Preparation of Methylcellulose Film-Based CO2 Indicator for Monitoring the Ripeness Quality of Mango Fruit cv. Nam Dok Mai Si Thong
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Ripeness Indicator Fabrication
2.2.1. Preparation of Color Indicator Solution
2.2.2. Preparation of the Color Indicator Label
2.3. Sensitivity of Mixed pH Dyeing Indicator
2.3.1. Measurement of Mixed pH Dyeing Solution Sensitivity
2.3.2. Color Changes in Indicator Labels Caused by Carbon Dioxide (CO2)
2.4. Applications of Ripeness Indicator Label with “Nam Dok Mai Si Thong” Mangoes
2.4.1. Experiment Setup
2.4.2. Quality Changes during the Fruits’ Ripening Period
2.4.3. Kinetics Study of Indicator Labels
2.5. Statistical Analysis
3. Results and Discussion
3.1. Color Change of Indicator Solution
3.2. Change in Color of Color Indicator Label
3.3. Color Changes and Kinetics Study of Indicator Labels during Mango Ripening
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kader, A.A.; Holcroft, D.M. Postharvest: An introduction to the physiology and handling of fruit, vegetables and ornamentals. Horttechnology 1999, 9, 299. [Google Scholar] [CrossRef]
- Oldoni, F.C.A.; Florencio, C.; Bertazzo, G.B.; Grizotto, P.A.; Junior, S.B.; Carneiro, R.L.; Colnago, L.A.; Ferreira, M.D. Fruit quality parameters and volatile compounds from ‘Palmer’mangoes with internal breakdown. Food Chem. 2022, 388, 132902. [Google Scholar] [CrossRef]
- Beauvoit, B.; Belouah, I.; Bertin, N.; Cakpo, C.B.; Colombié, S.; Dai, Z.; Gautier, H.; Génard, M.; Moing, A.; Roch, L. Putting primary metabolism into perspective to obtain better fruits. Ann. Bot. 2018, 122, 1–21. [Google Scholar] [CrossRef]
