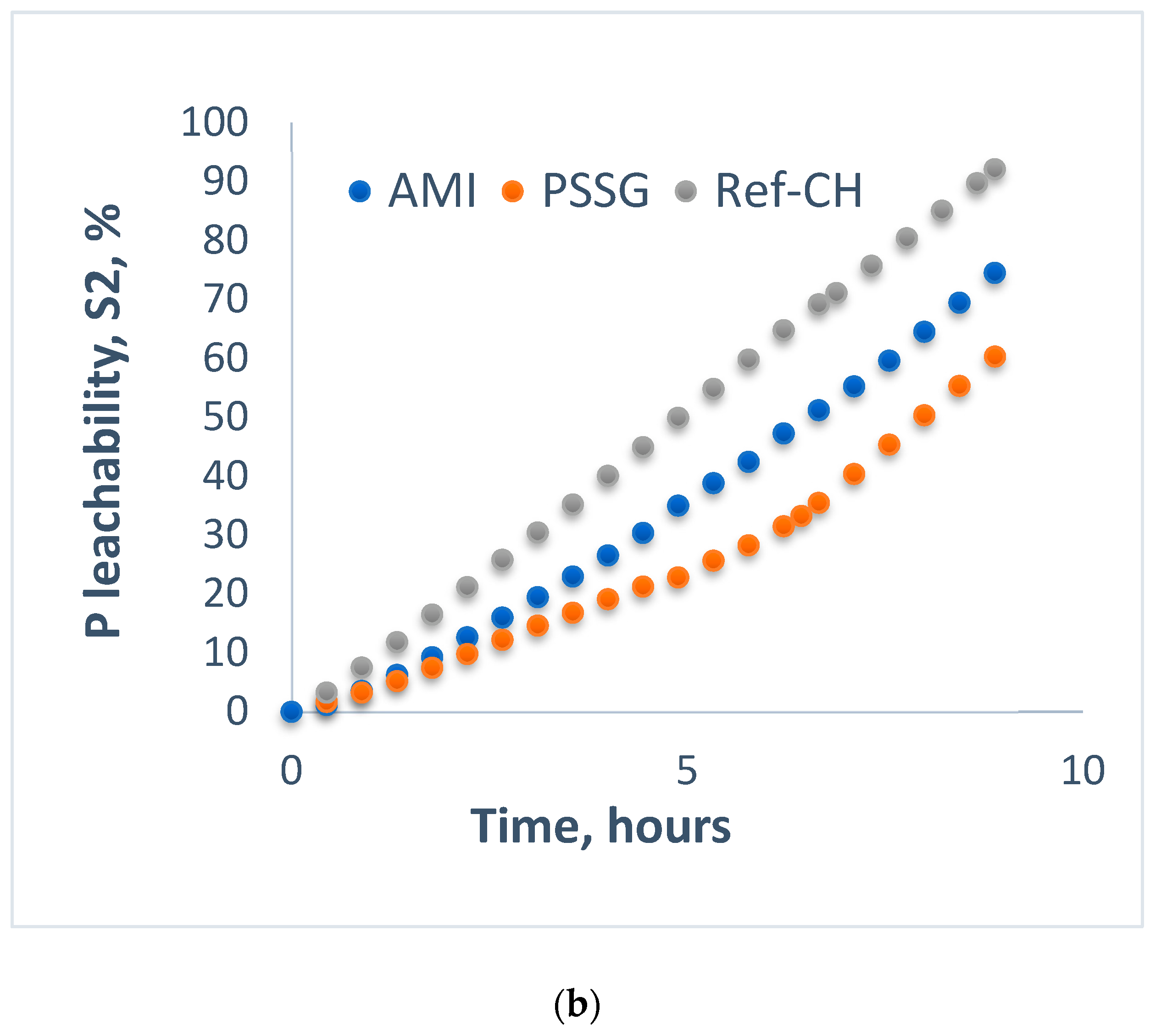
4.2. Behavior of Fertilizer Nutrients during Uptake and Leaching on Sand and Soils
4.3. Biological Amelioration of Soils’ Fertility
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Eurostat, Circular Economy in the EU, Record Recycling Rates and Use of Recycled Materials in the EU, 39/2019. Available online: https://ec.europa.eu/eurostat/documents/2995521/9629294/8-04032019-BP-EN.pdf/295c2302-4ed1-45b9-af86-96d1bbb7acb1 (accessed on 22 December 2020).
