Scalable Fabrication of Thermally Conductive Layered Nacre-like Self-Assembled 3D BN-Based PVA Aerogel Framework Nanocomposites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Characterizations
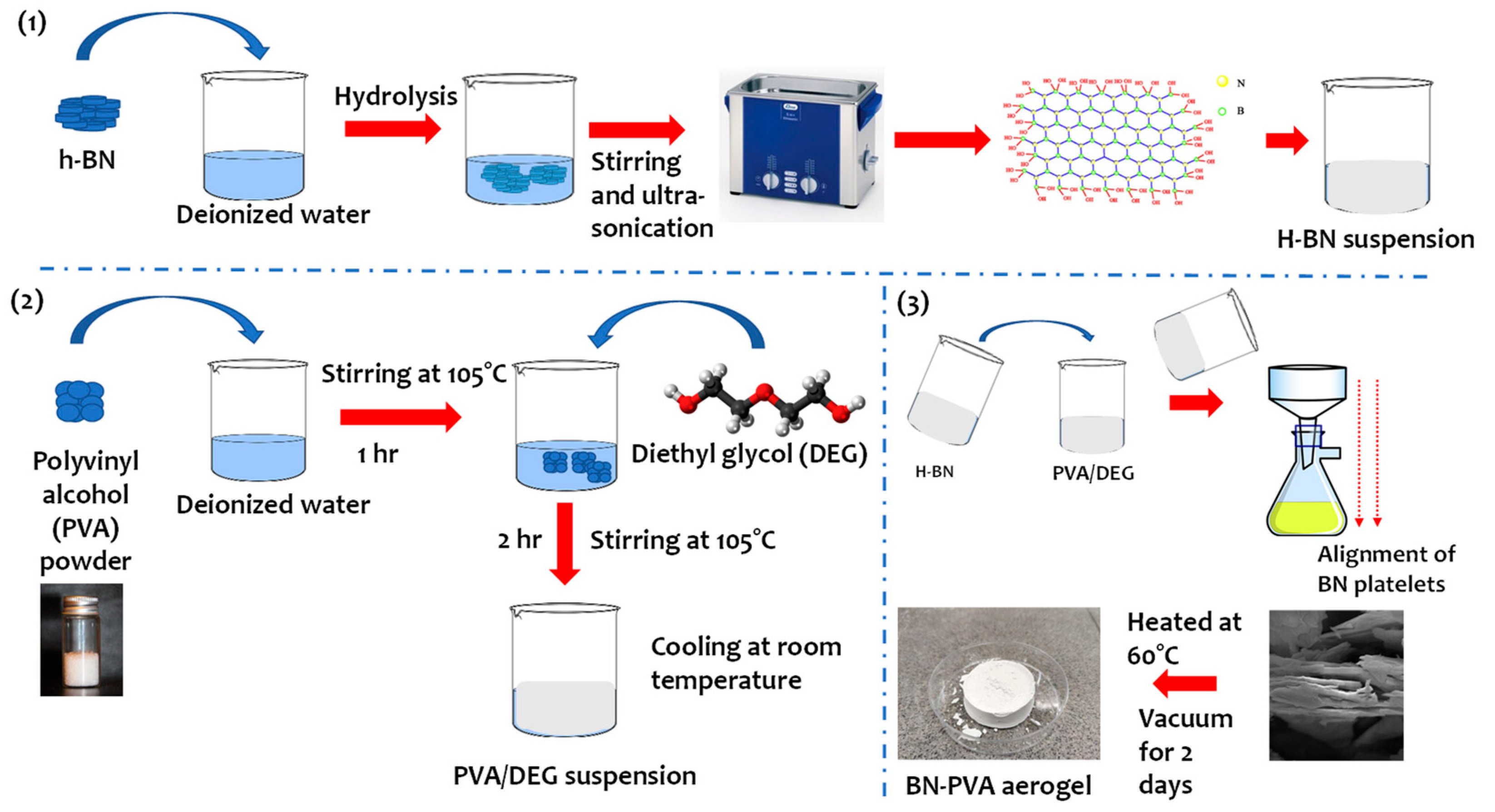
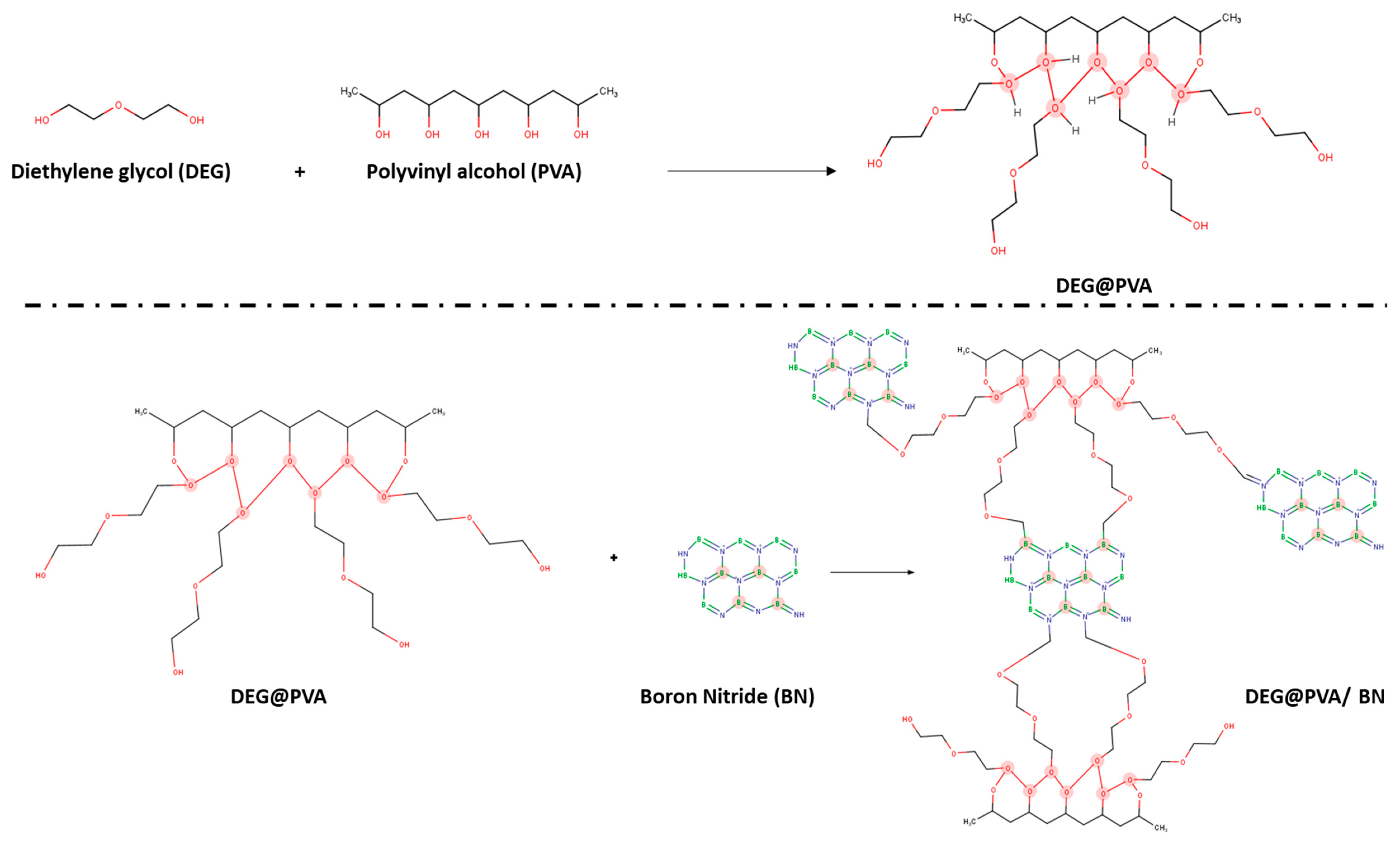
2.3. Methodology
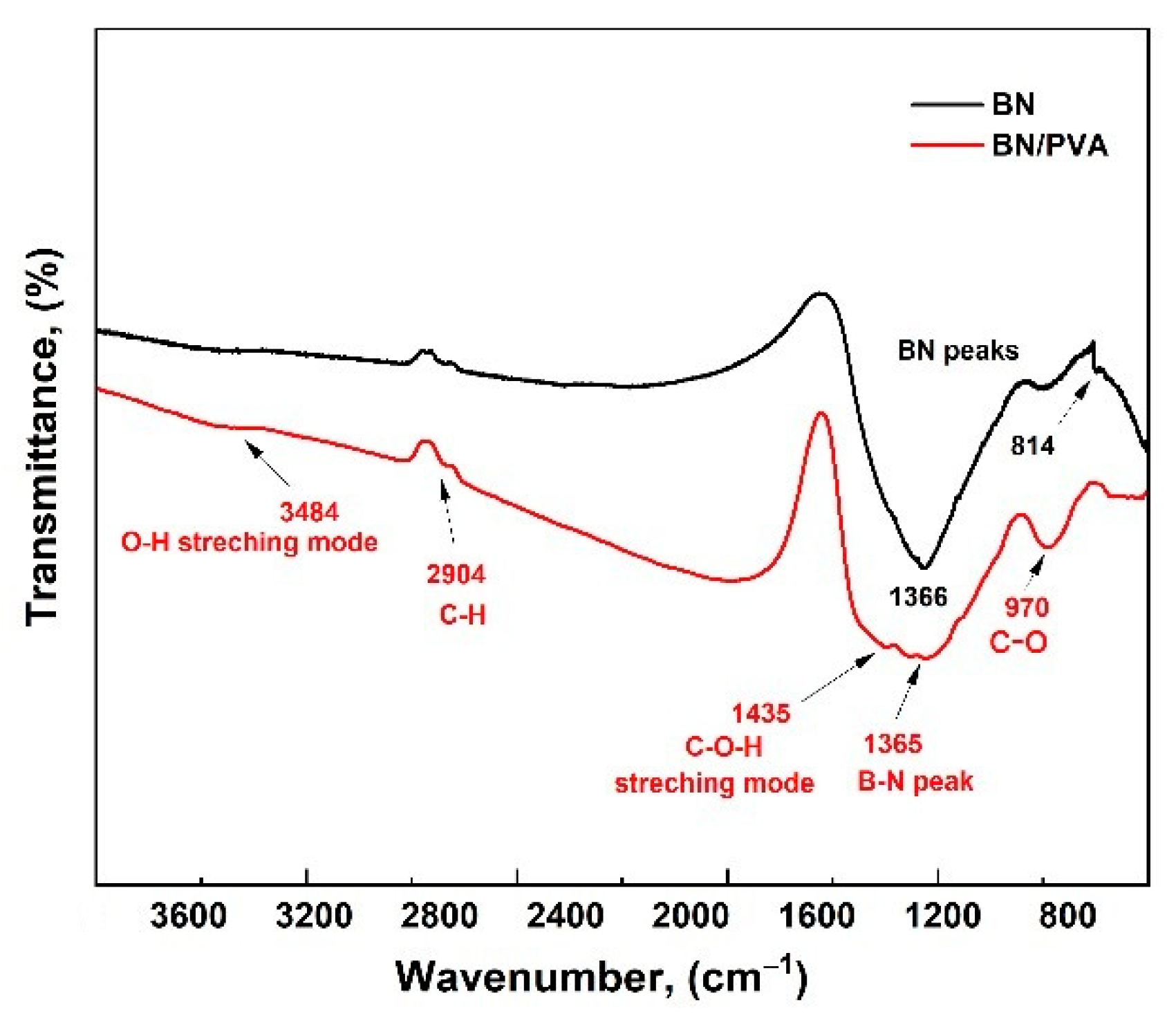
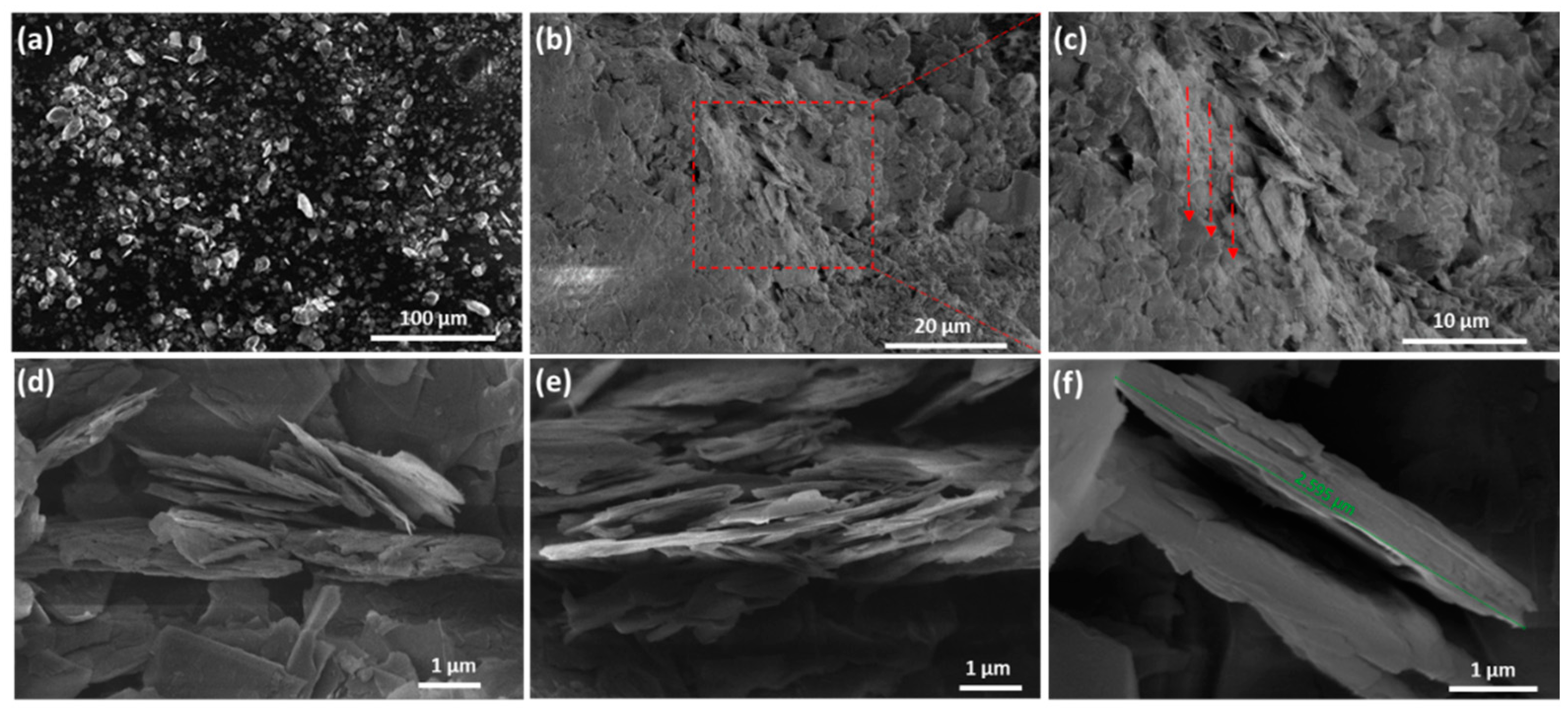
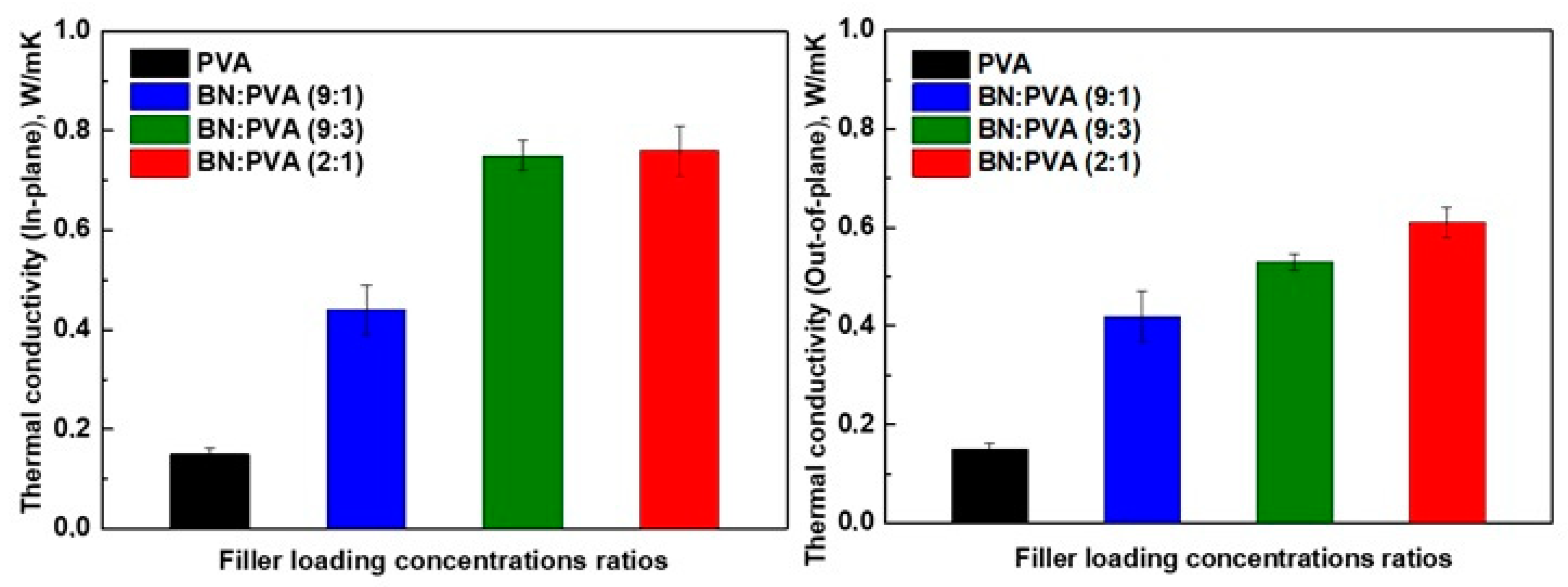
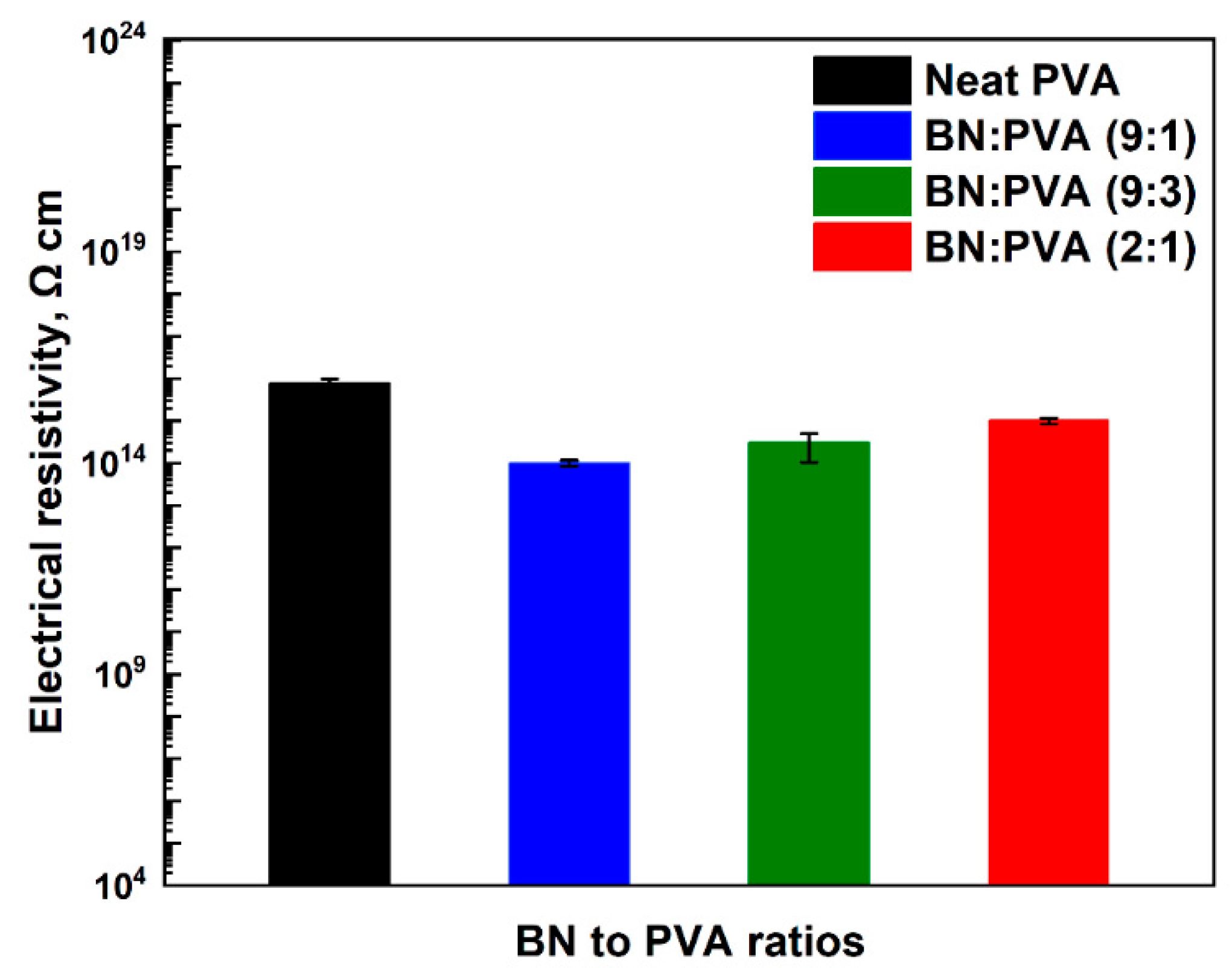
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Zhang, R.-C.; Huang, Z.; Huang, Z.; Zhong, M.; Zang, D.; Lu, A.; Lin, Y.; Millar, B.; Garet, G.; Turner, J. Uniaxially stretched polyethylene/boron nitride nanocomposite films with metal-like thermal conductivity. Compos. Sci. Technol. 2020, 196, 108154. [Google Scholar] [CrossRef]
- Mehra, N.; Mu, L.; Ji, T.; Yang, X.; Kong, J.; Gu, J.; Zhu, J. Thermal transport in polymeric materials and across composite interfaces. Appl. Mater. Today 2018, 12, 92–130. [Google Scholar] [CrossRef]
- Chen, H.; Ginzburg, V.V.; Yang, J.; Yang, Y.; Liu, W.; Huang, Y.; Du, L.; Chen, B. Thermal conductivity of polymer-based composites: Fundamentals and applications. Prog. Polym. Sci. 2016, 59, 41–85. [Google Scholar] [CrossRef]
- Li, Y.; Mehra, N.; Ji, T.; Yang, X.; Mu, L.; Gu, J.; Zhu, J. The stiffness-thermal conduction relationship at the composite interface: The effect of particle alignment on the long-range confinement of polymer chains monitored by scanning thermal microscopy. Nanoscale 2018, 10, 1695–1703. [Google Scholar] [CrossRef]
