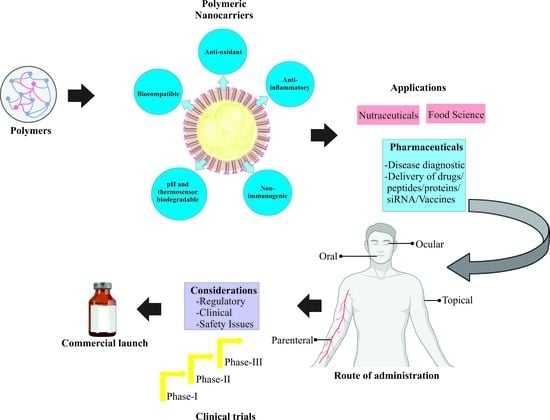
Insights on Development Aspects of Polymeric Nanocarriers: The Translation from Bench to Clinic
Abstract
:1. Introduction
2. Types of Polymer Based Nanocarriers
2.1. Polymer-Based Nanoparticles
2.2. Polymeric Micelles
2.3. Polymersomes
2.4. Dendrimers
2.5. Nanosized Hydrogels
2.6. Polymeric Cubosomes
3. Routes of Administration and Applications of Polymer Based Nanocarriers
3.1. Applications of Polymeric Nanocarriers in Oral Drug Delivery
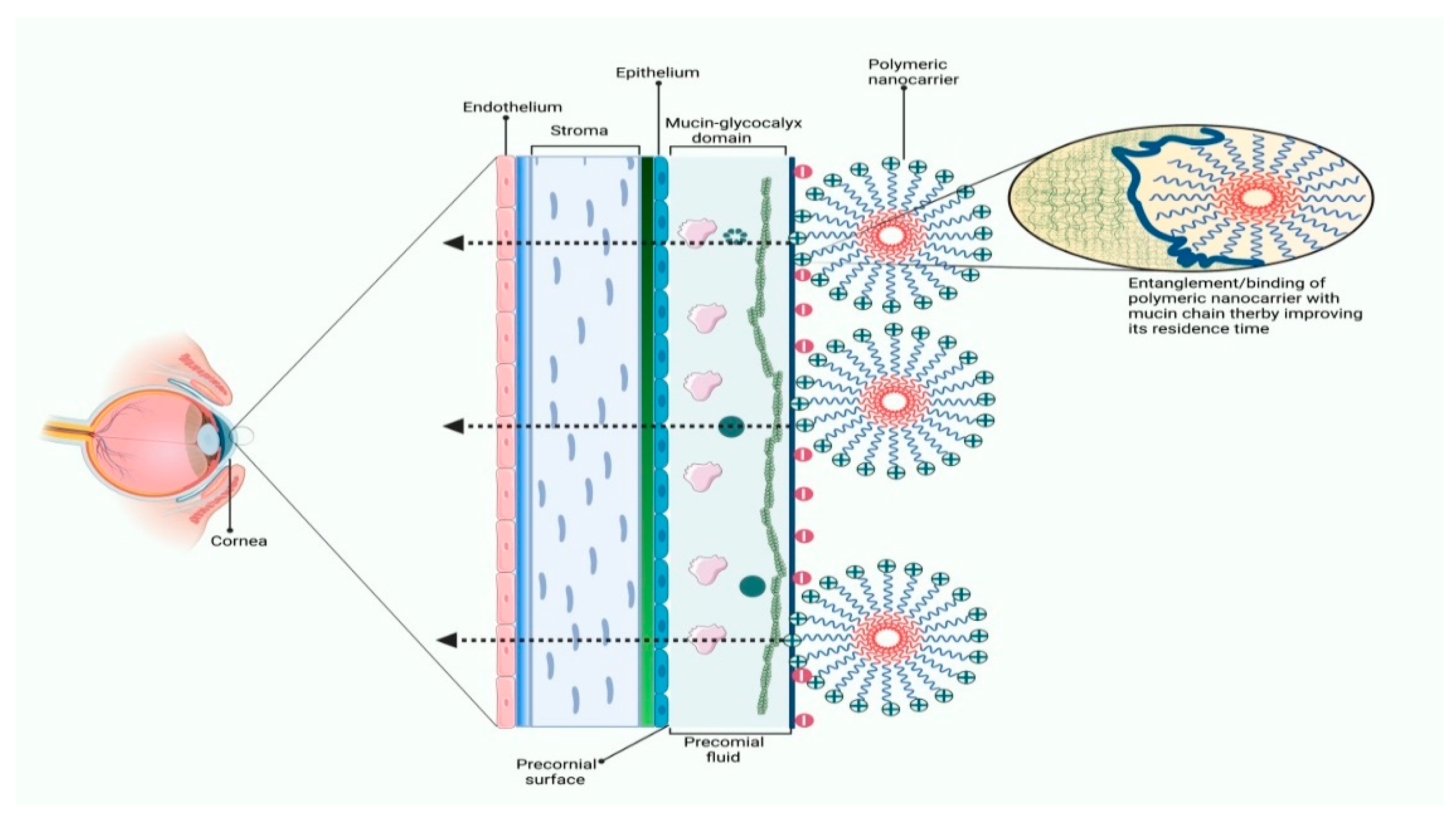
3.2. Applications of Polymeric Nanocarriers in Ocular Drug Delivery
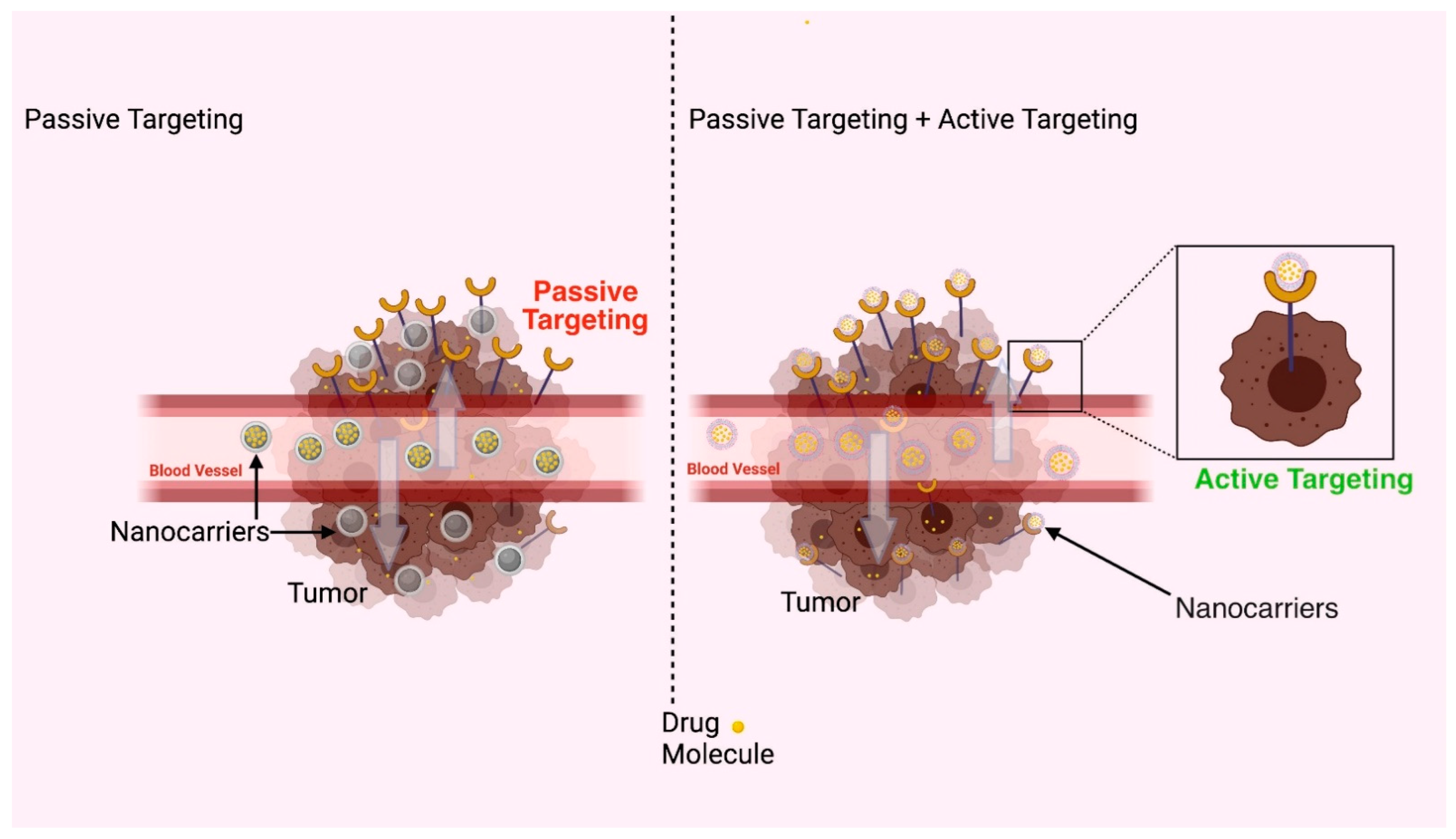
3.3. Applications of Polymeric Nanocarriers in Parenteral Drug Delivery
3.4. Applications of Polymeric Nanocarriers in Topical Drug Delivery
4. Practical and Regulatory Consideration
4.1. Toxicity Issues
4.2. Effects of the Route of Administration on Bioavailability
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DOX | Doxorubicin |
| NSM | Nanostructured materials |
| PCA | polycyanoacrylates |
| PCL | Polycaprolactone |
| PDLLA | Poly-DL-lactic acid |
| PEG | Polyethylene glycol |
| PGA | poly-glycolic acid |
| PLA | Polylactic acid |
| PLGA | Poly-lactic-co-glycolic acid |
| PVA | Polyvinyl alcohol |
| QDs | Quantum dots |
| USFDA | United States Food and Drug Administration. |
References
- Available online: https://www.brainkart.com/article/Historical-development-of-polymers_6358/ (accessed on 3 July 2022).
- Batool, S.A.; Ahmad, K.; Irfan, M.; Ur Rehman, M.A. Zn-Mn-doped mesoporous bioactive glass nanoparticle-loaded zein coatings for bioactive and antibacterial orthopedic implants. J. Funct Biomater. 2022, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Hyldbakk, A.; Mørch, Y.; Snipstad, S. Identification of novel cyanoacrylate monomers for use in nanoparticle drug delivery systems prepared by miniemulsion polymerisation—A multistep screening approach. Int. J. Pharm. X 2022, 4, 100124. [Google Scholar] [CrossRef] [PubMed]
- Weissig, V.; Pettinger, T.K.; Murdock, N. Nanopharmaceuticals (part 1): Products on the market. Int. J. Nanomed. 2014, 9, 4357–4373. [Google Scholar] [CrossRef] [PubMed]
- Suk, J.S.; Xu, Q.; Kim, N.; Hanes, J.; Ensign, L.M. PEGylation as a strategy for improving nanoparticle-based drug and gene delivery. Adv. Drug Deliv. Rev. 2016, 99, 28–51. [Google Scholar] [CrossRef]
- Ahmed, M.; Lukyanov, A.N.; Torchilin, V.; Tournier, H.; Schneider, A.N.; Goldberg, S.N. Combined radiofrequency ablation and adjuvant liposomal chemotherapy: Effect of chemotherapeutic agent, nanoparticle size, and circulation time. J. Vasc. Interv. Radiol. 2005, 16, 1365–1371. [Google Scholar] [CrossRef] [PubMed]
- Gabizon, A.; Shmeeda, H.; Barenholz, Y. Pharmacokinetics of pegylated liposomal Doxorubicin: Review of animal and human studies. Clin. Pharmacokinet. 2003, 42, 419–436. [Google Scholar] [CrossRef] [PubMed]
- Laginha, K.M.; Verwoert, S.; Charrois, G.J.; Allen, T.M. Determination of doxorubicin levels in whole tumor and tumor nuclei in murine breast cancer tumors. Clin. Cancer Res. 2005, 11, 6944–6949. [Google Scholar] [CrossRef]
- Aghemo, A.; Rumi, M.G.; Colombo, M. Pegylated interferons α2a and α2b in the treatment of chronic hepatitis C. Nature Rev. Gastroenterol. Hepatol. 2010, 7, 485–494. [Google Scholar] [CrossRef]
- Miele, E.; Spinelli, G.P.; Miele, E.; Tomao, F.; Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane® ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 2009, 4, 99–105. [Google Scholar] [CrossRef]
- Werner, M.E.; Cummings, N.D.; Sethi, M.; Wang, E.C.; Sukumar, R.; Moore, D.T.; Wang, A.Z. Preclinical evaluation of Genexol-PM, a nanoparticle formulation of paclitaxel, as a novel radiosensitizer for the treatment of non-small cell lung cancer. Int. J. Radiat. Oncol. Biol. Phys. 2013, 86, 463–468. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://clinicaltrials.gov/ct2/show/NCT00910741 (accessed on 18 January 2022).