- Eprikashvili, L.; Zautashvili, M.; Kordzakhia, T.; Pirtskhalava, N.; Dzagania, M.; Rubashvili, I.; Tsitsishvili, V. Intensification of bioproductivity of agricultural cultures by adding natural zeolites and brown coals into soils. Ann. Agrar. Sci. 2016, 14, 67–71. [Google Scholar] [CrossRef] [Green Version]
- He, L.L.; Zhong, Z.K.; Yang, H.M. Effects on soil quality of biochar and straw amendment in conjunction with chemical fertilizers. J. Integr. Agric. 2017, 16, 704–712. [Google Scholar] [CrossRef] [Green Version]
- Sharma, B.; Sarkar, A.; Singh, P.; Singh, R.P. Agricultural utilization of biosolids: A review on potential effects on soil and plant grown. Waste Manag. 2017, 64, 117–123. [Google Scholar] [CrossRef] [PubMed]
- De Albuquerque Nunes, W.A.G.; Menezes, J.F.S.; De Melo Benites, V.; De Lima, S.A., Jr.; Dos Santos Oliveir, A. Use of organic compost produced from slaughterhouse waste as fertilizer in soybean and corn crops. Sci. Agric. 2015, 72, 343–350. [Google Scholar] [CrossRef] [Green Version]
- Wuang, S.C.; Khin, M.C.; Chua, P.Q.D.; Luo, Y.D. Use of Spirulina biomass produced from treatment of aquaculture wastewater as agricultural fertilizers. Algal Res. 2016, 15, 59–64. [Google Scholar] [CrossRef]
- Nastac, M.; Negreanu-Pirjol, B.S.; Negreanu-Pirjol, T.; Meghea, A.; Gheorghiu, K.A.; Resteanu, A.N. Multicomposite Biologic Fertilizer. Romanian Patent RO131272 A2, reg. a2015 00061, 29 July 2016. [Google Scholar]
- Choi, W.J.; Kwak, J.H.; Lim, S.S.; Park, H.J.; Chang, S.X.; Lee, S.M.; Arshad, M.A.; Yun, S.I.; Kim, H.Y. Synthetic fertilizer and livestock manure differently affect δ15N in the agricultural landscape: A review. Agric. Ecosys. Environ. 2017, 237, 1–15. [Google Scholar] [CrossRef]