- Mehra, N.; Mu, L.; Zhu, J. Developing heat conduction pathways through short polymer chains in a hydrogen bonded polymer system. Compos. Sci. Technol. 2017, 148, 97–105. [Google Scholar] [CrossRef]
- Li, Q.; Guo, Y.; Li, W.; Qiu, S.; Zhu, C.; Wei, X.; Chen, M.; Liu, C.; Liao, S.; Gong, Y.; et al. Ultrahigh Thermal Conductivity of Assembled Aligned Multilayer Graphene/Epoxy Composite. Chem. Mater. 2014, 26, 4459–4465. [Google Scholar] [CrossRef]
- Xin, G.; Sun, H.; Hu, T.; Fard, H.R.; Sun, X.; Koratkar, N.; Borca-Tasciuc, T.; Lian, J. Large-area freestanding graphene paper for superior thermal management. Adv. Mater. 2014, 26, 4521–4526. [Google Scholar] [CrossRef]
- Wang, N.; Samani, M.K.; Li, H.; Dong, L.; Zhang, Z.; Su, P.; Chen, S.; Chen, J.; Huang, S.; Yuan, G. Tailoring the thermal and mechanical properties of graphene film by structural engineering. Small 2018, 14, 1801346. [Google Scholar] [CrossRef]
- Song, N.-J.; Chen, C.-M.; Lu, C.; Liu, Z.; Kong, Q.-Q.; Cai, R. Thermally reduced graphene oxide films as flexible lateral heat spreaders. J. Mater. Chem. A 2014, 2, 16563–16568. [Google Scholar] [CrossRef]
- Shen, B.; Zhai, W.; Zheng, W. Ultrathin flexible graphene film: An excellent thermal conducting material with efficient EMI shielding. Adv. Funct. Mater. 2014, 24, 4542–4548. [Google Scholar] [CrossRef]