- Hamaguchi, T.; Tsuji, A.; Yamaguchi, K.; Takeda, K.; Uetake, H.; Esaki, T.; Amagai, K.; Sakai, D.; Baba, H.; Kimura, M.; et al. A phase II study of NK012, a polymeric micelle formulation of SN-38, in unresectable, metastatic or recurrent colorectal cancer patients. Cancer Chemother. Pharmacol. 2018, 82, 1021–1029. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://clinicaltrials.gov/ct2/show/NCT01644890 (accessed on 18 January 2022).
- Sinha, R.; Kim, G.J.; Nie, S.; Shin, D.M. Nanotechnology in cancer therapeutics: Bioconjugated nanoparticles for drug delivery. Mol. Cancer Ther. 2006, 5, 1909–1917. [Google Scholar] [CrossRef] [PubMed]
- Blasco, C.; Pico, Y. Determining nanomaterials in food. Tends Anal. Chem. 2011, 30, 84–99. [Google Scholar] [CrossRef]
- Nikam, A.N.; More, M.P.; Pandey, A.P.; Patil, P.O.; Patil, A.G.; Deshmukh, P.K. Design and development of thiolated graphene oxide nanosheets for brain tumor targeting. Int. J. Polym. Mater. 2020, 69, 611–621. [Google Scholar] [CrossRef]
- Verma, R.; Kaushik, A.; Almeer, R.; Rahman, M.H.; Abdel-Daim, M.M.; Kaushik, D. Improved pharmacodynamic potential of rosuvastatin by self-nanoemulsifying drug delivery system: An in vitro and in vivo evaluation. Int. J. Nanomed. 2021, 16, 905–924. [Google Scholar] [CrossRef]
- Verma, R.; Kaushik, D. Design and optimization of candesartan loaded self-nanoemulsifying drug delivery system for improving its dissolution rate and pharmacodynamic potential. Drug Deliv. 2020, 27, 756–771. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Gunasekaran, T.; Haile, T.; Nigusse, T.; Dhanaraju, M.D. Nanotechnology: An effective tool for enhancing bioavailability and bioactivity of phytomedicine. Asian Pacific J. Trop. Biomed. 2014, 4, S1–S7. [Google Scholar] [CrossRef]
- De, R.; Mahata, M.K.; Kim, K.T. Structure-based varieties of polymeric nanocarriers and influences of their physicochemical properties on drug delivery profiles. Adv. Sci. 2022, 9, e2105373. [Google Scholar] [CrossRef]
- Simon, V.; Cavalu, S.; Simon, S.; Mocuta, H.; Vanea, E.; Prinz, M.; Neumann, M. Surface functionalisation of sol-gel derived aluminosilicates in simulated body fluids. Solid State Ionics 2009, 180, 764–769. [Google Scholar] [CrossRef]
- Nicolas, J.; Mura, S.; Brambilla, D.; Mackiewicz, N.; Couvreur, P. Design, functionalization strategies and biomedical applications of targeted biodegradable/biocompatible polymer-based nanocarriers for drug delivery. Chem. Soc. Rev. 2013, 42, 1147–1235. [Google Scholar] [CrossRef] [PubMed]
- De Jong, W.H.; Borm, P.J. Drug delivery and nanoparticles:applications and hazards. Int. J. Nanomed. 2008, 3, 133–149. [Google Scholar] [CrossRef] [PubMed]
- Peracchia, M.T.; Fattal, E.; Desmaele, D. Stealth PEGylated polycyanoacrylate nanoparticles for intravenous administration and splenic targeting. J. Control. Release 1999, 60, 121–128. [Google Scholar] [CrossRef]
- Rowe, R.C.; Sheskey, P.J.; Quinn, M.E. Handbook of Pharmaceutical Excipients, 6th ed.; Pharmaceutical Press: London, UK, 2009. [Google Scholar]
- Inactive Ingredient Search for Approved Drug Products, U.S. Food & Drug Administration. 2020. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm, (accessed on 6 March 2020).
- Bao, Y.; Maeki, M.; Ishida, A.; Tani, H.; Tokeshi, M. Preparation of size-tunable sub-200 nm PLGA-based nanoparticles with a wide size range using a microfluidic platform. PLoS ONE 2022, 17, e0271050. [Google Scholar] [CrossRef] [PubMed]
- Yaşar, M.; Yöntem, F.D.; Kahraman, M.V.; Apohan, N.K.; Aktaş, Z.; Öncül, M.O.; Akçakaya, H. Polymeric nanoparticles for selective protein recognition by using thiol-ene miniemulsion photopolymerization. J. Biomater. Sci. Polym. Ed. 2020, 31, 2044–2059. [Google Scholar] [CrossRef]
- Puri, R.; Berhe, S.A.; Akala, E.O. pH-Sensitive polymeric nanoparticles fabricated by dispersion polymerization for the delivery of bioactive agents. Pharm. Nanotechnol. 2017, 5, 44–66. [Google Scholar] [CrossRef]
- Popovici, C.; Popa, M.; Sunel, V.; Atanase, L.I.; Ichim, D.L. Drug delivery systems based on pluronic micelles with antimicrobial activity. Polymers 2022, 14, 3007. [Google Scholar] [CrossRef]
- Perumal, S.; Atchudan, R.; Lee, W. A review of polymeric micelles and their applications. Polymers 2022, 14, 2510. [Google Scholar] [CrossRef]
- Li, J.; Kataoka, K. Chemo-physical strategies to advance the in vivo functionality of targeted nanomedicine: The next generation. J. Am. Chem. Soc. 2021, 143, 538–559. [Google Scholar] [CrossRef]
- Varshosaz, J.; Taymouri, S.; Hassanzadeh, F.; Javanmard, S.H.; Rostami, M. Folated synperonic-cholesteryl hemisuccinate polymeric micelles for the targeted delivery of docetaxel in melanoma. Biomed. Res. Int. 2015, 2015, 746093. [Google Scholar] [CrossRef] [Green Version]
- Chida, T.; Miura, Y.; Cabral, H.; Nomoto, T.; Kataoka, K.; Nishiyama, N. Epirubicin-loaded polymeric micelles effectively treat axillary lymph nodes metastasis of breast cancer through selective accumulation and pH-triggered drug release. J. Control. Release 2018, 292, 130–140. [Google Scholar] [CrossRef] [PubMed]
- Bae, Y.; Fukushima, S.; Harada, A.; Kataoka, K. Design of environment-sensitive supramolecular assemblies for intracellular drug delivery: Polymeric micelles that are responsive to intracellular pH change. Angew. Chem. Int. Ed. Engl. 2003, 42, 4640–4643. [Google Scholar] [CrossRef] [PubMed]
- Leong, J.; Teo, J.Y.; Aakalu, V.K.; Yang, Y.Y.; Kong, H. Engineering polymersomes for diagnostics and therapy. Adv. Healthc. Mater. 2018, 7, e1701276. [Google Scholar] [CrossRef]
- Men, Y.; Li, W.; Tu, Y.; Peng, F.; Janssen, G.A.; Nolte, R.J.M.; Wilson, D.A. Nonequilibrium reshaping of polymersomes via polymer addition. ACS Nano. 2019, 13, 12767–12773. [Google Scholar] [CrossRef]
- Miere, F.; Fritea, L.; Cavalu, S.; Vicas, S.I. Formulation, characterization and advantages of using liposomes in multiple therapies. Pharmacophore 2020, 11, 1–12. Available online: https://pharmacophorejournal.com/WFzrTpR (accessed on 18 January 2022).