- Campitelli, P.A.; Velasco, M.I.; Ceppi, S.B. Chemical and physicochemical characteristics of humic acids extracted from compost, soil and amended soil. Talanta 2006, 69, 1234–1239. [Google Scholar] [CrossRef]
- Campitelli, P.; Ceppi, S. Effects of composting technologies on the chemical and physicochemical properties of humic acids. Geoderma 2008, 144, 325–333. [Google Scholar] [CrossRef]
- Garcia-Gil, J.C.; Ceppi, S.B.; Velasco, M.I.; Polo, A.; Senesi, N. Longterm effects of amendment with municipal solid waste compost on the elemental and acidic functional group composition and pH-buffer capacity of soil humic acids. Geoderma 2004, 121, 135–142. [Google Scholar] [CrossRef]
- Bajza, Z.; Vrucek, V. Thermal and enzymatic recovering of proteins from untanned leather waste. Waste Manag. 2001, 21, 79–84. [Google Scholar] [CrossRef]
- Rangel Serrano, A.; Maldonado, V.M.; Kosters, K. Characterization of waste materials in tanners for better ecological uses. J. Am. Leather Chem. Assoc. 2003, 98, 43–48. [Google Scholar]
- Puoci, F.; Iemma, F.; Spizzirri, U.G.; Cirillo, G.; Curcio, M.; Picci, N. Polymer in agriculture: A review. Am. J. Agric. Biol. Sci. 2008, 3, 299–314. [Google Scholar] [CrossRef] [Green Version]
- Ramli, N.K.; Mansor, N.; Man, Z. A comprehensive review on biodegradable polymers and their blends used in controlled release fertilizer processes. Rev. Chem. Eng. 2015, 31, 69–95. [Google Scholar] [CrossRef]
- Gomez-Sagasti, M.T.; Hernandes, A.; Artetxe, U.; Garbisu, C.; Becerril, J.M. How valuable are organic amendments as tool for the phytomanagement of degradated soils? The knowns, known unknows, and unknowns. Front. Sustain. Food Syst. 2018, 2, 68. [Google Scholar] [CrossRef] [Green Version]