- Noiwan, D.; Suppakul, P.; Joomwong, A.; Uthaibutra, J.; Rachtanapun, P. Kinetics of mango fruits (Mangifera indica cv.‘Nam Dok Mai Si Thong’) quality changes during storage at various temperatures. J. Agric. Sci. 2017, 9, 199–212. [Google Scholar] [CrossRef]
- Busatto, N.; Vittani, L.; Farneti, B.; Khomenko, I.; Caffini, M.; Faccini, S.; Boschetti, M.; Costa, F. Physiological and molecular characterization of the late ripening stages in Mangifera indica cv Keitt. Postharvest Biol. Technol. 2022, 183, 111746. [Google Scholar] [CrossRef]
- Matulaprungsan, B.; Wongs-Aree, C.; Penchaiya, P.; Boonyaritthongchai, P.; Srisurapanon, V.; Kanlayanarat, S. Analysis of Critical Control Points of Post-Harvest Diseases in the Material Flow of Nam Dok Mai Mango Exported to Japan. Agriculture 2019, 9, 200. [Google Scholar] [CrossRef]
- Baek, S.; Maruthupandy, M.; Lee, K.; Kim, D.; Seo, J. Preparation and characterization of a poly (ether-block-amide) film–based CO2 indicator for monitoring kimchi quality. React. Funct. Polym. 2018, 131, 75–83. [Google Scholar] [CrossRef]
- Abbott, J.A. Quality measurement of fruits and vegetables. Postharvest Biol. Technol. 1999, 15, 207–225. [Google Scholar] [CrossRef]
- Ziosi, V.; Noferini, M.; Fiori, G.; Tadiello, A.; Trainotti, L.; Casadoro, G.; Costa, G. A new index based on vis spectroscopy to characterize the progression of ripening in peach fruit. Postharvest Biol. Technol. 2008, 49, 319–329. [Google Scholar] [CrossRef]
- Peris, M.; Escuder-Gilabert, L. A 21st century technique for food control: Electronic noses. Anal. Chim. Acta 2009, 638, 1–15. [Google Scholar] [CrossRef]