- Gao, C.; Qi, S.; Liu, K. Functional characterization of Brassica napus DNA topoisomerase Iα-1 and its effect on flowering time when expressed in Arabidopsis thaliana. Biochem. Biophys. Res. Commun. 2017, 486, 124–129. [Google Scholar] [CrossRef]
- Zavvar, T.; Babaei, M.; Abnous, K. Synthesis of multimodal polymersomes for targeted drug delivery and MR/fluorescence imaging in metastatic breast cancer model. Int. J. Pharm. 2020, 578, 119091. [Google Scholar] [CrossRef]
- Zhou, Y.; Chen, R.; Yang, H.; Bao, C.; Fan, J.; Wang, C.; Lin, Q.; Zhu, L. Light-responsive polymersomes with a charge-switch for targeted drug delivery. J. Mater. Chem. B. 2020, 8, 727–735. [Google Scholar] [CrossRef]
- Li, J.; Xiao, S.; Xu, Y.; Zuo, S.; Zha, Z.; Ke, W.; He, C.; Ge, Z. Smart asymmetric vesicles with triggered availability of inner cell-penetrating shells for specific intracellular drug delivery. ACS Appl Mater. Interfaces 2017, 9, 17727–17735. [Google Scholar] [CrossRef]
- Li, J.; Dirisala, A.; Ge, Z.; Wang, Y.; Yin, W.; Ke, W.; Toh, K.; Xie, J.; Matsumoto, Y.; Anraku, Y.; et al. vesicular nanoreactors with tumor-specific activation and self-destruction for synergistic tumor ablation. Angew. Chem. Int. Ed. Engl. 2017, 56, 14025–14030. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Wang, Y.; Ke, W.; Chen, W.; Wang, W.; Ge, Z. Polymer prodrug-based nanoreactors activated by tumor acidity for orchestrated oxidation/chemotherapy. Nano Lett. 2017, 17, 6983–6990. [Google Scholar] [CrossRef] [PubMed]
- Koide, A.; Kishimura, A.; Osada, K.; Jang, W.D.; Yamasaki, Y.; Kataoka, K. Semipermeable polymer vesicle (PICsome) self-assembled in aqueous medium from a pair of oppositely charged block copolymers: Physiologically stable micro-/nanocontainers of water-soluble macromolecules. J. Am. Chem. Soc. 2006, 128, 5988–5989. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Anraku, Y.; Kataoka, K. Self-boosting catalytic nanoreactors integrated with triggerable crosslinking membrane networks for initiation of immunogenic cell death by pyroptosis. Angew. Chem. Int. Ed. Engl. 2020, 59, 13526–13530. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol Eng. 2022, 16, 18. [Google Scholar] [CrossRef] [PubMed]
- Mittal, P.; Saharan, A.; Verma, R. Dendrimers: A new race of pharmaceutical nanocarriers. Biomed. Res. Int. 2021, 2021, 8844030. [Google Scholar] [CrossRef] [PubMed]
- Bober, Z.; Bartusik-Aebisher, D.; Aebisher, D. Application of dendrimers in anticancer diagnostics and therapy. Molecules 2022, 27, 3237. [Google Scholar] [CrossRef]
- Pillay, N.S.; Daniels, A.; Singh, M. Folate-targeted transgenic activity of dendrimer functionalized selenium nanoparticles in vitro. Int. J. Mol. Sci. 2020, 21, 7177. [Google Scholar] [CrossRef]
- Bartusik-Aebisher, D.; Chrzanowski, G.; Bober, Z.; Aebisher, D. An analytical study of Trastuzumab-dendrimer-fluorine drug delivery system in breast cancer therapy in vitro. Biomed. Pharmacother. 2021, 133, 111053. [Google Scholar] [CrossRef]
- Mota, P.; Pires, R.F.; Serpa, J.; Bonifácio, V.D.B. l-buthionine sulfoximine detection and quantification in polyurea dendrimer nanoformulations. Molecules 2019, 24, 3111. [Google Scholar] [CrossRef]
- Zhang, S.; Lloveras, V.; Lope-Piedrafita, S.; Calero-Pérez, P.; Wu, S.; Candiota, A.P.; Vidal-Gancedo, J. Metal-free radical dendrimers as MRI contrast agents for glioblastoma diagnosis: Ex vivo and in vivo approaches. Biomacromolecules 2022, 23, 2767–2777. [Google Scholar] [CrossRef]
- Knauer, N.; Arkhipova, V.; Li, G.; Hewera, M.; Pashkina, E.; Nguyen, P.H.; Meschaninova, M.; Kozlov, V.; Zhang, W.; Croner, R.S.; et al. In vitro validation of the therapeutic potential of dendrimer-based nanoformulations against tumor stem cells. Int. J. Mol. Sci. 2022, 23, 5691. [Google Scholar] [CrossRef] [PubMed]
- Chabria, Y.; Duffy, G.P.; Lowery, A.J.; Dwyer, R.M. Hydrogels: 3D drug delivery systems for nanoparticles and extracellular vesicles. Biomedicines 2021, 9, 1694. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.J.; Moon, Y.J.; Chun, H.J.; Yang, D.H. Doxorubicin·hydrochloride/cisplatin-loaded hydrogel/nanosized (2-hydroxypropyl)-beta-cyclodextrin local drug-delivery system for osteosarcoma treatment in vivo. Nanomaterials 2019, 9, 1652. [Google Scholar] [CrossRef] [PubMed]
- Szwajca, A.; Juszczyńska, S.; Jarzębski, M.; Baryła-Pankiewicz, E. Incorporation of fluorescent fluorinated methacrylate nano-sized particles into chitosan matrix formed as a membranes or beads. Polymers 2022, 14, 2750. [Google Scholar] [CrossRef]
- Liu, L.; Gao, Q.; Lu, X.; Zhou, H. In situ forming hydrogels based on chitosan for drug delivery and tissue regeneration. Asian J. Pharm. Sci. 2016, 11, 673–683. [Google Scholar] [CrossRef]
- Kim, J.; Yoon, M.; Jin, S.M.; Lee, J.; La, Y.; Lee, E.; Kim, K.T. Polymer cubosomes of block copolymers having cross-linkable soft hydrophobic blocks. Polym. Chem. 2019, 10, 3778–3785. [Google Scholar] [CrossRef]
- Sungmin, H.A.; Yunju, L.A.; Kyoung, T.K. Polymer cubosomes: Infinite cubic mazes and possibilities. Acc. Chem. Res. 2020, 53, 620–631. [Google Scholar] [CrossRef]
- Boge, L.; Bysell, H.; Ringstad, L. Lipid-based liquid crystals as carriers for antimicrobial peptides: Phase behavior and antimicrobial effect. Langmuir 2016, 32, 4217–4228. [Google Scholar] [CrossRef]
- Angelova, A.; Garamus, V.M.; Angelov, B.; Tian, Z.; Li, Y.; Zou, A. Advances in structural design of lipid-based nanoparticle carriers for delivery of macromolecular drugs, phytochemicals and anti-tumor agents. Adv. Colloid Interface Sci. 2017, 249, 331–345. [Google Scholar] [CrossRef]
- Zerkoune, L.; Lesieur, S.; Putaux, J.L. Mesoporous self-assembled nanoparticles of biotransesterified cyclodextrins and nonlamellar lipids as carriers of water-insoluble substances. Soft Matter. 2016, 12, 7539–7550. [Google Scholar] [CrossRef]
- Zou, A.; Li, Y.; Chen, Y. Self-assembled stable sponge-type nanocarries for Brucea javanica oil delivery. Colloids Surf. B Biointerfaces 2017, 153, 310–319. [Google Scholar] [CrossRef] [PubMed]
- Wakaskar, R.R. General overview of lipid-polymer hybrid nanoparticles, dendrimers, micelles, liposomes, spongosomes and cubosomes. J. Drug Target. 2018, 26, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Nasr, M.; Saber, S.; Bazeed, A.Y.; Ramadan, H.A.; Ebada, A.; Ciorba, A.L.; Cavalu, S.; Elagamy, H.I. Advantages of Cubosomal Formulation for Gatifloxacin Delivery in the Treatment of Bacterial Keratitis: In Vitro and In Vivo Approach Using Clinical Isolate of Methicillin-Resistant Staphylococcus aureus. Materials 2022, 15, 3374. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, J.; Tian, D.; Sun, L.; Wang, X.; Tian, M. Theranostic combinatorial drug-loaded coated cubosomes for enhanced targeting and efficacy against cancer cells. Cell Death Dis. 2020, 11, 1. [Google Scholar] [CrossRef]
- Boge, L.; Hallstensson, K.; Ringstad, L.; Johansson, J.; Andersson, T.; Davoudi, M.; Larsson, P.T.; Mahlapuu, M.; Håkansson, J.; Andersson, M. Cubosomes for topical delivery of the antimicrobial peptide LL-37. Eur. J. Pharm. Biopharm. 2019, 134, 60–67. [Google Scholar] [CrossRef]
- Ahmed, H.M.; Roy, A.; Wahab, M.; Ahmed, M.; Othman-Qadir, G.; Elesawy, B.H.; Khandaker, M.U.; Islam, M.N.; Emran, T.B. Applications of nanomaterials in agrifood and pharmaceutical industry. J. Nanomater. 2021, 2021, 1472096. [Google Scholar] [CrossRef]
- Brocchini, S.; Duncan, R. Pendent drugs release from polymers. In Encyclopedia of Controlled Drug Delivery, 1st ed.; John Wiley and Sons: New York, NY, USA, 1999; p. 561. [Google Scholar]
- Pillai, O.; Panchagnula, R. Polymers in drug delivery. Curr. Opin. Chem. Biol. 2001, 5, 447–451. [Google Scholar] [CrossRef]
- Maham, A.; Tang, Z.; Wu, H.; Wang, J.; Lin, Y. Protein-based nanomedicine platforms for drug delivery. Small 2009, 5, 1706–1721. [Google Scholar] [CrossRef]
- Cavalu, S.; Bisboaca, S.; Mates, I.M.; Pasca, P.M.; Laslo, V.; Costea, T.; Fritea, L.; Vicas, S. Novel Formulation Based on Chitosan-Arabic Gum Nanoparticles Entrapping Propolis Extract Production, physico-chemical and structural characterization. Rev. De Chim. 2018, 69, 3756–3760. [Google Scholar] [CrossRef]
- Avcu, E.; Bastan, F.E.; Guney, M.; Avcu, Y.Y.; Rehman, M.A.U.; Boccaccini, A.R. Biodegradable polymer matrix composites containing graphene-related materials for antibacterial applications: A critical review. Acta Biomater. 2022, in press. [CrossRef]
- Arif, Z.U.; Khalid, M.Y.; Noroozi, R.; Sadeghianmaryan, A.; Jalalvand, M.; Hossain, M. Recent advances in 3D-printed polylactide and polycaprolactone-based biomaterials for tissue engineering applications. Int. J. Biol. Macromol. 2022, 218, 930–968. [Google Scholar] [CrossRef] [PubMed]
- Kenry, L.; Liu, B. Recent advances in biodegradable conducting polymers and their biomedical applications. Biomacromolecules 2018, 19, 1783–1803. [Google Scholar] [CrossRef] [PubMed]
- Rai, P.; Mehrotra, S.; Priya, S.; Gnansounou, E.; Sharma, S.K. Recent advances in the sustainable design and applications of biodegradable polymers. Bioresour. Technol. 2021, 325, 124739. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Park, T.; Yoo, H. Biodegradable polymer composites for electrophysiological signal sensing. Polymers 2022, 14, 2875. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J.; Rajendran, R.R.; Mohanto, S.; Agarwal, U.; Panda, K.; Dhotre, K.; Manne, R.; Deepak, A.; Zafar, A.; Yasir, M.; et al. Poly(N-isopropylacrylamide)-based hydrogels for biomedical applications: A review of the state-of-the-art. Gels 2022, 8, 454. [Google Scholar] [CrossRef]
- Mashak, A.; Rahimi, A. Silicone polymers in controlled drug delivery systems: A review. Iran. Polymer J. 2009, 18, 279–295. [Google Scholar]
- Priya James, H.; John, R.; Alex, A.; Anoop, K.R. Smart polymers for the controlled delivery of drugs—A concise overview. Acta Pharm. Sin. B 2014, 4, 120–127. [Google Scholar] [CrossRef]
- Teodorescu, M.; Bercea, M. Poly (vinylpyrrolidone)—A versatile polymer for biomedical and beyond medical applications. Polym. Plast. Technol. Eng. 2015, 54, 923–943. [Google Scholar] [CrossRef]
- Kaushik, R.; Budhwar, V.; Kaushik, D. An Overview on recent patents and technologies on solid dispersion. Recent Pat. Drug Deliv. Formul. 2020, 14, 63–74. [Google Scholar] [CrossRef]
- Karthika, C.; Hari, B.; Mano, V.; Radhakrishnan, A.; Janani, S.K.; Akter, R.; Kaushik, D.; Rahman, M.H. Curcumin as a great contributor for the treatment and mitigation of colorectal cancer. Exp. Gerontol. 2021, 152, 111438. [Google Scholar] [CrossRef]
- Chouhan, N.; Mittal, V.; Kaushik, D.; Khatkar, A.; Raina, M. Self emulsifying drug delivery system (SEDDS) for phytoconstituents: A review. Curr. Drug Deliv. 2015, 12, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.; Yoo, H.; Lee, E.K. New Opportunities for organic semiconducting polymers in biomedical applications. Polymers 2022, 14, 2960. [Google Scholar] [CrossRef]