- Tahat, M.M.; Alananbeh, M.K.; Othman, A.Y.; Leskovar, I.D. Soil Health and Sustainable Agriculture. Sustainability 2020, 12, 4859. [Google Scholar] [CrossRef]
- Ashmead, H.H. Synergistic Plant Regulatory Compositions. U.S. Patent 4169717, 1979. [Google Scholar]
- Ashmead, H.H.; Hsu, H.-H. Potassium Polyphosphate Protein Hydrolysate Fertilizer. U.S. Patent 4491464, 1985. [Google Scholar]
- Gu, J.L.; Lee, E. Mineral Collagen Chelates and Methods of Making and Using Same. U.S. Patent 2010/004183, 2010. [Google Scholar]
- Tzoumani, I.; Lainioti, G.C.; Aletras, A.J.; Zainescu, G.; Stefan, S.; Meghea, A.; Kallitsis, J.K. Modification of collagen derivatives with water-soluble polymers for the development of cross-linked hydrogels for controlled release. Materials 2019, 12, 4067. [Google Scholar] [CrossRef] [Green Version]
- Stefan, D.S.; Zainescu, G.; Manea-Saghin, A.-M.; Triantaphyllidou, I.-E.; Tzoumani, I.; Tatoulis, T.I.; Syriopoulos, G.T.; Meghea, A. Collagen-based hydrogels composites from hide waste to produce smart fertilizers. Materials 2020, 13, 4396. [Google Scholar] [CrossRef]
- Stefan, D.S.; Bosomiu, M.; Constantinescu, R.R.; Ignat, M. Composite polymers from hide waste to produce smart fertilizers. Polymers 2021, 13, 4351. [Google Scholar] [CrossRef]
- Zainescu, G.A.; Albu, L.; Constantinescu, R. Study of collagen hydrogel biodegradability over time. Rev. Chem. 2018, 69, 101–104. [Google Scholar] [CrossRef]
- Zainescu, G.; Constantinescu, R.; Sirbu, C. Smart hydrogels with collagen structure made of pelt waste. Rev. Chem. 2017, 68, 393–395. [Google Scholar] [CrossRef]