- Zha, X.-H.; Zhou, J.; Zhou, Y.; Huang, Q.; He, J.; Francisco, J.S.; Luo, K.; Du, S. Promising electron mobility and high thermal conductivity in Sc2CT2 (T = F, OH) MXenes. Nanoscale 2016, 8, 6110–6117. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Z.; Chen, Y.; Lu, Y.; Liu, L.; Hu, S.; Wen, S.; Mao, Y.; Zhang, L. Fabrication of highly oriented hexagonal boron nitride nanosheet/elastomer nanocomposites with high thermal conductivity. Small 2015, 11, 1655–1659. [Google Scholar] [CrossRef] [PubMed]
- Owais, M.; Zhao, J.; Imani, A.; Wang, G.; Zhang, H.; Zhang, Z. Synergetic effect of hybrid fillers of boron nitride, graphene nanoplatelets, and short carbon fibers for enhanced thermal conductivity and electrical resistivity of epoxy nanocomposites. Compos. Part A Appl. Sci. Manuf. 2019, 117, 11–22. [Google Scholar] [CrossRef]
- Renteria, J.D.; Ramirez, S.; Malekpour, H.; Alonso, B.; Centeno, A.; Zurutuza, A.; Cocemasov, A.I.; Nika, D.L.; Balandin, A.A. Strongly anisotropic thermal conductivity of free-standing reduced graphene oxide films annealed at high temperature. Adv. Funct. Mater. 2015, 25, 4664–4672. [Google Scholar] [CrossRef]
- Zhu, H.; Li, Y.; Fang, Z.; Xu, J.; Cao, F.; Wan, J.; Preston, C.; Yang, B.; Hu, L. Highly thermally conductive papers with percolative layered boron nitride nanosheets. ACS Nano 2014, 8, 3606–3613. [Google Scholar] [CrossRef]