- Win, K.Y.; Feng, S.S. Effects of particle size and surface coating on cellular uptake of polymeric nanoparticles for oral delivery of anticancer drugs. Biomaterials 2005, 26, 2713–2722. [Google Scholar] [CrossRef]
- He, C.; Yin, L.; Tang, C.; Yin, C. Size-dependent absorption mechanism of polymeric nanoparticles for oral delivery of protein drugs. Biomaterials 2012, 33, 8569–8578. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, S.A.; Feng, S.S. Effects of particle size and surface modification on cellular uptake and biodistribution of polymeric nanoparticles for drug delivery. Pharm Res. 2013, 30, 2512–2522. [Google Scholar] [CrossRef]
- Pandey, R.; Ahmad, Z.; Sharma, S.; Khuller, G.K. Nano-encapsulation of azole antifungals: Potential applications to improve oral drug delivery. Int. J. Pharm. 2005, 301, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Ahlin, P.; Kristl, J.; Kristl, A.; Vrecer, F. Investigation of polymeric nanoparticles as carriers of enalaprilat for oral administration. Int. J. Pharm. 2002, 239, 113–120. [Google Scholar] [CrossRef]
- Pandey, R.; Zahoor, A.; Sharma, S.; Khuller, G.K. Nanoparticle encapsulated antitubercular drugs as a potential oral drug delivery system against murine tuberculosis. Tuberculosis 2003, 83, 373–378. [Google Scholar] [CrossRef]
- Roger, E.; Kalscheuer, S.; Kirtane, A.; Guru, B.R.; Grill, A.E.; Whittum-Hudson, J.; Panyam, J. Folic acid functionalized nanoparticles for enhanced oral drug delivery. Mol. Pharm. 2012, 9, 2103–2110. [Google Scholar] [CrossRef]
- Damgé, C.; Maincent, P.; Ubrich, N. Oral delivery of insulin associated to polymeric nanoparticles in diabetic rats. J. Contr. Release 2007, 117, 163–170. [Google Scholar] [CrossRef]
- Jain, A.K.; Swarnakar, N.K.; Das, M.; Godugu, C.; Singh, R.P.; Rao, P.R.; Jain, S. Augmented anticancer efficacy of doxorubicin-loaded polymeric nanoparticles after oral administration in a breast cancer induced animal model. Mol. Pharm. 2011, 8, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Jana, S.; Maji, N.; Nayak, A.K.; Sen, K.K.; Basu, S.K. Development of chitosan-based nanoparticles through inter-polymeric complexation for oral drug delivery. Carbohydr. Polym. 2013, 98, 870–876. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Yin, L.; Tang, C.; Yin, C. Multifunctional polymeric nanoparticles for oral delivery of TNF-α siRNA to macrophages. Biomaterials 2013, 34, 2843–2854. [Google Scholar] [CrossRef] [PubMed]
- Cavalu, S.; Antoniac, I.V.; Mohan, A.; Bodog, F.; Doicin, C.; Mates, I.; Ulmeanu, M.; Murzac, R.; Semenescu, A. Nanoparticles and Nanostructured Surface Fabrication for Innovative Cranial and Maxillofacial Surgery. Materials 2020, 13, 5391. [Google Scholar] [CrossRef] [PubMed]
- Cohen, H.; Levy, R.J.; Gao, J.; Fishbein, I.; Kousaev, V.; Sosnowski, S.; Slomkowski, S.; Golomb, G. Sustained delivery and expression of DNA encapsulated in polymeric nanoparticles. Gene Ther. 2000, 7, 1896–1905. [Google Scholar] [CrossRef]
- Jain, A.K.; Swarnakar, N.K.; Godugu, C.; Singh, R.P.; Jain, S. The effect of the oral administration of polymeric nanoparticles on the efficacy and toxicity of tamoxifen. Biomaterials 2011, 32, 503–515. [Google Scholar] [CrossRef]
- Malik, B.; Goyal, A.K.; Markandeywar, T.S.; Rath, G.; Zakir, F.; Vyas, S.P. Microfold-cell targeted surface engineered polymeric nanoparticles for oral immunization. J. Drug Target. 2012, 20, 76–84. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, X.; Zhou, Y.; Wang, X.; Fan, Y.; Huang, Y.; Liu, Y. Preparation and evaluation of poly(ethylene glycol)-poly(lactide) micelles as nanocarriers for oral delivery of cyclosporine A. Nanoscale Res. Lett. 2010, 5, 917–925. [Google Scholar] [CrossRef]
- Ghosh, R.; Mondal, S.; Mukherjee, D.; Adhikari, A.; Ahmed, S.A.; Alsantali, R.I. Oral drug delivery using a polymeric nanocarrier: Chitosan nanoparticles in the delivery of rifampicin. Mater. Adv. 2022, 3, 4622–4628. [Google Scholar] [CrossRef]
- Akib, A.A.; Rumon, M.H.; Moniruzzaman, M.; Chowdhury, A.N.; Roy, C.K. PLA-PEG Diblock Copolymer Micelle as Nanocarrier for Anti-Obesity Drug Delivery Dystem. SPAST. 2021 Abstracts, 1(01). Available online: https://spast.org/techrep/article/view/1901 (accessed on 11 March 2022).
- Chen, T.; Tu, L.; Wang, G.; Qi, N.; Wu, W.; Zhang, W.; Feng, J. Multi-functional chitosan polymeric micelles as oral paclitaxel delivery systems for enhanced bioavailability and anti-tumor efficacy. Int. J. Pharm. 2020, 578, 119105. [Google Scholar] [CrossRef]
- Zhu, C.; Gong, S.; Ding, J.; Yu, M.; Ahmad, E.; Feng, Y.; Gan, Y. Supersaturated polymeric micelles for oral silybin delivery: The role of the Soluplus-PVPVA complex. Acta Pharm. Sin. B 2019, 9, 107–117. [Google Scholar] [CrossRef] [PubMed]
- Salimi, A.; Makhmal, S.; Zadeh, B.; Kazemi, M. Preparation and optimization of polymeric micelles as an oral drug delivery system for deferoxamine mesylate: In vitro and ex vivo studies. Res. Pharm. Sci. 2019, 14, 293–307. [Google Scholar] [CrossRef] [PubMed]
- Woraphatphadung, T.; Sajomsang, W.; Rojanarata, T. Development of chitosan-based pH-sensitive polymeric micelles containing curcumin for colon-targeted drug delivery. AAPS Pharm. Sci. Tech. 2018, 19, 991–1000. [Google Scholar] [CrossRef] [PubMed]
- Motwani, S.K.; Chopra, S.; Talegaonkar, S.; Kohli, K.; Ahmad, F.J.; Khar, R.K. Chitosan-sodium alginate nanoparticles as submicroscopic reservoirs for ocular delivery: Formulation, optimisation and in vitro characterisation. Eur. J. Pharm. Biopharm. 2008, 68, 513–525. [Google Scholar] [CrossRef]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Nagarwal, R.C.; Kant, S.; Singh, P.N.; Maiti, P.; Pandit, J.K. Polymeric nanoparticulate system: A potential approach for ocular drug delivery. J. Contr Releas. 2009, 136, 2–13. [Google Scholar] [CrossRef]
- Sharma, U.K.; Verma, A.; Prajapati, S.K.; Pandey, H.; Pandey, A.C. In vitro, in vivo and pharmacokinetic assessment of amikacin sulphate laden polymeric nanoparticles meant for controlled ocular drug delivery. Appl. Nanosci. 2014, 5, 143–155. [Google Scholar] [CrossRef]
- Mahor, A.; Prajapati, S.K.; Verma, A.; Gupta, R.; Iyer, A.K.; Kesharwani, P. Moxifloxacin loaded gelatin nanoparticles for ocular delivery: Formulation and in vitro, in vivo evaluation. J. Colloid Interface Sci. 2016, 483, 132–138. [Google Scholar] [CrossRef]
- Mittal, N.; Kaur, G. Investigations on polymeric nanoparticles for ocular delivery. Adv. Polym. 2019, 2019, 1316249. [Google Scholar] [CrossRef]
- Duxfield, L.; Sultana, R.; Wang, R.; Englebretsen, V.; Deo, S.; Swift, S.; Rupenthal, I.; Al-Kassas, R. Development of gatifloxacin-loaded cationic polymeric nanoparticles for ocular drug delivery. Pharm. Dev. Technol. 2016, 21, 172–179. [Google Scholar] [CrossRef]
- Musumeci, T.; Bucolo, C.; Carbone, C.; Pignatello, R.; Drago, F.; Puglisi, G. Polymeric nanoparticles augment the ocular hypotensive effect of melatonin in rabbits. Int. J. Pharm. 2013, 440, 135–140. [Google Scholar] [CrossRef]
- Costa, J.R.; Silva, N.C.; Sarmento, B.; Pintado, M. Potential chitosan-coated alginate nanoparticles for ocular delivery of daptomycin. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1255–1262. [Google Scholar] [CrossRef] [PubMed]
- Aksungur, P.; Demirbilek, M.; Denkbaş, E.B.; Vandervoort, J.; Ludwig, A.; Unlü, N. Development and characterization of cyclosporine A loaded nanoparticles for ocular drug delivery: Cellular toxicity, uptake and kinetic studies. J. Control. Release 2011, 151, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Yoncheva, K.; Vandervoort, J.; Ludwig, A. Development of mucoadhesive poly(lactide-co-glycolide) nanoparticles for ocular application. Pharm. Dev. Technol. 2011, 16, 29–35. [Google Scholar] [CrossRef]
- Gadad, A.P.; Chandra, P.S.; Dandagi, P.M.; Mastiholimath, V.S. Moxifloxacin loaded polymeric nanoparticles for sustained ocular drug delivery. Int. J. Pharm. Sci. Nanotechnol. 2012, 5, 1727–1734. [Google Scholar] [CrossRef]
- Singh, K.H.; Shinde, U.A. Development and evaluation of novel polymeric nanoparticles of brimonidine tartrate. Curr. Drug Deliv. 2010, 7, 244–251. [Google Scholar] [CrossRef]
- Jain, K.; Kumar, R.S.; Sood, S.; Dhyanandhan, G. Betaxolol hydrochloride loaded chitosan nanoparticles for ocular delivery and their anti-glaucoma efficacy. Curr. Drug Deliv. 2013, 10, 493–499. [Google Scholar] [CrossRef]
- Başaran, E.; Demirel, M.; Sirmagül, B.; Yazan, Y. Polymeric cyclosporine-A nanoparticles for ocular application. J. Biomed. Nanotechnol. 2011, 7, 714–723. [Google Scholar] [CrossRef]
- Nasr, F.H.; Khoee, S.; Dehghan, M.M.; Chaleshtori, S.S.; Shafiee, A. Preparation and evaluation of contact lenses embedded with polycaprolactone-based nanoparticles for ocular drug delivery. Biomacromolecules 2016, 17, 485–495. [Google Scholar] [CrossRef]
- Shmueli, R.B.; Sunshine, J.C.; Xu, Z.; Duh, E.J.; Green, J.J. Gene delivery nanoparticles specific for human microvasculature and macrovasculature. Nanomedicine 2012, 8, 1200–1207. [Google Scholar] [CrossRef]
- Sah, A.K.; Suresh, P.K.; Verma, V.K. PLGA nanoparticles for ocular delivery of loteprednol etabonate: A corneal penetration study. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1156–1164. [Google Scholar] [CrossRef] [PubMed]
- Basaran, E.; Senel, B.; Kirimlioglu, G.Y.; Guven, U.M.; Yazan, Y. Ornidazole incorporated chitosan nanoparticles for ocular application. Latin Am. J. Pharm. 2015, 34, 1180–1188. [Google Scholar]
- Zhang, P.; Liu, X.; Hu, W.; Bai, Y.; Zhang, L. Preparation and evaluation of naringenin-loaded sulfobutylether-β-cyclodextrin/chitosan nanoparticles for ocular drug delivery. Carbohydr. Polym. 2016, 149, 224–230. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Xu, X.; Chen, H.; Li, X. Ocular biocompatibility and tolerance study of biodegradable polymeric micelles in the rabbit eye. Colloids Surf. B Biointerfaces 2013, 112, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Yousry, C.; Elkheshen, S.A.; El-Laithy, H.M.; Essam, T.; Fahmy, R.H. Studying the influence of formulation and process variables on vancomycin-loaded polymeric nanoparticles as potential carrier for enhanced ophthalmic delivery. Eur. J. Pharm. Sci. 2017, 100, 142–154. [Google Scholar] [CrossRef]
- Gupta, H.; Aqil, M.; Khar, R.K.; Ali, A.; Bhatnagar, A.; Mittal, G. Sparfloxacin-loaded PLGA nanoparticles for sustained ocular drug delivery. Nanomedicine 2010, 6, 324–333. [Google Scholar] [CrossRef]
- Salama, A.H.; Shamma, R.N. Tri/tetra-block co-polymeric nanocarriers as a potential ocular delivery system of lornoxicam: In vitro characterization.; and in vivo estimation of corneal permeation. Int. J. Pharm. 2015, 492, 28–39. [Google Scholar] [CrossRef]