- Rusu, M.; Marghitas, M.; Oroian, I.; Mihaiescu, T.C.; Dumitras, A. Treatease of Agrochemistry; Ed Ceres: Bucharest, Romania, 2005. [Google Scholar]
- Stoica, E.; Rauta, C.; Florea, N. Methods for Chemical Analysis of Soil; ICPA: Bucharest, Romania, 1988; 487p. (In Romanian) [Google Scholar]
- Furtak, K.; Gajda, A.M. Activity and variety of soil microorganisms depending on the diversity of the tillage system, Chapter 3. In Sustainability of Agrosystems; de Oliveira, A.B., Ed.; IntechOpen: London, UK, 2018; pp. 45–61. [Google Scholar] [CrossRef] [Green Version]
- Garland, J.; Millis, A. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1991, 57, 2351–2359. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-Based Hydrogels for Organ 3D Bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [Green Version]
- Nadtoka, O.; Kutsevol, N.; Krysa, V.; Krysa, B. Hybrid polyacryamide hydrogels: Synthesis, properties and prospects of application. Mol. Cryst. Liq. Cryst. 2018, 672, 1–10. [Google Scholar] [CrossRef]
- Nawaz, H.; Waheed, R.; Nawaz, M.; Shahwar, D. Physical and Chemical Modifications in Starch Structure and Reactivity. In Chemical Properties of Starch; Emeje, M., Ed.; IntechOpen: London, UK, 2020. [Google Scholar] [CrossRef] [Green Version]
- Gilalni, Q.F.; Ahmad, F.; Mutalib, M.I.A.; Arogundade, A. Effect of Dolomite Clay on Thermal Performance and Char Morphology of Expandable Graphite Based Intumescent Fire Retardant Coatings. Procedia Eng. 2016, 148, 146–150. [Google Scholar] [CrossRef] [Green Version]