- Chen, J.; Huang, X.; Zhu, Y.; Jiang, P. Cellulose nanofiber supported 3D interconnected BN nanosheets for epoxy nanocomposites with ultrahigh thermal management capability. Adv. Funct. Mater. 2017, 27, 1604754. [Google Scholar] [CrossRef]
- Wang, M.; Zhang, T.; Mao, D.; Yao, Y.; Zeng, X.; Ren, L.; Cai, Q.; Mateti, S.; Li, L.H.; Zeng, X. Highly compressive boron nitride nanotube aerogels reinforced with reduced graphene oxide. ACS Nano 2019, 13, 7402–7409. [Google Scholar] [CrossRef]
- Jiang, F.; Cui, S.; Song, N.; Shi, L.; Ding, P. Hydrogen bond-regulated boron nitride network structures for improved thermal conductive property of polyamide-imide composites. ACS Appl. Mater. Interfaces 2018, 10, 16812–16821. [Google Scholar] [CrossRef]
- Jing, L.; Li, H.; Tay, R.Y.; Sun, B.; Tsang, S.H.; Cometto, O.; Lin, J.; Teo, E.H.T.; Tok, A.I.Y. Biocompatible hydroxylated boron nitride nanosheets/poly (vinyl alcohol) interpenetrating hydrogels with enhanced mechanical and thermal responses. ACS Nano 2017, 11, 3742–3751. [Google Scholar] [CrossRef]
- Lao, J.; Xie, H.; Shi, Z.; Li, G.; Li, B.; Hu, G.-H.; Yang, Q.; Xiong, C. Flexible regenerated cellulose/boron nitride nanosheet high-temperature dielectric nanocomposite films with high energy density and breakdown strength. ACS Sustain. Chem. Eng. 2018, 6, 7151–7158. [Google Scholar] [CrossRef]