- Pignatello, R.; Ricupero, N.; Bucolo, C.; Maugeri, F.; Maltese, A.; Puglisi, G. Preparation and characterization of Eudragit retard nanosuspensions for the ocular delivery of cloricromene. AAPS Pharm. Sci. Tech. 2006, 7, 192–198. [Google Scholar] [CrossRef]
- Vega, E.; Gamisans, F.; García, M.L.; Chauvet, A.; Lacoulonche, F.; Egea, M.A. PLGA nanospheres for the ocular delivery of flurbiprofen: Drug release and interactions. J. Pharm. Sci. 2008, 97, 5306–5317. [Google Scholar] [CrossRef]
- Katara, R.; Majumdar, D.K. Eudragit RL 100-based nanoparticulate system of aceclofenac for ocular delivery. Colloids Surf. B Biointerfaces 2013, 103, 455–462. [Google Scholar] [CrossRef]
- Salama, A.H.; Mahmoud, A.A.; Kamel, R. A novel method for preparing surface-modified fluocinolone acetonide loaded PLGA nanoparticles for ocular use: In vitro and in vivo evaluations. AAPS Pharm. Sci. Tech. 2016, 17, 1159–1172. [Google Scholar] [CrossRef] [PubMed]
- Khin, S.Y.; Soe, H.M.S.H.; Chansriniyom, C.; Pornputtapong, N.; Asasutjarit, R.; Loftsson, T.; Jansook, P. Development of fenofibrate/randomly methylated β-cyclodextrin-loaded Eudragit® RL 100 nanoparticles for ocular delivery. Molecules 2022, 27, 4755. [Google Scholar] [CrossRef]
- Jacinto, T.A.; Oliveira, B.; Miguel, S.P.; Ribeiro, M.P.; Coutinho, P. Ciprofloxacin-loaded zein/hyaluronic acid nanoparticles for ocular mucosa delivery. Pharmaceutics 2022, 14, 1557. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, H.B.; Attia Shafie, M.A.; Mekkawy, A.I. Chitosan nanoparticles for meloxicam ocular delivery: Development, in vitro characterization, and in vivo evaluation in a rabbit eye model. Pharmaceutics 2022, 14, 893. [Google Scholar] [CrossRef] [PubMed]
- Prosperi-Porta, G.; Kedzior, S.; Muirhead, B.; Sheardown, H. Phenylboronic-acid-based polymeric micelles for mucoadhesive anterior segment ocular drug delivery. Biomacromolecules 2016, 17, 1449–1457. [Google Scholar] [CrossRef]
- Pignatello, R.; Corsaro, R.; Bonaccorso, A.; Zingale, E.; Carbone, C.; Musumeci, T. Soluplus® polymeric nanomicelles improve solubility of BCS-class II drugs. Drug Deliv. Transl. Res. 2022, 12, 1991–2006. [Google Scholar] [CrossRef]
- Binkhathlan, Z.; Alomrani, A.H.; Hoxha, O.; Ali, R.; Kalam, M.A.; Alshamsan, A. Development and characterization of PEGylated fatty acid-block-poly(ε-caprolactone) novel block copolymers and their self-assembled nanostructures for ocular delivery of cyclosporine A. Polymers 2022, 14, 1635. [Google Scholar] [CrossRef]
- Safwat, M.A.; Mansour, H.F.; Hussein, A.K.; Abdelwahab, S.; Soliman, G.M. Polymeric micelles for the ocular delivery of triamcinolone acetonide: Preparation and in vivo evaluation in a rabbit ocular inflammatory model. Drug Deliv. 2020, 27, 1115–1124. [Google Scholar] [CrossRef]
- Varela-Fernández, R.; García-Otero, X.; Díaz-Tomé, V.; Regueiro, U.; López-López, M.; González-Barcia, M.; Isabel Lema, M.; Otero-Espinar, F.J. Mucoadhesive PLGA nanospheres and nanocapsules for lactoferrin controlled ocular delivery. Pharmaceutics 2022, 14, 799. [Google Scholar] [CrossRef]
- Silva-Abreu, M.; Calpena, A.C.; Espina, M. Optimization, biopharmaceutical profile and therapeutic efficacy of pioglitazone-loaded PLGA-PEG nanospheres as a novel strategy for ocular inflammatory disorders. Pharm Res. 2018, 35, 11. [Google Scholar] [CrossRef]
- Muthu, M.S. Nanoparticles based on PLGA and its co-polymer: An overview. Asian J. Pharm. Sci. 2009, 3, 266–273. [Google Scholar] [CrossRef]
- Ulbrich, K.; Holá, K.; Šubr, V.; Bakandritsos, A.; Tuček, J.; Zbořil, R. Targeted drug delivery with polymers and magnetic nanoparticles: Covalent and noncovalent approaches, release control, and clinical studies. Chem. Rev. 2016, 116, 5338–5431. Available online: https://pubs.acs.org/doi/full/10.1021/acs.chemrev.5b00589 (accessed on 4 March 2022). [CrossRef] [PubMed]
- Tosi, G.; Bortot, B.; Ruozi, B.; Dolcetta, D.; Vandelli, M.A.; Forni, F.; Severini, G.M. Potential use of polymeric nanoparticles for drug delivery across the blood-brain barrier. Curr. Med. Chem. 2013, 20, 2212–2225. [Google Scholar] [CrossRef]
- Muthu, M.S.; Singh, S. Studies on biodegradable polymeric nanoparticles of risperidone: In vitro and in vivo evaluation. Nanomedicine 2008, 3, 305–319. [Google Scholar] [CrossRef]
- Muthu, M.S.; Rawat, M.K.; Mishra, A.; Singh, S. PLGA nanoparticle formulations of risperidone: Preparation and neuropharmacological evaluation. Nanomedicine 2009, 5, 323–333. [Google Scholar] [CrossRef] [PubMed]
- Bisht, S.; Mizuma, M.; Feldmann, G.; Ottenhof, N.A.; Hong, S.M.; Pramanik, D.; Chenna, V.; Karikari, C.; Sharma, R.; Goggins, M.G.; et al. Systemic administration of polymeric nanoparticle-encapsulated curcumin (NanoCurc) blocks tumor growth and metastases in preclinical models of pancreatic cancer. Mol. Cancer Ther. 2010, 9, 2255–2264. [Google Scholar] [CrossRef] [PubMed]
- Nanjwade, B.K.; Singh, J.; Parikh, K.A.; Manvi, F.V. Preparation and evaluation of carboplatin biodegradable polymeric nanoparticles. Int. J. Pharm. 2010, 385, 176–180. [Google Scholar] [CrossRef]
- Song, L.; Shen, Y.; Hou, J.; Lei, L.; Guo, S.; Qian, C. Polymeric micelles for parenteral delivery of curcumin: Preparation.; characterization and in vitro evaluation. Colloids Surf. 2011, 390, 25–32. [Google Scholar] [CrossRef]
- Richter, A.; Olbrich, C.; Krause, M.; Hoffmann, J.; Kissel, T. Polymeric micelles for parenteral delivery of sagopilone: Physicochemical characterization, novel formulation approaches and their toxicity assessment in vitro as well as in vivo. Eur. J. Pharm. Biopharm. 2010, 75, 80–89. [Google Scholar] [CrossRef]
- Haggag, Y.A.; Abosalha, A.K.; Tambuwala, M.M.; Silva, A.M.; Gimeno, A.; Egea, M.A.; García, M.L. Polymeric nanoencapsulation of zaleplon into PLGA nanoparticles for enhanced pharmacokinetics and pharmacological activity. Biopharm. Drug Dispos. 2021, 42, 12–23. [Google Scholar] [CrossRef]
- Ford, C.A.; Spoonmore, T.J.; Gupta, M.K.; Duvall, C.L.; Guelcher, S.A.; Cassat, J.E. Diflunisal-loaded poly(propylene sulfide) nanoparticles decrease S. aureus-mediated bone destruction during osteomyelitis. J. Orthop. Res. 2021, 39, 426–437. [Google Scholar] [CrossRef] [PubMed]
- Elmowafy, M.; Alruwaili, N.K.; Shalaby, K.; Alharbi, K.S.; Altowayan, W.M.; Ahmad, N.; Zafar, A.; Elkomy, M. Long-acting paliperidone parenteral formulations based on polycaprolactone nanoparticles; the influence of stabilizer and chitosan on in vitro release, protein adsorption, and cytotoxicity. Pharmaceutics 2020, 12, 160. [Google Scholar] [CrossRef] [PubMed]
- Sang, M.M.; Liu, F.L.; Wang, Y.; Luo, R.J.; Huan, X.X.; Han, L.F.; Zhang, Z.T.; Feng, F.; Qu, W.; Liu, W.; et al. A novel redox/pH dual-responsive and hyaluronic acid-decorated multifunctional magnetic complex micelle for targeted gambogic acid delivery for the treatment of triple negative breast cancer. Drug Deliv. 2018, 25, 1846–1857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Sau, S.; Alsaab, H.O.; Iyer, A.K. CD44 directed nanomicellar payload delivery platform for selective anticancer effect and tumor specific imaging of triple negative breast cancer. Nanomedicine 2018, 14, 1441–1454. [Google Scholar] [CrossRef] [PubMed]
- Hiroshi, M. Macromolecular therapeutics in cancer treatment: The EPR effect and beyond. J. Control. Release 2012, 164, 138–144. [Google Scholar] [CrossRef]
- Akhtar, N.; Verma, A.; Pathak, K. Topical delivery of drugs for the effective treatment of fungal infections of skin. Curr. Pharm. Des. 2015, 21, 2892–2913. [Google Scholar] [CrossRef]
- Akhtar, N.; Verma, A.; Pathak, K. Exploring preclinical and clinical effectiveness of nanoformulations in the treatment of atopic dermatitis: Safety aspects and patent reviews. Bull. Fac. Pharm. Cairo Univ. 2017, 55, 3. [Google Scholar] [CrossRef]
- Kim, D.G.; Jeong, Y.I.; Choi, C.; Roh, S.H.; Kang, S.K.; Jang, M.K.; Nah, J.W. Retinol-encapsulated low molecular water-soluble chitosan nanoparticles. Int. J. Pharm. 2006, 319, 130–138. [Google Scholar] [CrossRef]
- Elmowafy, M.; Samy, A.; Raslan, M.A.; Salama, A.; Said, R.A.; Abdelaziz, A.E.; El-Eraky, W.; El Awdan, S.; Viitala, T. Enhancement of bioavailability and pharmacodynamic effects of thymoquinone via nanostructured lipid carrier (NLC) formulation. AAPS Pharm. Sci. Tech. 2016, 17, 663–672. [Google Scholar] [CrossRef]
- Chen, Y.S.; Alany, R.G.; Young, S.A.; Green, C.R.; Rupenthal, I.D. In vitro release characteristics and cellular uptake of poly(D.;L-lactic-co-glycolic acid) nanoparticles for topical delivery of antisense oligodeoxynucleotides. Drug Deliv. 2011, 18, 493–501. [Google Scholar] [CrossRef]
- Youngren, S.R.; Tekade, R.K.; Gustilo, B.; Hoffmann, P.R.; Chougule, M.B. STAT6 siRNA matrix-loaded gelatin nanocarriers: Formulation, characterization, and ex vivo proof of concept using adenocarcinoma cells. Biomed. Res. Int. 2013, 2013, 858946. [Google Scholar] [CrossRef] [PubMed]
- Balzus, B.; Sahle, F.F.; Hönzke, S.; Gerecke, C.; Schumacher, F.; Hedtrich, S.; Kleuser, B.; Bodmeier, R. Formulation and ex vivo evaluation of polymeric nanoparticles for controlled delivery of corticosteroids to the skin and the corneal epithelium. Eur. J. Pharm. Biopharm. 2017, 115, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Saadallah, M.A.; Hamid, O. Formulation and evaluation of rosuvastatin calcium polymeric nanoparticles-loaded transdermal patch. Iraqi J. Pharm. 2022, 18, 22–38. [Google Scholar] [CrossRef]
- Albanese, A.; Tang, P.S.; Chan, W.C. The effect of nanoparticle size, shape, and surface chemistry on biological systems. Annu. Rev. Biomed. Eng. 2012, 14, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Kamaly, N.; Xiao, Z.; Valencia, P.M.; Radovic-Moreno, A.F.; Farokhzad, O.C. Targeted polymeric therapeutic nanoparticles: Design.; development and clinical translation. Chem. Soc. Rev. 2012, 41, 2971–3010. [Google Scholar] [CrossRef]
- Etheridge, M.L.; Campbell, S.A.; Erdman, A.G.; Haynes, C.L.; Wolf, S.M.; McCullough, J. The big picture on nanomedicine: The state of investigational and approved nanomedicine products. Nanomedicine 2013, 9, 1–14. [Google Scholar] [CrossRef]
- Cavalu, S.; Ratiu, C.; Ponta, O.; Simon, V.; Rugina, D.; Miclaus, V.; Akin, I.; Goller, G. Improving osseointegration of alumina/zirconia ceramic implants by fluoride surface treatment. Dig. J. Nanomater. Biostructures 2014, 9, 797–808. [Google Scholar]
- Bobo, D.; Robinson, K.J.; Islam, J.; Thurecht, K.J.; Corrie, S.R. Nanoparticle-based medicines: A review of FDA-approved materials and clinical trials to date. Pharm. Res. 2016, 33, 2373–2387. [Google Scholar] [CrossRef]
- Duncan, R. Polymer therapeutics: Top 10 selling pharmaceuticals—What next? J. Control. Release 2014, 190, 371–380. [Google Scholar] [CrossRef]
- Lang, L. FDA approves Cimzia to treat Crohn’s disease. Gastroenterology 2008, 134, 1819. [Google Scholar] [CrossRef]
- Krystexxa Pegloticase. 2020. Available online: https://www.krystexxa.com/ (accessed on 6 March 2020).