- Watts, D.B.; Torbert, H.A.; Prior, S.A.; Huluka, G. Long-term tillage and poultry litter impacts soil carbon and nitrogen mineralization and fertility. Soil Sci. Soc. Am. J. 2010, 74, 1239–1247. [Google Scholar] [CrossRef] [Green Version]
- Anghel, A.; Lacatusu, A.-R.; Lacatusu, R.; Iancu, S.; Lungu, M.; Lazar, R.; Vrinceanu, A. The effect of prolonged release mineral fertilizers coated with co-polyester films from pet waste recycling on maize plants. Sci. Papers. Ser. A Agron. 2012, 55, 123–128. [Google Scholar]
- Lima, D.Q.; Oliveira, L.C.A.; Bastos, A.R.R.; Carvalho, G.S.; Marques, J.G.S.M.; Carvalho, J.G.; Souza, G.A. Leather industry solid waste as nitrogen source for growth of common bean plants. Appl. Environ. Soil Sci. 2010, 2010, 703842. [Google Scholar] [CrossRef]





| Chemical Analysis, % | Ref-CH | PSSG | POLY | AMI | DO |
|---|---|---|---|---|---|
| Nitrogen (N) | 9.91 | 9.86 | 10.70 | 9.90 | 9.89 |
| Phosphorus (P) | 5.50 | 5.20 | 5.25 | 5.30 | 5.22 |
| Potassium (K) | 10.07 | 9.55 | 9.50 | 9.28 | 9.95 |
| Initial Fertilizers | Concentration in Suspension | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Nr. Crt. | Parameter | Units | AMI | DO | 0.5% | 1% | 5% | 10% | ||||
| AMI | DO | AMI | DO | AMI | DO | AMI | DO | |||||
| 1 | Total nitrogen (Nt) | % | 6.97 | 10.13 | ||||||||
| 2 | Total phosphorus (P2O5) | % | 1.50 | 3.43 | ||||||||
| 3 | Potassium, water-soluble (K2O) | % | 21.97 | 2.35 | ||||||||
| 4 | Total content of soluble salts, extraction ratio 1:10 | g/100 g | 32.80 | 21.91 | ||||||||
| 5 | pH solution conc. | pH units | 7.60 | 3.70 | 7.55 | 3.63 | 7.43 | 3.50 | 7.37 | 3.43 | ||
| 6 | Conductivity solution conc. | mS/cm | 3.49 | 2.49 | 6.51 | 4.63 | 20 | 18.97 | 47.20 | 32.90 | ||