- Wang, Y.; Kong, D.; Shi, W.; Liu, B.; Sim, G.J.; Ge, Q.; Yang, H.Y. Ice templated free-standing hierarchically WS2/CNT-rGO aerogel for high-performance rechargeable lithium and sodium ion batteries. Adv. Energy Mater. 2016, 6, 1601057. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, E.; Li, X.; Zhang, Y.; Qu, J.; Yu, Z.-Z. Cellulose/graphene aerogel supported phase change composites with high thermal conductivity and good shape stability for thermal energy storage. Carbon 2016, 98, 50–57. [Google Scholar] [CrossRef]
- Yang, J.; Qi, G.-Q.; Liu, Y.; Bao, R.-Y.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Hybrid graphene aerogels/phase change material composites: Thermal conductivity, shape-stabilization and light-to-thermal energy storage. Carbon 2016, 100, 693–702. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Jiao, L.; Bian, H.; Yang, W.; Wu, W.; Xiao, H.; Dai, H. Aerogel perfusion-prepared h-BN/CNF composite film with multiple thermally conductive pathways and high thermal conductivity. Nanomaterials 2019, 9, 1051. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Tang, L.-S.; Bao, R.-Y.; Bai, L.; Liu, Z.-Y.; Yang, W.; Xie, B.-H.; Yang, M.-B. Largely enhanced thermal conductivity of poly (ethylene glycol)/boron nitride composite phase change materials for solar-thermal-electric energy conversion and storage with very low content of graphene nanoplatelets. Chem. Eng. J. 2017, 315, 481–490. [Google Scholar] [CrossRef]
- Ahankari, S.; Paliwal, P.; Subhedar, A.; Kargarzadeh, H. Recent Developments in Nanocellulose-Based Aerogels in Thermal Applications: A Review. ACS Nano 2021, 15, 3849–3874. [Google Scholar] [CrossRef]