- Kolb-Mäurer, A.; Sunderkötter, C.; Kukowski, B.; Meuth, S.G. An update on peginterferon beta-1a management in multiple sclerosis: Results from an interdisciplinary board of German and Austrian neurologists and dermatologists. BMC Neurol. 2019, 19, 130. [Google Scholar] [CrossRef] [PubMed]
- Prime, B.I. CHMP Recommends EU Marketing Authorization for ADYNOVI® [Antihemophilic Factor (Recombinant), PEGylated] for Adults and Adolescents with Hemophilia, A. 2020. Available online: https://www.globenewswire.com/news-release/2017/11/13/1184850/0/en/CHMP-recommends-EU-marketing-authorization-for-ADYNOVI-Antihemophilic-Factor-Recombinant-PEGylated-for-adults-and-adolescents-with-Hemophilia-A.html (accessed on 8 July 2020).
- Piedmonte, D.M.; Treuheit, M.J. Formulation of Neulasta. Adv. Drug Deliv. Rev. 2008, 60, 50–58. [Google Scholar] [CrossRef] [PubMed]
- Rajender Reddy, K.; Modi, M.W.; Pedder, S. Use of peginterferon alfa-2a (40 KD) (Pegasys) for the treatment of hepatitis C. Adv. Drug Deliv. Rev. 2002, 54, 571–586. [Google Scholar] [CrossRef]
- Ge, Y.; Grossman, R.I.; Udupa, J.K.; Fulton, J.; Constantinescu, C.S.; Gonzales–Scarano, F.; Babb, J.S.; Mannon, L.J.; Kolson, D.L.; Cohen, J.A. Glatiramer acetate (Copaxone) treatment in relapsing-remitting MS: Quantitative MR assessment. Neurology 2000, 54, 813–817. [Google Scholar] [CrossRef]
- Oerlemans, C.; Bult, W.; Bos, M.; Storm, G.; Nijsen, J.F.; Hennink, W.E. Polymeric micelles in anticancer therapy: Targeting, imaging and triggered release. Pharm. Res. 2010, 27, 2569–2589. [Google Scholar] [CrossRef]
- Chiechi, L.M. Estrasorb. I Drugs 2004, 7, 860–864. [Google Scholar]
- U.S. National Library of Medicine. 2020. Available online: https://clinicaltrials.gov/ (accessed on 8 July 2020).
- Unites States Patent and Trademark Office. 2020. Available online: https://www.uspto.gov/ (accessed on 8 July 2020).
- Sharma, N.; Madan, P.; Lin, S. Effect of process and formulation variables on the preparation of parenteral paclitaxel-loaded biodegradable polymeric nanoparticles: A co-surfactant study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef]
- Ahlawat, J.; Henriquez, G.; Narayan, M. Enhancing the delivery of chemotherapeutics: Role of biodegradable polymeric nanoparticles. Molecules 2018, 23, 2157. [Google Scholar] [CrossRef]
- Caster, J.M.; Stephanie, K.Y.; Patel, A.N.; Newman, N.J.; Lee, Z.J.; Warner, S.B.; Wagner, K.T.; Roche, K.C.; Tian, X.; Min, Y.; et al. Effect of particle size on the biodistribution, toxicity, and efficacy of drug-loaded polymeric nanoparticles in chemoradiotherapy. Nanomedicine 2017, 13, 1673–1683. [Google Scholar] [CrossRef]
- Singh, R.P.; Ramarao, P. Accumulated polymer degradation products as effector molecules in cytotoxicity of polymeric nanoparticles. Toxicol. Sci. 2013, 136, 131–143. [Google Scholar] [CrossRef]
- Grabowski, N.; Hillaireau, H.; Vergnaud, J.; Tsapis, N.; Pallardy, M.; Kerdine-Römer, S.; Fattal, E. Surface coating mediates the toxicity of polymeric nanoparticles towards human-like macrophages. Int. J. Pharm. 2015, 482, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Rejinold, N.S.; Muthunarayanan, M.; Divyarani, V.V.; Sreerekha, P.R.; Chennazhi, K.P.; Nair, S.V.; Tamura, H.; Jayakumar, R. Curcumin-loaded biocompatible thermoresponsive polymeric nanoparticles for cancer drug delivery. J. Colloid Interface Sci. 2011, 360, 39–51. [Google Scholar] [CrossRef] [PubMed]
- Dandekar, P.; Dhumal, R.; Jain, R.; Tiwari, D.; Vanage, G.; Patravale, V. Toxicological evaluation of pH-sensitive nanoparticles of curcumin: Acute.; sub-acute and genotoxicity studies. Food Chem. Toxicol. 2010, 48, 2073–2089. [Google Scholar] [CrossRef] [PubMed]
- Karabasz, A.; Bzowska, M.; Szczepanowicz, K. Biomedical applications of multifunctional polymeric nanocarriers: A review of current literature. Int. J. Nanomed. 2020, 15, 8673–8696. [Google Scholar] [CrossRef]
- Homayun, B.; Lin, X.; Choi, H.J. Challenges and recent progress in oral drug delivery systems for Biopharmaceuticals. Pharmaceutics 2019, 11, 129. [Google Scholar] [CrossRef] [Green Version]
- Nabi, B.; Rehman, S.; Baboota, S.; Ali, J. Insights on oral drug delivery of lipid nanocarriers: A win-win solution for augmenting bioavailability of antiretroviral drugs. AAPS Pharm. Sci. Tech. 2019, 20, 60. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Mangla, B.; Javed, S.; Sultan, M.H.; Ahsan, W.; Aggarwal, G.; Kohli, K. Nanocarriers-assisted needle-free vaccine delivery through oral and intranasal transmucosal routes: A novel therapeutic conduit. Front. Pharmacol. 2022, 12, 757761. [Google Scholar] [CrossRef]
- Tsai, C.H.; Wang, P.Y.; Lin, I.C.; Huang, H.; Liu, G.S.; Tseng, C.L. Ocular drug delivery: Role of degradable polymeric nanocarriers for ophthalmic application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef]
- Grund, S.; Bauer, M.; Fischer, D. Polymers in drug delivery-state of the art and future trends. Adv. Eng. Mater. 2011, 13, 61–87. [Google Scholar] [CrossRef]
- Bennet, D.; Kim, S. Polymer nanoparticles for smart drug delivery. In Application of Nanotechnology in Drug Delivery; Sezer, A.D., Ed.; Intech: Rijeka, Croatia, 2014. [Google Scholar] [CrossRef]
- Luk, B.T.; Zhang, L. Current advances in polymer-based nanotheranostics for cancer treatment and diagnosis. ACS Appl. Mater. Interfaces 2014, 6, 21859–21873. [Google Scholar] [CrossRef] [PubMed]
- Vaut, L.; Juszczyk, J.J.; Kamguyan, K.; Jensen, K.E.; Tosello, G.; Boisen, A. 3D printing of reservoir devices for oral drug delivery: From concept to functionality through design improvement for enhanced mucoadhesion. ACS Biomater. Sci. Eng. 2020, 6, 2478–2486. [Google Scholar] [CrossRef] [PubMed]
- Vauthier, C.; Bouchemal, K. Processing and scale-up of polymeric nanoparticles. In Intracellular Delivery; Springer: Berlin/Heidelberg, Germany, 2011; pp. 433–456. [Google Scholar]
- Liechty, W.B.; Kryscio, D.R.; Slaughter, B.V.; Peppas, N.A. Polymers for drug delivery systems. Annu. Rev. Chem. Biomol. Eng. 2010, 1, 149–173. [Google Scholar] [CrossRef]
- Narang, A.S.; Chang, R.K.; Hussain, M.A. Pharmaceutical development and regulatory considerations for nanoparticles and nanoparticulate drug delivery systems. J. Pharm. Sci. 2013, 102, 3867–3882. [Google Scholar] [CrossRef] [PubMed]




| Classification | Name of Polymers | Applications of Polymer | Ref. |
|---|---|---|---|
| Natural polymers | |||
| Protein based | Soy, whey, collagen, gelatin | Gene delivery, nanoparticles | [74] |
| Polysaccharides | Alginate, pectin, guar gum, chitosan, chondroitin, heparin, hyaluronic acid, cyclodextrins | Binding and film coating agents in tablets, mucoadhesive, controlled release of drugs | [75] |
| Synthetic polymers | |||
| Biodegradable | |||
| Polyamides | Polyamino acids, poly(iminocarbonates) and their copolymers | Sutures, catheters for angioplasty, controlled and sustained drug delivery | [76] |
| Cellulose | Carboxy methylcellulose, ethyl cellulose, cellulose acetate, HPMC | Binder, coating, emulsifying, disintegrants in tablets and capsules | [77] |
| Polyanhydrides | Poly(adipic) acid, poly(sebacic) acid, and their copolymers | Controlled release coatings, medical implants | [78] |
| Polyesters | Poly (lactic acid), poly (glycolic acid), poly(dioxanes) and their copolymers | Protein delivery, dialysis membrane | [79] |
| Others | Polyurethane, polyortho esters, poly(cyano) acrylates, polystyrenes | Sutures, stents, drug delivery devices, dialysis media | |
| Non-biodegradable | |||
| Silicones | Colloidal silica, polydimethylsiloxane | Therapeutic devices, implants, medical grade adhesive for transdermal delivery | [82] |
| Acrylic polymers | Polymethacrylates, polyhydroxy (ethyl acrylates) | Thermo-gelling acrylamide derivatives, its balance of hydrogen bonding, and hydrophobic association changes with temperature (smart polymers), film-forming agent | [83] |
| Others | PVP, poloxamers, ethyl vinyl acetate | Tablet granulation, plasma replacement | [84] |
| Polymer | Delivery System | Therapeutic Molecule | Summary | Ref. |
|---|---|---|---|---|
| PLGA | NPs | Coumarin-6 | Surface modification of PLGA nanoparticles with vitamin E TPGS notably improved the cellular uptake to promote oral chemotherapy. | [89] |
| Carboxylated chitosan | NPs | Bovine serum albumin | Chitosan grafted nanoparticles showed increased intestinal absorption due to higher mucoadhesion, cellular uptake, and systemic biodistribution after oral administration. | [90] |
| Polystyrene | NPs | NA | Vitamin E TPGS modified polystyrene nanoparticles evidenced increased cellular uptake by Caco-2 and MDCK cells employed as an in vitro model for gastro-intestinal and blood-brain barrier respectively. In vivo investigation displayed that vitamin E TPGS coated nanoparticles (<200 nm) can escape RES and thus enhance half-life. | [91] |
| PLGA and alginate stabilized chitosan | NPs | Clotrimazole and econazole | Oral administration of polymeric nanoparticle encapsulated drugs showed controlled drug delivery for 5–6 days compared to unencapsulated drugs which were cleared within 3–4 h following oral or IV administration. Bioavailability of drugs was remarkably improved and was detected in the lung, liver, and spleen tissues till 6–8 days compared to free drugs which were cleared by 12 h. | [92] |
| PLGA and polymethylmethacrylate | NPs | Enalaprilat | The in vitro study across rat jejunum showed that the apparent permeability coefficient of enalaprilat-loaded PLGA nanoparticles was not significantly improved compared to the enalaprilat solution. | [93] |
| PLG | NPs | Rifampicin, isoniazid and pyrazinamide | Oral administration of drug-loaded nanoparticles in mice showed prolonged blood circulation for up to 6 days for rifampicin and up to 9 days for isoniazid and pyrazinamide. The therapeutic concentration of the drug in the tissues was maintained for 9–11 days. | [94] |
| N-isopropyl acrylamide, methylmethacrylate and acrylic acid in 60:20:20 | NPs | Rapamycin | Significant blood levels of rapamycin were observed within 30 min after oral administration of rapamycin-loaded polymeric nanoparticles and continue to be detected in bloodstream up to 24 h. | [88] |