- Lebrun, M.; Plotto, A.; Goodner, K.; Ducamp, M.-N.; Baldwin, E. Discrimination of mango fruit maturity by volatiles using the electronic nose and gas chromatography. Postharvest Biol. Technol. 2008, 48, 122–131. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, J.; Ye, S. Predictions of acidity, soluble solids and firmness of pear using electronic nose technique. J. Food Eng. 2008, 86, 370–378. [Google Scholar] [CrossRef]
- Zakaria, A.; Shakaff, A.Y.M.; Masnan, M.J.; Saad, F.S.A.; Adom, A.H.; Ahmad, M.N.; Jaafar, M.N.; Abdullah, A.H.; Kamarudin, L.M. Improved maturity and ripeness classifications of magnifera indica cv. harumanis mangoes through sensor fusion of an electronic nose and acoustic sensor. Sensors 2012, 12, 6023–6048. [Google Scholar] [CrossRef]
- Hor, S.; Léchaudel, M.; Mith, H.; Bugaud, C. Fruit density: A reliable indicator of sensory quality for mango. Sci. Hortic. 2020, 272, 109548. [Google Scholar] [CrossRef]
- Gomez, A.H.; Wang, J.; Hu, G.; Pereira, A.G. Discrimination of storage shelf-life for mandarin by electronic nose technique. LWT-Food Sci. Technol. 2007, 40, 681–689. [Google Scholar] [CrossRef]
- Gómez, A.H.; Wang, J.; Hu, G.; Pereira, A.G. Monitoring storage shelf life of tomato using electronic nose technique. J. Food Eng. 2008, 85, 625–631. [Google Scholar] [CrossRef]
- Mizrach, A. Determination of avocado and mango fruit properties by ultrasonic technique. Ultrasonics 2000, 38, 717–722. [Google Scholar] [CrossRef]
- Mizrach, A. Ultrasonic technology for quality evaluation of fresh fruit and vegetables in pre-and postharvest processes. Postharvest Biol. Technol. 2008, 48, 315–330. [Google Scholar] [CrossRef]