- Owais, M.; Javed, M.H.; Akram, M.Z.; Paxton, W.F.; Akhatov, I.S.; Abaimov, S.G. Review—Recent Advances in Thermally Conductive Paper-Like Films. ECS J. Solid State Sci. Technol. 2021, 10, 033001. [Google Scholar] [CrossRef]
- Wu, W.; Liu, H.; Wang, Z.; Lv, P.; Hu, E.; Zheng, J.; Yu, K.; Wei, W. Formation of thermal conductive network in boron nitride/polyvinyl alcohol by ice-templated self-assembly. Ceram. Int. 2021, 47, 33926–33929. [Google Scholar] [CrossRef]
- Zeng, X.; Ye, L.; Yu, S.; Sun, R.; Xu, J.; Wong, C.-P. Facile Preparation of Superelastic and Ultralow Dielectric Boron Nitride Nanosheet Aerogels via Freeze-Casting Process. Chem. Mater. 2015, 27, 5849–5855. [Google Scholar] [CrossRef]
- Yang, X.; Guo, Y.; Han, Y.; Li, Y.; Ma, T.; Chen, M.; Kong, J.; Zhu, J.; Gu, J. Significant improvement of thermal conductivities for BNNS/PVA composite films via electrospinning followed by hot-pressing technology. Compos. Part B Eng. 2019, 175, 107070. [Google Scholar] [CrossRef]
- Datsyuk, V.; Trotsenko, S.; Reich, S. Carbon-nanotube–polymer nanofibers with high thermal conductivity. Carbon 2013, 52, 605–608. [Google Scholar] [CrossRef]
- Shen, H.; Guo, J.; Wang, H.; Zhao, N.; Xu, J. Bioinspired modification of h-BN for high thermal conductive composite films with aligned structure. ACS Appl. Mater. Interfaces 2015, 7, 5701–5708. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Liu, Y.; Raghavan, S.; Moon, K.-S.; Sitaraman, S.K.; Wong, C.