| PLGA | NPs | Paclitaxel | Orally administered paclitaxel-loaded PLGA nanoparticles displayed a 5-fold increase in apparent permeability across Caco-2 cells, employed as in vitro model compared to free paclitaxel. Surface functionalization with folic acid further increased the transport of nanoparticles by 8-fold. | [95] |
| Eudragit® RS | NPs | Insulin | Oral delivery of polymeric nanoparticles preserved the biological activity of encapsulated insulin and showed increased serum insulin level for a prolonged period due to the mucoadhesive property of polycationic polymer (Eudragit® RS) facilitating insulin intestinal uptake. | [96] |
| PLGA | NPs | Doxorubicin | Time and concentration-dependent increase in cellular uptake of doxorubicin-loaded polymeric nanoparticles was observed across Caco-2 cells compared to doxorubicin solution. Orally administered nanoparticles displayed reduced cardiotoxicity compared with the intravenously injected free drug solution. | [97] |
| Chitosan | NPs | Alprazolam | The cationic chitosan polymer was intercomplexed with anionic egg albumin and stabilized with PEG 400 to develop nanoparticles which demonstrated sustained drug delivery up to 24 h. | [98] |
| Trimethyl chitosan | NPs | TNF-α siRNA | Following oral administration mannose functionalized chitosan-cysteine conjugate nanoparticles enhanced siRNA stability in physiological fluid and promoted its transport across intestinal epithelium leading to siRNA uptake by macrophages through endocytosis and cytoplasmic siRNA release. | [99] |
| PCL, PLGA and Eudrajit® RS and RL | NPs | Heparin | Orally administered polymeric nano-particulate heparin showed prolonged anti-Xa activity compared to the heparin solution injected intravenously. A 2-fold increase in activated partial thromboplastin time was reported. | [100] |
| Poly-(DL-lactide-co-glycolide) | NPs | pDNA (alkaline phosphatase, a reporter gene) | pDNA loaded in PLGA polymer particles showed sustained release of pDNA while maintaining its structural and functional integrity for over a month. | [101] |
| PLGA | NPs | Tamoxifen | The oral bioavailability of tamoxifen was increased by 3.84 times compared to free tamoxifen citrate and 11.19 times compared to free tamoxifen when formulated in PLGA nanoparticles. Histopathological studies evidenced the low toxicity of tamoxifen encapsulated nanoparticles compared to free drug. | [102] |
| Chitosan | NPs | Antigen (bovine serum albumin) | Antigen-loaded chitosan nanoparticles; surface engineered with Ulex europaeus agglutinin lectin displayed efficient systemic and mucosal immune responses. | [103] |
| PEG-poly(lactide) diblock copolymers | Micelles | Cyclosporine A | Enhanced stability and intestinal absorption of cyclosporine A-loaded polymeric micelles were reported compared to commercial tablet formulation of cyclosporine A. | [104] |
| Chitosan | NPs | Rifampicin | pH-dependent drug release (75%) at simulated intestinal pH over a period of 24 h | [105] |
| Poly lactic acid-co-PEG | Micelles | Fenofibrate | Micelles having a size range of 158 to 249 nm were prepared as a carrier for oral administration. | [106] |
| Chitosan | Micelles | Paclitaxel | 3.80-fold enhanced bioavailability of paclitaxel micelles compared to Taxol® | [107] |
| Polyvinyl caprolactam-polyvinyl acetate-PEG graft copolymer | Micelles | Silybin | Significant increase in absorption of Silybin following oral administration of drug-loaded polymeric micelles in rats. | [108] |
| Carbomer 934 and poloxamer P 407 | Micelles | Deferoxamine mesylate | Polymeric micelle exhibited 2.5 times increased drug permeation across intestine compared to control. | [109] |
| N-naphthyl-N,O-succinyl chitosan | Micelles | Curcumin | pH-responsive polymeric micelles exhibited a significantly increased amount of drug release in simulated colonic fluid compared to free drug. | [110] |
| Polymer | Delivery System | Therapeutic Molecule | Summary | Ref. |
|---|---|---|---|---|
| Eudragit® RL and RS (50:50) | NPs | Gatifloxacin | Prolonged-release rate and antimicrobial activity. | [117] |
| PLGA and PLGA–PEG | NPs | Melatonin | PLGA–poly (ethylene glycol) loaded melanin displayed prolonged pharmacological effect (reduced intraocular pressure) up to 8 h. | [118] |
| Chitosan-alginate | NPs | Daptomycin | In vitro ocular permeability study of daptomycin-loaded chitosan-alginate nanoparticles showed increased epithelial retention compared to free drug. | [119] |
| PLGAwith Eudragit®RL or coated with Carbopol® | NPs | Cyclosporin A | Polymeric nanoparticles displayed biphasic release i.e., initial burst followed by slow drug release up to 24 h. PLGA with Eudragit®RL showed the highest degree of cellular uptake, tear film concentration, and ocular bioavailability. | [120] |
| PLGA | NPs | Pilocarpine | Chitosan-coated PLGA nanoparticles of pilocarpine displayed prolonged residence time after topical ocular application. | [121] |
| PLGA | NPs | Moxifloxacin | PLGA nanoparticles loaded with moxifloxacin showed higher drug permeation compared to conventional eye drops. Sustained drug release was observed up to 24 h, thereby could avoid frequent administration of dosage. | [122] |
| Gelatin | NPs | Moxifloxacin | Moxifloxacin-loaded gelatin nanoparticles exhibited burst release in the first hour followed by controlled release up to 12 h in an in vitro experiment. | [115] |
| Sodium alginate | NPs | Brimonidine tartrate | In vivo experiment in albino rats displayed prolonged drug release up to 8 h following topical application of brimonidine tartrate loaded gelatin nanoparticles. | [123] |
| Chitosan | NPs | Betaxolol hydrochloride | In vitro drug release study showed an initial burst followed by sustained release up to 12 h. This could be due to mucoadhesiveness of chitosan leading to improved pre-corneal residence time and hence corneal permeability. | [124] |
| Eudragit® RS 100 | NPs | Cyclosporin A | In vitro experiment of cyclosporin A loaded positively charged Eudragit® RS 100 nanoparticles displayed extended drug release. In vivo results showed prolonged residence time of drug and polymeric nanoparticles in vitreous humor. | [125] |
| Poly-ε-caprolactone, 2-hydroxy ethyl methacrylate, PEG diacrylate | NPs | Loteprednol | A drug release study of hydrogel-embedded polymeric nanoparticles showed extended release for up to 12 days. | [126] |
| Chitosan-sodium alginate | NPs | Gatifloxacin | Mucoadhesive polymeric nanoparticles showed fast release during 1 h followed by gradual release up to 24 h. | [105] |
| Poly(beta-amino esters) | NPs | Genes | Polymeric nanoparticles showed transfection efficiency of up to 85% for human reticuloendothelial cells and up to 65% for human umbilical vein endothelial cells. | [127] |
| Poly(D,L-lactide co-glycolide) (PLGA) | NPs | Loteprednol etabonate | Ex vivo trans corneal permeation study across goat cornea revealed an improved permeation profile of formulated drug product compared to the plain dug owing to an increased residence time of PLGA nanoparticles. | [128] |
| Chitosan | NPs | Ornidazole | In vitro drug release study revealed initial burst release followed by gradual release up to 24 h of the ornidazole-loaded mucodhesive chitosan nanoparticles. | [129] |
| Chitosan | NPs | Naringenin | In vitro study of naringenin-loaded chitosan nanoparticles revealed a moderate sustained-release effect. In vivo experiment exhibited prolonged residence time of polymeric nanoparticles compared to naringenin suspension which could be accountable for its improved bioavailability in aqueous humor. | [130] |
| Methoxy poly(ethylene glycol)–poly(ɛ-caprolactone) | Micelles | Not applicable | In vitro studies of polymeric micelles did not show any cytotoxicity against human corneal epithelial cells, human lens epithelial cells, and retinal pigment epithelial cells at micellar concentrations of 0–2 mg/mL. | [131] |
| Eudragit® RS100 | NPs | Vancomycin | Prolonged residence time and Cmax of vancomycin-loaded polymeric nanoparticle was observed resulting in a more than two-fold increment in bioavailability (AUC0.25–24) over control group. | [132] |
| Poly(dl-lactide-co-glycolide) (PLGA) | NPs | Sparfloxacin | In vitro release study exhibited an extended drug release profile. Gamma scintigraphy study in albino rabbits showed prolonged precorneal retention of the radiolabelled sparfloxacin-loaded polymeric nanoparticles compared to the marketed formulation. The formulation displayed non-irritant properties in the Hen egg test-chorioallantoic membrane test. | [133] |
| Poly(ethylene oxide)-poly(propylene oxide) | Micelles | Lornoxicam | Confocal laser studies evidenced the appreciable corneal penetrating power of the polymeric micelles. | [134] |
| Eudragit® RS100 and Eudragit® RL100 | NPs | Cloricromene | In vitro studies showed modified release of drug from the polymer matrix. | [135] |
| Poly(D,L-lactide-co-glycolide) | Nanospheres | Flurbiprofen | Polymeric nanospheres showed a two-fold increment in drug permeation compared to commercial eye drops formulation in an ex vivo experiment. | [136] |
| Eudragit® RL100 | NPs | Aceclofenac | In vitro transcorneal permeability study across excised goat cornea revealed a 2-fold increment in drug permeation from polymeric nanoparticles compared to aqueous drug solution. No signs of corneal damage were reported. | [137] |
| PLGA and chitosan | NPs | Fluocinolone acetonide | Polymeric nanoparticles showed good mucoadhesion characteristics and exhibited rapid and extended drug delivery to the eye evidenced in the pharmacokinetic experiment. | [138] |
| Methylated β-cyclodextrin | NPs | Fenofibrate | Polymeric NPs resulted in low cytotoxicity, low hemolytic potential, and moderately irritable to the eyes. | [139] |
| Zein and hyaluronic acid | NPs | Ciprofloxacin | The developed NPs were biocompatible, had high %EE, and prolonged release of the drug. These can be employed for the treatment of conjunctivitis. | [140] |
| Chitosan | NPs | Meloxicam | Polymeric nanoparticles showed sustained drug release behavior and improved permeation through the cornea. | [141] |
| Poly(L-lactide)-b-poly(methacrylic acid-co-3-acrylamidophenylboronic acid) | Polymeric micelles | Cyclosporin A | The developed micelles resulted in low cytotoxicity, reduction of dose, and improve bioavailability of the therapeutic molecule. | [142] |
| Soluplus | Polymeric micelles | Ibuprofen, idebenone, and miconazole | The developed nanomicelles have potential applications in ocular delivery. | [143] |