- Vergara, A.; Llobet, E.; Ramírez, J.; Ivanov, P.; Fonseca, L.; Zampolli, S.; Scorzoni, A.; Becker, T.; Marco, S.; Wöllenstein, J. An RFID reader with onboard sensing capability for monitoring fruit quality. Sens. Actuators B Chem. 2007, 127, 143–149. [Google Scholar] [CrossRef]
- Hussain, A.; Pu, H.; Sun, D.-W. Innovative nondestructive imaging techniques for ripening and maturity of fruits–a review of recent applications. Trends Food Sci. Technol. 2018, 72, 144–152. [Google Scholar] [CrossRef]
- Li, B.; Lecourt, J.; Bishop, G. Advances in non-destructive early assessment of fruit ripeness towards defining optimal time of harvest and yield prediction—A review. Plants 2018, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Yam, K.L.; Takhistov, P.T.; Miltz, J. Intelligent packaging: Concepts and applications. J. Food Sci. 2005, 70, R1–R10. [Google Scholar] [CrossRef]
- Kuswandi, B.; Murdyaningsih, E.A. Simple on package indicator label for monitoring of grape ripening process using colorimetric pH sensor. J. Food Meas. Charact. 2017, 11, 2180–2194. [Google Scholar] [CrossRef]
- Kuswandi, B.; Maryska, C.; Abdullah, A.; Heng, L.Y. Real time on-package freshness indicator for guavas packaging. J. Food Meas. Charact. 2013, 7, 29–39. [Google Scholar] [CrossRef]
- Niponsak, A.; Laohakunjit, N.; Kerdchoechuen, O.; Wongsawadee, P. Development of smart colourimetric starch–based indicator for liberated volatiles during durian ripeness. Food Res. Int. 2016, 89, 365–372. [Google Scholar] [CrossRef] [PubMed]