| Parameter | Result (cfu/g) | Method | Conditions | ||
|---|---|---|---|---|---|
| S1-L | S2-CL | S3-SiCL | |||
| Aerobic mesophilic bacteria count | 500,000 | 900,000 | 700,000 | IH O:36141 | PCA/Aerobic/30 °C/24–72 h |
| Proteolytic bacteria | <10 | <10 | <10 | IH O:36143 | PCA-SM/Aerobic/30 °C/24–72 h |
| Yeasts | <50 | 1500 | <50 | IH O:43842 | RBCA/Aerobic/20.5 °C/3–5 d |
| Molds | 3000 | 15,000 | 1000 | IH O:43842 | RBCA/Aerobic/20.5 °C/3–5 d |
| Actinomyces | 150 | 15,000 | 150 | IH:55151 | SBA/Anaerobic/35 °C/3–7 d |
| Indicator | ||||||||
|---|---|---|---|---|---|---|---|---|
| VARIANT | Aerobic Cultivable Mesophilic Bacteria Number | Cultivable Fungi Number | Soil Respiration | Microbial Biomass | ||||
| mil cfu/g soil | % | mil cfu/g soil | % | mg CO2/100 g soil | % | mg CO2/100 g sol | % | |
| Moist soil (S4-SCL) | 4644 | 100 | 6490 | 100 | 7977 | 100 | 372.8 | 100 |
| Hydrogel AMI 0.1% | 7488 | 161 | 4722 | 73 | 9322 | 117 | 445.2 | 119 |
| Hydrogel AMI 0.2% | 8416 | 181 | 4666 | 72 | 9250 | 91 | 301.8 | 81 |
| Soil | Ref-CH | PSSG | AMI | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| P | N | P | N | P | N | |||||||
| Amax | t, h | Amax | t, h | Amax | t, h | Amax | t, h | Amax | t, h | Amax | t, h | |
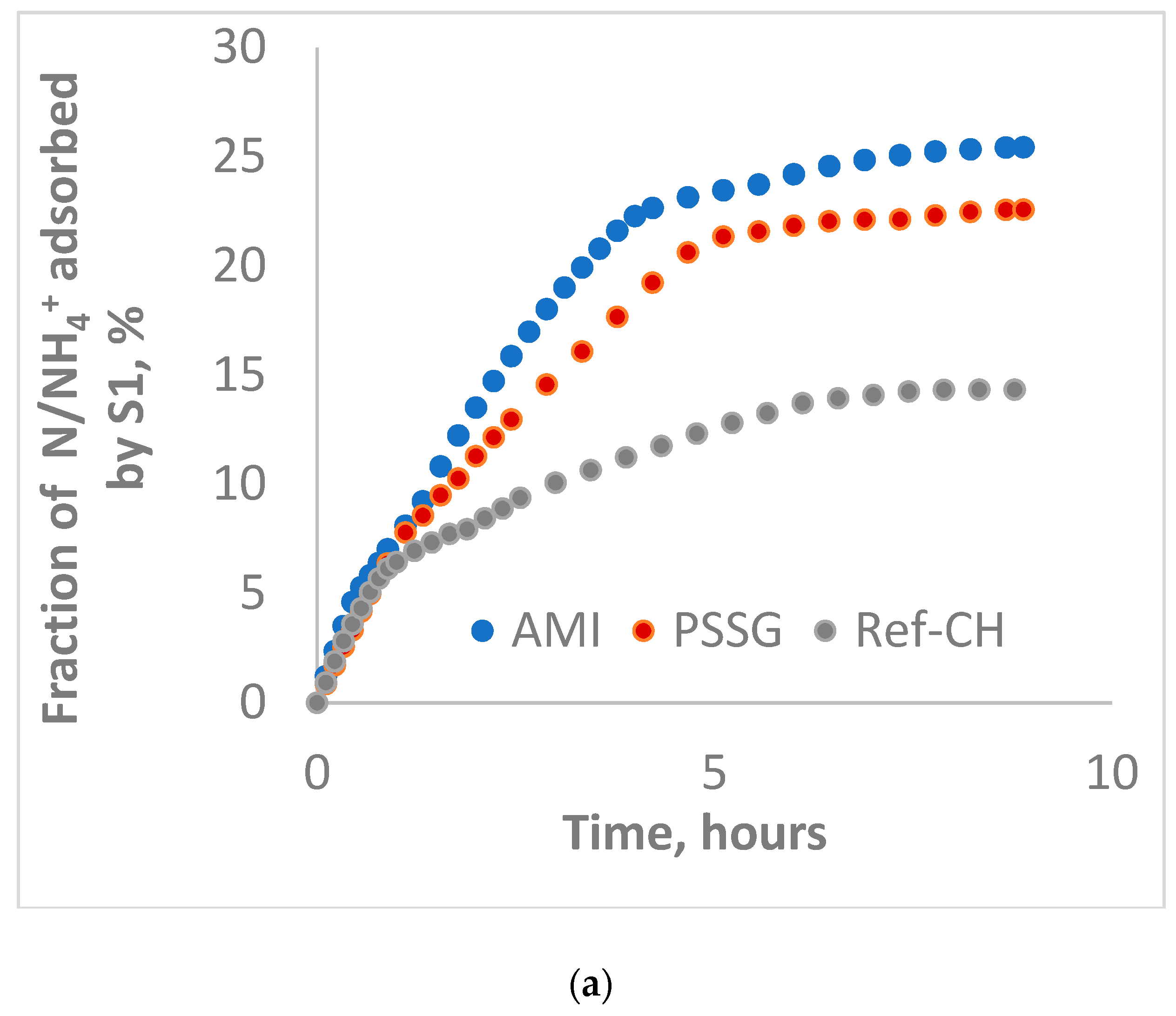
| S1-L | 1.989 | 2 | 6.10 | 6 | 0.228 | 6 | 13.08 | 6 | 0.1105 | 1 | 16.50 | 6 |
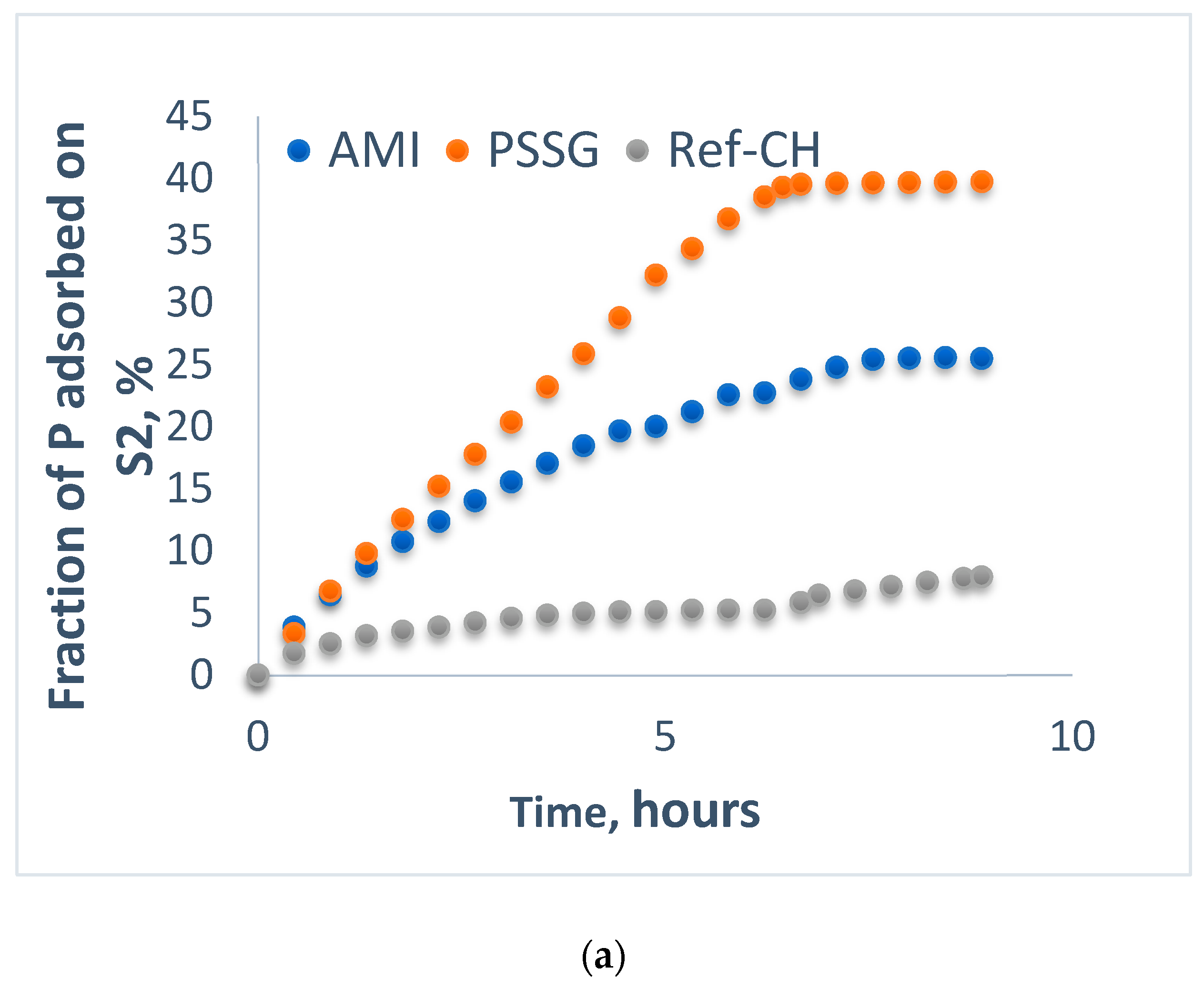
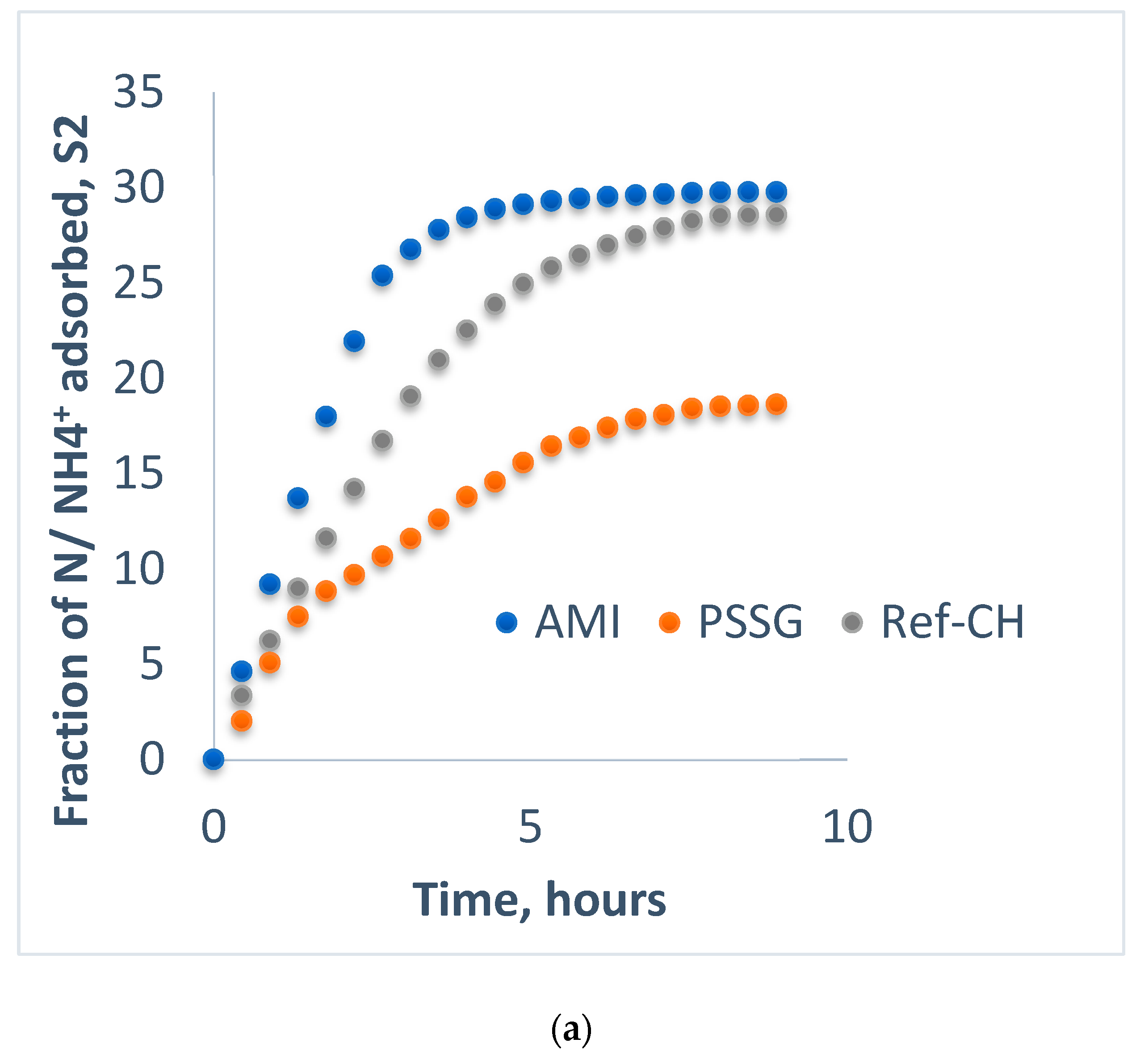
| S2-CL | 1.775 | 4 | 20.17 | 8 | 5.700 | 7 | 10.80 | 7 | 0.9560 | 7 | 19.26 | 4 |
| Crt. | Functionalization Agents | Possible Structure of the Composite Fertilizers | ||
|---|---|---|---|---|
| Name | Structure | Active Groups | ||