| PEGylated fatty acid-block-poly(ε-caprolactone) | Polymeric micelles | Cyclosporin A | The developed nanomicelles showed sustained drug release behavior and can be employed for ocular drug delivery. | [144] |
| PEG-b-PLA | Polymeric micelles | Triamcinolone acetonide | The developed micelles enhanced anti-inflammatory action. | [145] |
| PLGA | Polymeric nanospheres | Lactoferrin | In vitro and in vivo investigations revealed a significant increment in residence time of developed formulation on the eye surface. | [146] |
| PLGA-PEG | Polymeric nanospheres | Pioglitazone | The ex vivo investigations of developed nanospheres revealed that permeation and retention via sclera were greater than corneal and non-irritant for the eye. | [147] |
| Polymer | Delivery System | Therapeutic Molecule | Summary | Ref. |
|---|---|---|---|---|
| PLGA | NPs | pDNA (alkaline phosphatase, a reporter gene) | pDNA loaded in PLGA particles showed sustained release of pDNA while maintaining its structural and functional integrity for over a month. | [101] |
| PCL | NPs | Risperidone | In vivo studies evidenced the prolonged antipsychotic effect of risperidone-loaded biodegradable polymeric nanoparticles compared to risperidone solution administered through intravenous route. | [151] |
| PLGA | NPs | Risperidone | In vivo studies in mice showed the prolonged antipsychotic effect of risperidone-loaded polymeric nanoparticles up to 72 h with fewer extrapyramidal side effects compared to risperidone solution administered subcutaneously. | [152] |
| N-iso propylacrylamide, PVP, and acrylic acid in the ratio of 60:20:20 | NPs | Curcumin | Polymeric nanoparticles of curcumin displayed higher systemic bioavailability in plasma and tissues compared to free curcumin after parenteral administration. No systemic adverse action was reported. | [153] |
| Sodium alginate | Nanoparticles | Carboplatin | Prolonged drug release up to 12 h compared to the pure drug (up to 3 h). The drug was detected in the liver, lungs, and spleen after parenteral administration in Laca mice, thereby showing the potential of sodium alginate nanoparticles as a promising tool for targeted drug delivery. | [154] |
| Methoxypoly (ethylene glycol)-b-poly(ε-caprolactone-co-p-dioxanone) | Micelles | Curcumin | Polymeric micelles showed slow drug release and dose-dependent inhibition of PC-3 human prostate cancer cells. | [155] |
| PEG-b-PLA, PEG-b-PCL | Micelles | Sagopilone | Sagopilone-loaded polymeric micelles were found stable up to 24 h at 37 °C. In vitro studies showed high antiproliferative activity (IC50 < 1 nM). No carrier-related side effects were observed In vivo. | [156] |
| PLGA | Nanoparticles | Zaleplon | The developed NPs showed greater ant-convulsant potential in contrast to free drug. | [157] |
| Poly(propylenesulfide) | Nanoparticles | Diflunisal | The developed NPs resulted in a significant decline in S. aureus-mediated bone degradation and aided in establishing the possibility of systematic delivery of anti-viral agents to treat osteomyelitis. | [158] |
| Chitosan and PCL | Nanoparticles | Paliperidone | The developed formulations showed minimum cellular toxicity. | [159] |
| Hexadecanol-modified chitosan oligosaccharide | Nanomicelles | Gambogic acid | It was reported that nanomicelles resulted in improved cellular uptake and quick drug release. | [160] |
| Hyaluronic acid, vitamin E and styrene maleic anhydride | Nanomicelles | Curcumin analogues | The developed nanomicelles revealed excellent TNBC accumulation with minimum spleen and liver retention. | [161] |
| Polymer | Delivery System | Therapeutic Molecule | Summary | Ref. |
|---|---|---|---|---|
| Chitosan | NPs | Retinol | Encapsulation of retinol in chitosan nanoparticles improved retinol stability and minimized its irritation and toxicity. | [165] |
| PCL | NPs | Indomethacin | Ex vivo permeation study of polymeric nanoparticles displayed higher flux across human skin compared to marketed gel formulation, Indotopic® gel. | [166] |
| PLGA | NPs | Antisense oligodeoxynucleotides | Polymeric nanoparticles displayed 25 to 32% drug release within one day via a diffusion-controlled process followed by PLGA degradation-controlled drug release (39% to 70%) after 14 days. | [167] |
| Gelatin | NPs | STAT6 siRNA | Gelatin nanoparticles exhibited stable and biocompatible formulation for topical delivery of siRNA. | [168] |
| Ethyl cellulose, Eudragit® RS | Nanospheres/Nanocapsules | Dexamethasone | In an ex vivo study, polymeric nanoparticles displayed slower drug release and penetration compared to conventional cream and could be an efficient way to control the release and penetration of dexamethasone on the skin and mucous membrane. | [169] |
| HPMC K15 & PEG 200 | NPs | Rosuvastatin | The ex vivo permeation study exhibited significantly higher permeation via rat skin. | [170] |
| Name | Formulation | Indications | Role | Ref |
|---|---|---|---|---|
| Polymeric nanoparticles | ||||
| Cimzia® | PEGylated antibody fragment (Certolizumab) | Crohn’s disease, rheumatoid arthritis, Ankylosing spondylitis | Improved circulation time and greater stability In vivo. | [177] |
| Krystexxa® | Polymer-protein conjugate (PEGylated porcine-like uricase) | Chronic gout | Improved stability of protein through PEGylation | [178] |
| Plegridy® | Polymer-protein conjugate (PEGylated INF) | Multiple sclerosis | Improved protein stability by PEGylation | [179] |
| Adenovate | Polymer-protein conjugate (PEGylated Factor VIII) | Hemophilia | [180] | |
| Neulasta® | PEGylated GCSF protein | Neutropenia | [181] | |
| Pegasys® | PEGylated IFN alpha-2a protein | Hepatitis B and C | [182] | |
| Copaxone® | Copolymer of L-glutamic acid, L-alanine, L-lysine, and L-tyrosine | Immunomodulator in multiple sclerosis | Improved biocompatibility/solubility | [183] |
| Polymeric micelles | ||||
| Estrasorb™ | Micellar estradiol | Menopausal therapy | Controlled delivery | [184] |
| Product Developed | Therapeutic Agent | Targeted Disease | Objective | Clinical Trial Status/Verification Date |
|---|---|---|---|---|
| Polymeric nanoparticle surface modified with somatostatin analog | Cetuximab | Colon cancer and colorectal cancer | To evaluate the bioavailability and therapeutic window. | Phase 1 (Recruiting)/October 2019 |
| Polymeric micelle | Docetaxel | Esophageal Carcinoma | To determine the effects and safety. | Phase 2 (Recruiting)/July 2018 |
| Polymeric micelle | Paclitaxel | Recurrent breast cancer | To examine the response rate. | Phase 4/June 2009 |
| Polymer basednanoparticles | Docetaxel | Advanced solid malignancies | To determine the maximum tolerated dose and evaluate the safety and pharmacokinetics | Phase 1 (completed)/May 2017 |
| Polymeric micelle | Docetaxel | Head and neck squamous cell carcinoma | To determine safety and efficacy | Phase 2 (Recruiting)/April 2017 |
| Polymeric micelle | Paclitaxel | Ovarian cancer | To determine the maximum tolerated dose and evaluate the safety/efficacy | Phase 1 & 2/Dec 2009 |
| Polymeric micelle | Paclitaxel | Non-small cell lung cancer | To examine the response rate. | Phase 2 (completed)/May 2017 |
| Polymeric micelle | Paclitaxel | Bladder cancer and ureter cancer | To examine safety and efficacy | Phase 2 (completed)/December 2011 |
| Patent Number, Year | Title of the Patent | Description |
|---|---|---|
| US 20150353676 A1, 2015 | Polymeric nanoparticles and a process of preparation thereof | Disclosed composition and method of preparation for emulsifier-free biodegradable polymeric nanoparticles made of a block copolymer having a size range between 30–120 nm. |
| US 20150320856 A1, 2015 | Method for providing polymeric synthetic nanocarriers for generating antigen-specific tolerance immune responses | Described composition and method of preparation for immunosuppressant loaded pH-sensitive polymeric synthetic nanocarrier, surface engineered with APC antigen for site-specific and controlled drug delivery. |
| US 20160324966 A1, 2016 | Polymeric nanocarriers with a light-triggered release mechanism | Highlighted a method for light-triggered release of PLGA polymeric nanocarrier for biomedical applications. |
| US 20150079005 A1, 2015 | Polymeric nanocarriers with a linear dual response mechanism | Depicted a method for preparation of pH-sensitive polymeric nanocarrier which degrades at lower pH and/or reactive oxygen species. |
| US 20090258078 A1, 2009 | Antioxidant polymer nanocarriers for use in preventing oxidative injury | Presented a composition and preparation method for protein encapsulated polymeric nanocarrier with an intent to protect the protein from protease degradation and increase the therapeutic half-life for topical delivery. |
| US8613951 B2, 2013 | Therapeutic polymeric nanoparticles with mTOR inhibitors and methods of making and using the same | Reveals a preparation method for mTOR inhibitors loaded polymeric nanoparticles made of the diblock copolymer. |
| US8715741 B2, 2014 | Water-dispersible oral parenteral, and topical formulations for poorly water-soluble drugs using smart polymeric nanoparticles | Discloses composition for poorly water-soluble drug encapsulated polymeric nanocarrier conversant with mucoadhesive, oral bioavailability and multifunctional systemic targeting characteristics. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tewari, A.K.; Upadhyay, S.C.; Kumar, M.; Pathak, K.; Kaushik, D.; Verma, R.; Bhatt, S.; Massoud, E.E.S.; Rahman, M.H.; Cavalu, S. Insights on Development Aspects of Polymeric Nanocarriers: The Translation from Bench to Clinic. Polymers 2022, 14, 3545. https://doi.org/10.3390/polym14173545
Tewari AK, Upadhyay SC, Kumar M, Pathak K, Kaushik D, Verma R, Bhatt S, Massoud EES, Rahman MH, Cavalu S. Insights on Development Aspects of Polymeric Nanocarriers: The Translation from Bench to Clinic. Polymers. 2022; 14(17):3545. https://doi.org/10.3390/polym14173545
Chicago/Turabian StyleTewari, Akhilesh Kumar, Satish Chandra Upadhyay, Manish Kumar, Kamla Pathak, Deepak Kaushik, Ravinder Verma, Shailendra Bhatt, Ehab El Sayed Massoud, Md. Habibur Rahman, and Simona Cavalu. 2022. "Insights on Development Aspects of Polymeric Nanocarriers: The Translation from Bench to Clinic" Polymers 14, no. 17: 3545. https://doi.org/10.3390/polym14173545