- Lang, C.; Hübert, T. A colour ripeness indicator for apples. Food Bioprocess Technol. 2012, 5, 3244–3249. [Google Scholar] [CrossRef]
- Ebnesajjad, S. Handbook of Adhesives and Surface Preparation: Technology, Applications and Manufacturing; William Andrew: Norwich, NY, USA, 2010. [Google Scholar]
- Baek, S.; Maruthupandy, M.; Lee, K.; Kim, D.; Seo, J. Freshness indicator for monitoring changes in quality of packaged kimchi during storage. Food Packag. Shelf Life 2020, 25, 100528. [Google Scholar] [CrossRef]
- Alavi, S.; Thomas, S.; Sandeep, K.; Kalarikkal, N.; Varghese, J.; Yaragalla, S. Polymers for Packaging Applications; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Nopwinyuwong, A.; Trevanich, S.; Suppakul, P. Development of a novel colorimetric indicator label for monitoring freshness of intermediate-moisture dessert spoilage. Talanta 2010, 81, 1126–1132. [Google Scholar] [CrossRef]
- Monisha, S.; Niveditha, A.; Pavithra, D.; Chidanand, D.; Sunil, C.; Rawson, A. Effect of microwave treatment on colour of turmeric (Curcuma longa L.). Int. J. Sci. Environ. Technol. 2016, 5, 2062–2070. [Google Scholar]
- Sivakumar, D.; Naudé, Y.; Rohwer, E.; Korsten, L. Volatile compounds, quality attributes, mineral composition and pericarp structure of South African litchi export cultivars Mauritius and McLean’s Red. J. Sci. Food Agric. 2008, 88, 1074–1081. [Google Scholar] [CrossRef]
- AOAC. Acidity (Titratable) of Fruitproducts with AOAC Official Method 920, 17th ed.; AOAC: Scottsdale, AZ, USA, 2002. [Google Scholar]
- Taoukis, P. Modelling the use of time-temperature indicators in distribution and stock rotation PS Taoukis, National Technical University of Athens. Food Process. Model. 2001, 59, 402–431. [Google Scholar]