-P. Magnetic alignment of hexagonal boron nitride platelets in polymer matrix: Toward high performance anisotropic polymer composites for electronic encapsulation. ACS Appl. Mater. Interfaces 2013, 5, 7633–7640. [Google Scholar] [CrossRef] [PubMed]
- Wieme, T.; Tang, D.; Delva, L.; D’hooge, D.R.; Cardon, L. The relevance of material and processing parameters on the thermal conductivity of thermoplastic composites. Polym. Eng. Sci. 2018, 58, 466–474. [Google Scholar] [CrossRef]
- Popov, E.; Goncharov, A.; Popov, Y.; Spasennykh, M.; Chekhonin, E.; Shakirov, A.; Gabova, A. Advanced techniques for determining thermal properties on rock samples and cuttings and indirect estimating for atmospheric and formation conditions. IOP Conf. Ser. Earth Environ. Sci. 2019, 367, 012017. [Google Scholar] [CrossRef]
- Popov, Y.; Beardsmore, G.; Clauser, C.; Roy, S. ISRM Suggested Methods for Determining Thermal Properties of Rocks from Laboratory Tests at Atmospheric Pressure. Rock Mech. Rock Eng. 2016, 49, 4179–4207. [Google Scholar] [CrossRef]
- Luo, F.; Zhang, M.; Chen, S.; Xu, J.; Ma, C.; Chen, G. Sandwich-structured PVA/rGO films from self-construction with highthermal conductivity and electrical insulation. Compos. Sci. Technol. 2021, 207, 108707. [Google Scholar] [CrossRef]


| Pristine BN | |||||
|---|---|---|---|---|---|
| Element | At. No | Mass (%) | Atom (%) | Abs. Error (%) (1 Sigma) | Rel. Error (%) (1 Sigma) |