| 1 | Poly-acryl amide, PAM -synthetic polymers -acrylic resin | 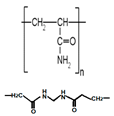 diacrylamide [31] | -NH2 (amino groups) >C=O (carbonyl groups) | 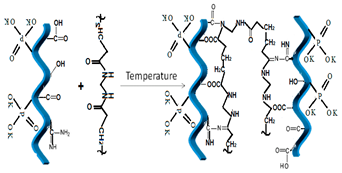 |
| 2 | Poly(sodium 4-styrenesulfonate-co-glycidyl methacrylate) (P(SSNa-co-GMAx), synthetic polymer | 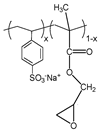 [21] | >C=O (carbonyl groups) | 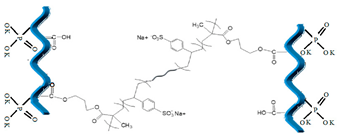 |
| 3 | Starch, natural polymer | 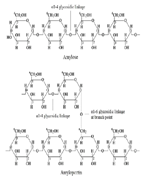 [32] | -OH hydroxyl -CH2-O- CH2- glycoside linkages | 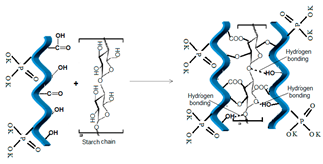 |
| 4 | Dolomite, natural ore | 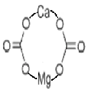 [33] | CO3− | 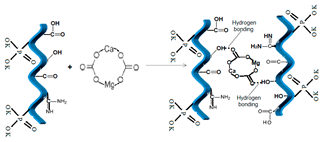 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stefan, D.S.; Manea-Saghin, A.-M.; Triantaphyllidou, I.-E.; Tzoumani, I.; Meghea, I. Advanced Collagen-Based Composites as Fertilizers Obtained by Recycling Lime Pelts Waste Resulted during Leather Manufacture. Polymers 2022, 14, 3169. https://doi.org/10.3390/polym14153169
Stefan DS, Manea-Saghin A-M, Triantaphyllidou I-E, Tzoumani I, Meghea I. Advanced Collagen-Based Composites as Fertilizers Obtained by Recycling Lime Pelts Waste Resulted during Leather Manufacture. Polymers. 2022; 14(15):3169. https://doi.org/10.3390/polym14153169
Chicago/Turabian StyleStefan, Daniela Simina, Ana-Maria Manea-Saghin, Irene-Eva Triantaphyllidou, Ioanna Tzoumani, and Irina Meghea. 2022. "Advanced Collagen-Based Composites as Fertilizers Obtained by Recycling Lime Pelts Waste Resulted during Leather Manufacture" Polymers 14, no. 15: 3169. https://doi.org/10.3390/polym14153169