- Mook, W.; Rozanski, K. Environmental Isotopes in the Hydrological Cycle. Principles and Applications, Volumes I, IV and V; IAEA Publish: New York, NY, USA, 2000. [Google Scholar]
- Sabnis R, W. Handbook Of Acid Base Indicators; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Puligundla, P.; Jung, J.; Ko, S. Carbon dioxide sensors for intelligent food packaging applications. Food Control. 2012, 25, 328–333. [Google Scholar] [CrossRef]
- Peleg, M.; Bagley, E.B. Physical properties of foods. In Proceedings of the IFT Basic Symposium Series (USA), Las Vegas, NV, USA, 1 January 1983. [Google Scholar]
- Rukchon, C.; Nopwinyuwong, A.; Trevanich, S.; Jinkarn, T.; Suppakul, P. Development of a food spoilage indicator for monitoring freshness of skinless chicken breast. Talanta 2014, 130, 547–554. [Google Scholar] [CrossRef]
- Diaz-Perez, J.C.; Bautista, S.; Villanueva, R. Quality changes in sapote mamey fruit during ripening and storage. Postharvest Biol. Technol. 2000, 18, 67–73. [Google Scholar] [CrossRef]
- Rumainum, I.M.; Worarad, K.; Srilaong, V.; Yamane, K. Fruit quality and antioxidant capacity of six Thai mango cultivars. Agric. Nat. Resour. 2018, 52, 208–214. [Google Scholar] [CrossRef]
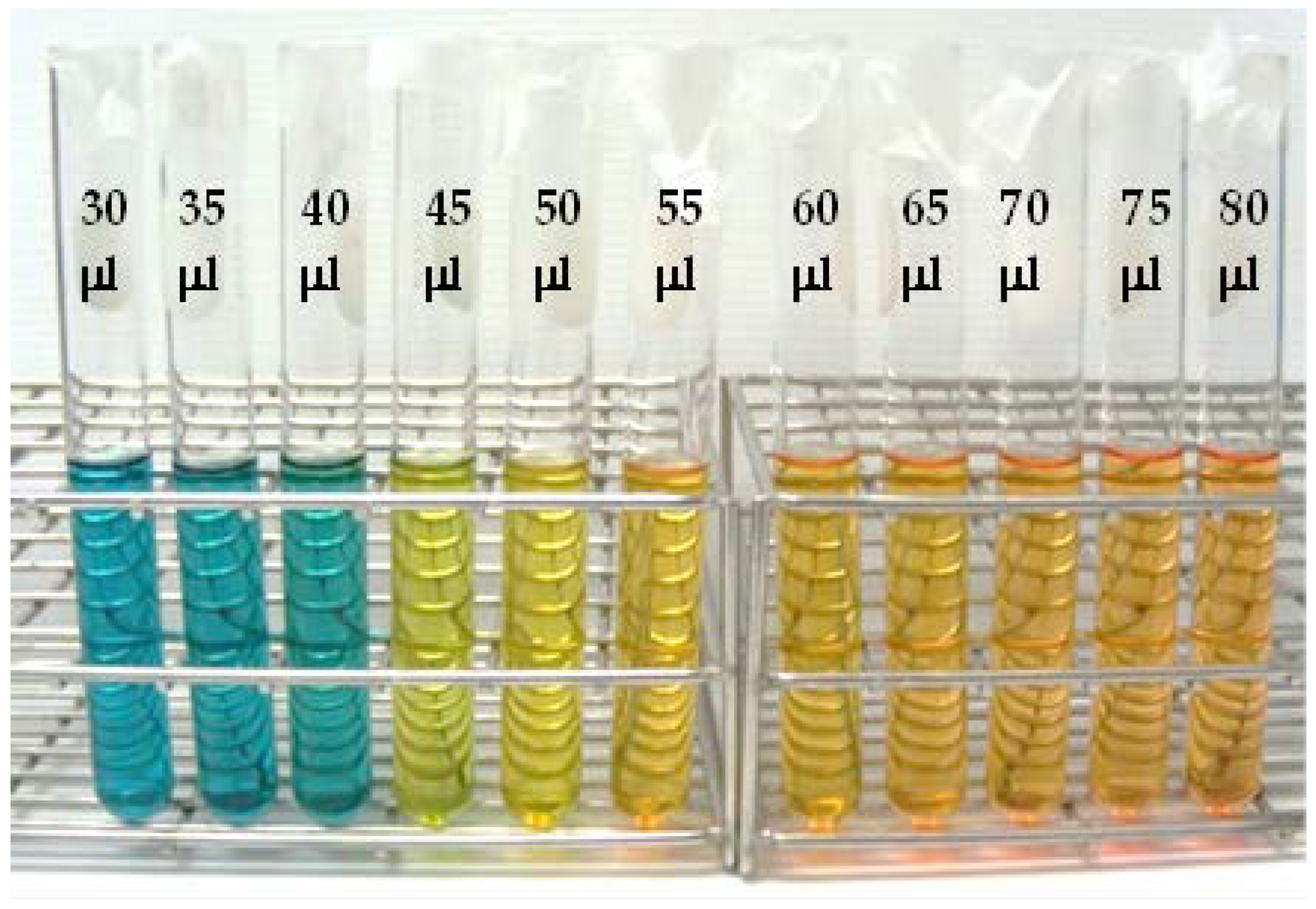

| 0.1 M Acetic Acid (µL) | pH | Absorbance (416 nm) |
|---|---|---|
| 30 | 10.59 | 1.4191 |
| 35 | 10.04 | 1.5552 |
| 40 | 9.04 | 1.6053 |
| 45 | 8.1 | 1.6082 |
| 50 | 7.64 | 1.6065 |
| 55 | 7.04 | 1.6009 |
| 60 | 6.66 | 1.5987 |
| 65 | 6.35 | 1.5765 |
| 70 | 6.17 | 1.5624 |
| 75 | 6.01 | 1.5616 |
| 80 | 5.91 | 1.5524 |