| Nitrogen | 7 | 53.92 | 48.47 | 5.77 | 10.70 |
| Boron | 5 | 38.10 | 44.38 | 4.19 | 11.00 |
| Oxygen | 8 | 4.63 | 3.65 | 0.59 | 12.73 |
| Carbon | 6 | 3.34 | 3.50 | 0.43 | 12.80 |
| BN/PVA Composite | |||||
|---|---|---|---|---|---|
| Element | At. No | Mass (%) | Atom (%) | Abs. Error (%) (1 Sigma) | Rel. Error (%) (1 Sigma) |
| Nitrogen | 7 | 45.40 | 40.99 | 5.01 | 11.04 |
| Boron | 5 | 31.84 | 37.25 | 3.65 | 11.47 |
| Oxygen | 8 | 8.42 | 6.66 | 1.05 | 12.51 |
| Carbon | 6 | 14.34 | 15.10 | 1.65 | 11.51 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Owais, M.; Shiverskii, A.; Sulimov, A.; Ostrizhiniy, D.; Popov, Y.; Mahato, B.; Abaimov, S.G. Scalable Fabrication of Thermally Conductive Layered Nacre-like Self-Assembled 3D BN-Based PVA Aerogel Framework Nanocomposites. Polymers 2022, 14, 3316. https://doi.org/10.3390/polym14163316
Owais M, Shiverskii A, Sulimov A, Ostrizhiniy D, Popov Y, Mahato B, Abaimov SG. Scalable Fabrication of Thermally Conductive Layered Nacre-like Self-Assembled 3D BN-Based PVA Aerogel Framework Nanocomposites. Polymers. 2022; 14(16):3316. https://doi.org/10.3390/polym14163316
Chicago/Turabian StyleOwais, Mohammad, Aleksei Shiverskii, Artem Sulimov, Dmitriy Ostrizhiniy, Yuri Popov, Biltu Mahato, and Sergey G. Abaimov. 2022. "Scalable Fabrication of Thermally Conductive Layered Nacre-like Self-Assembled 3D BN-Based PVA Aerogel Framework Nanocomposites" Polymers 14, no. 16: 3316. https://doi.org/10.3390/polym14163316