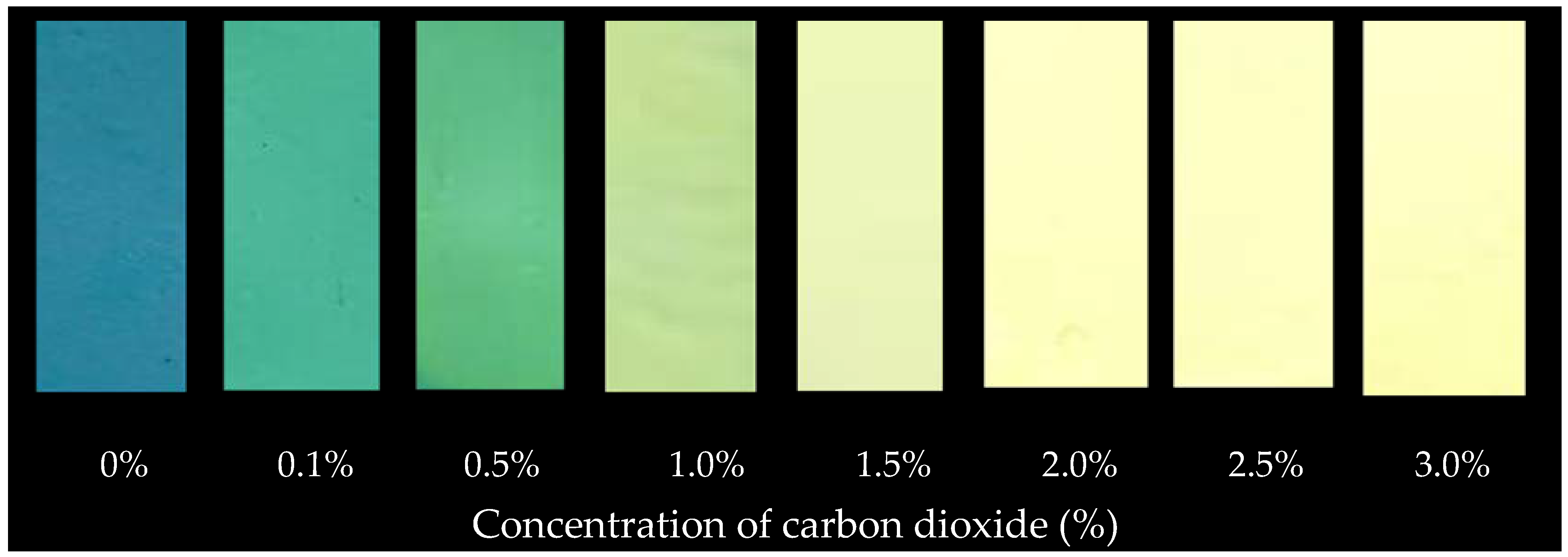
| Carbon Dioxide (%) | L* | a* | b* |
|---|---|---|---|
| 0 | 40.38 | −16.3 | −19.14 |
| 0.1 | 41.52 | −21.9 | −6.18 |
| 0.5 | 49.34 | −25.74 | −4.57 |
| 1 | 58.09 | −22.38 | 10.18 |
| 1.5 | 62.72 | −18.22 | 17.28 |
| 2 | 70.84 | −8.34 | 26.78 |
| 2.5 | 74.93 | −3.81 | 29.6 |
| 3 | 78.41 | −0.28 | 34.09 |
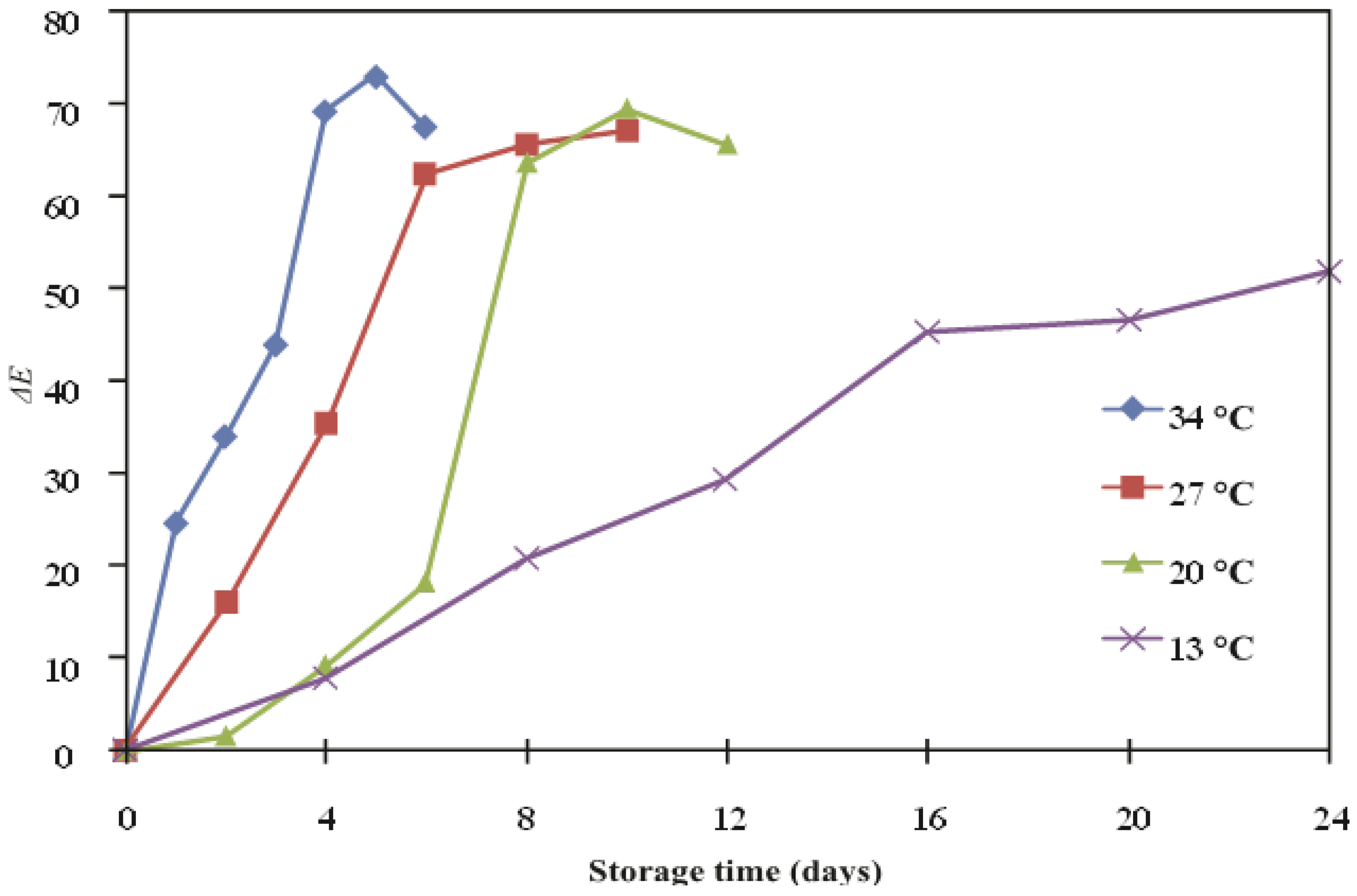
| Temperature (°C) | ∆E | ||
|---|---|---|---|
| k (day−1) | R2 | Ea (kJ/mol) | |
| 13 | 0.77 | 0.9737 | 52.3 |
| 20 | 0.16 | 0.9417 | |
| 27 | 0.249 | 0.9845 | |
| 34 | 0.271 | 0.915 | |
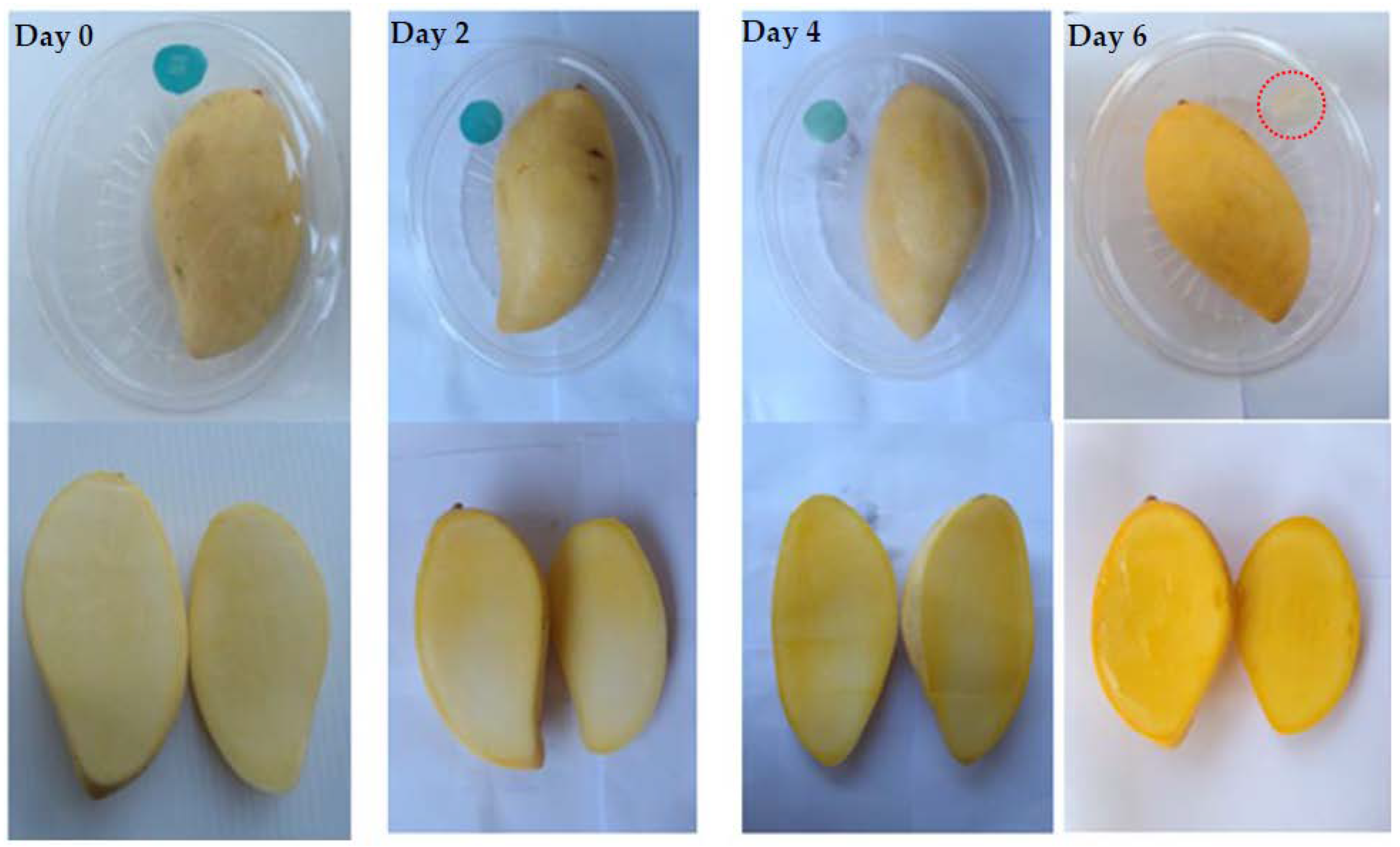
| Storage Time (Days) | 0 | 2 | 4 | 6 |
|---|---|---|---|---|
| SSC (%) | 10.70 | 11.78 | 14.82 | 18.26 |
| TA (%) | 2.84 | 2.38 | 1.29 | 0.21 |
| firmness (N) | 44.54 | 12.94 | 3.44 | 2.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noiwan, D.; Suppakul, P.; Rachtanapun, P. Preparation of Methylcellulose Film-Based CO2 Indicator for Monitoring the Ripeness Quality of Mango Fruit cv. Nam Dok Mai Si Thong. Polymers 2022, 14, 3616. https://doi.org/10.3390/polym14173616
Noiwan D, Suppakul P, Rachtanapun P. Preparation of Methylcellulose Film-Based CO2 Indicator for Monitoring the Ripeness Quality of Mango Fruit cv. Nam Dok Mai Si Thong. Polymers. 2022; 14(17):3616. https://doi.org/10.3390/polym14173616
Chicago/Turabian StyleNoiwan, Duangjai, Panuwat Suppakul, and Pornchai Rachtanapun. 2022. "Preparation of Methylcellulose Film-Based CO2 Indicator for Monitoring the Ripeness Quality of Mango Fruit cv. Nam Dok Mai Si Thong" Polymers 14, no. 17: 3616. https://doi.org/10.3390/polym14173616






